Abstract
The bacterial cell envelope is the interface between a bacterium and its environment and is constantly exposed to environmental changes. The BaeSR two-component system regulates one of six envelope stress responses in Escherichia coli and is induced by spheroplasting, overexpression of the pilin subunit PapG, and exposure to indole. The known BaeR regulon is small, consisting of eight genes, mdtABCD-baeSR, acrD, and spy, two of which encode the BaeSR two-component system itself. In this study, we investigated the molecular nature of the BaeS-inducing cue and the cellular role of the BaeSR envelope stress response. We demonstrated that at least two flavonoids and sodium tungstate are novel inducers of the BaeSR response. Interestingly, flavonoids and sodium tungstate led to much stronger induction of the BaeSR response in an mdtA efflux pump mutant, while indole did not. These findings are consistent with the hypothesis that flavonoids and sodium tungstate are natural substrates of the MdtABC efflux pump. Indole has recently been implicated in cell-cell signaling and biofilm repression through a putative interaction with the LuxR homologue SdiA. Using genetic analyses, we found that induction of the BaeSR response by indole occurs via a pathway separate from the SdiA biofilm pathway. Further, we demonstrated that the BaeSR response does not influence biofilm formation, nor is it involved in indole-mediated inhibition of biofilm formation. We hypothesize that the main function of the Bae response is to upregulate efflux pump expression in response to specific envelope-damaging agents.
INTRODUCTION
The Gram-negative envelope is composed of an inner membrane (IM), an outer membrane (OM), and a peptidoglycan-containing periplasmic space between them (17, 32). The envelope is a unique compartment of the bacterial cell and acts as the interface between the intracellular space and the extracellular environment. As such, the envelope is exposed to environmental changes and stresses, including changes in temperature, pH, and osmolarity; exposure to toxic compounds; and oxidative stress. To adapt to such stresses, bacteria have evolved a number of envelope stress responses that sense specific cues and prompt the cell to respond appropriately, usually by regulating gene expression.
To date, six envelope stress responses have been identified in Escherichia coli. They are the Cpx, sigma E, Bae, Psp (phage shock protein), Rcs, and vesicle release responses (31). The Cpx and sigma E pathways are thought to sense misfolded proteins destined for the periplasm and outer membrane, respectively (28), and subsequently to upregulate various envelope protein folding and degrading factors (28, 31). The Psp response is thought to be induced by and to mediate adaptation to changes to the proton motive force across the inner membrane (12). The Rcs pathway was discovered as a “regulator of capsular synthesis” (cps genes) and more recently has been implicated in the response to damaged peptidoglycan (13, 33, 38). The recently discovered vesicle release pathway is thought to be a more general envelope stress response that collects and disposes of misfolded proteins and other unwanted molecules in the envelope by packaging them into outer membrane vesicles that are released into the environment (20, 21).
The BaeSR-regulated response was first discovered in a screen for additional two-component systems in E. coli and was later classified as an envelope stress response by Raffa and Raivio, who showed that induction of spy gene expression during spheroplasting, exposure to indole, and overexpression of the pilin subunit PapG in the absence of its chaperone were partially attributable to BaeSR pathway activity (27). All three of these inducers have been shown to also induce other stress responses, such as the sigma E response (PapG overexpression) and the Cpx pathway (spheroplasting, indole, and PapG overexpression) (9). However, in the case of indole at least, the majority of spy gene induction occurs through BaeSR (27).
The BaeSR pathway is a classical two-component system and as such consists of an inner-membrane-bound sensor histidine kinase (HK), BaeS, containing a periplasmic sensing domain, and a cytoplasmic response regulator (RR), BaeR (27). BaeS communicates with BaeR using phosphotransfer between the histidine of BaeS and the conserved aspartate residue in the BaeR receiver domain (23). Phosphorylation of BaeR to BaeR∼P results in an active output (DNA binding) domain, and BaeR∼P acts as a dimer to regulate transcription of the BaeR regulon.
The confirmed regulon of BaeR consists of the acrD gene, the spy gene, and the mdtABCD-baeSR operon. These genes encode two RND (resistance, nodulation, and cell division) multidrug efflux pumps, MdtABC and AcrD; a predicted MFS (major facilitator superfamily) multidrug efflux pump, MdtD; the BaeS and BaeR proteins; and the periplasmic protein Spy, whose function is unknown (27). It has also been shown that CpxR∼P, of the CpxAR system can bind to the mdtABCD-baeSR, acrD, and spy promoters, but in the cases of mdtABCD and acrD, this binding is thought to be auxiliary to BaeR∼P binding, and in the case of spy, these pathways are believed to act independently of one another (8). Another putative regulon member is ycaC, encoding a predicted cysteine hydrolase (25). Although ycaC does appear to be directly regulated by BaeR, its activation is weak, and the predicted binding site is far from consensus (50%) compared to other regulon members (mdtABCD-baeSR, acrD, and spy have 94%, 94%, and 100% consensus binding sites, respectively) (25). Additional putatively Bae-regulated genes from recent studies include tolC, which encodes an outer membrane protein that is utilized by many multidrug efflux pumps, as well as several other conserved protein-encoding genes of unknown function, yicO, ygcL, ynjA, ynjB, and yeeN (2, 3, 25). The tolC gene lacks a BaeR-binding box, and all six genes are changed less than 3-fold upon Bae activation, so they may represent more weakly regulated members of the BaeR regulon (2, 3, 25).
The nature of the signal(s) generated by indole, PapG overexpression, and spheroplasting that result in BaeS induction is unknown. Two recent studies have suggested additional inducing cues of the Bae pathway. In one, a phenotypic microarray found that a BaeSR mutant was more sensitive to growth in the presence of sodium tungstate, myricetin, gallic acid, and nickel chloride (43). A more recent study of global gene expression changes in the presence of plant secondary metabolites, called condensed tannins, discovered genes regulated by the Bae pathway to be upregulated in their presence (44). Zoetendal and colleagues also found that BaeSR mutants were more sensitive to growth in the presence of condensed tannins and that overexpression of the MdtABC efflux pump aided in survival under these conditions (44). These studies suggest novel inducing cues of the BaeSR pathway, and also that induction of the MdtABC efflux pump may be a major means by which the BaeSR pathway mediates adaptation to these compounds.
Interestingly, the known Bae inducer indole has recently been implicated in cell-cell signaling, more prevalently at lower temperatures (39). Recent work has shown that indole interacts with the LuxR homologue SdiA to negatively influence early biofilm formation by affecting SdiA-mediated transcription of genes such as ftsQ (6, 14, 15, 16, 40). The involvement of indole in these processes suggests that the Bae pathway might also be related to biofilm formation. The goal of this study was to identify and further characterize the inducing cues of the BaeSR two-component system and to clarify its physiological role with regard to the envelope stress response of E. coli and biofilm formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The wild-type (WT) laboratory strain of E. coli used was MC4100 (4) (for a complete strain list, see Table 1). All strains were grown at 37°C in Luria-Bertani (LB) broth or on LB plates (1% Bacto Tryptone, 0.5% Bacto yeast extract, 0.5% NaCl, and 1.5% agar for plates). The KEIO library was used as a source of kanamycin (Kn)-resistant deletion strains, and deletion strains for this study were constructed by generalized P1 transduction, as described previously (1). The antibiotic concentrations used were 30 μg/ml kanamycin, 25 μg/ml chloramphenicol, and 100 μg/ml ampicillin.
Table 1.
Bacterial strains, plasmids, and phages used in this study
| Name | Genotype or description | Reference |
|---|---|---|
| Strain | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 (Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | 4 |
| 2K1056 | F− λ−IN(rrnD-rrnE)1rph-1Δ (argF-lac)U169 | 26 |
| TR530 | MC4100 λRS88[spy-lacZ] | 29 |
| TR776 | MC4100 baeS1::Tn10cam | 27 |
| fimA::Kn | 2K1056 ΔfimA::Kn | This study |
| MC4100-amp | MC4100(pUC19) | This study |
| ΔbaeR-amp | TR1162(pUC19) | This study |
| CO4 | MC4100 mdtA::λplacMu53 | This study |
| SL99 | 2K1056 ΔbaeR::Kn | This study |
| SL100 | TR530 ΔbaeR::Kn | This study |
| SL102 | TR530 ΔmdtA::Kn | This study |
| SL103 | TR530 Δmtr::Kn | This study |
| SL113 | TR530 ΔtnaA::Kn | This study |
| SL114 | TR530 ΔsdiA::Kn | This study |
| SL119 | 2K1056 baeS1::Tn10cam | This study |
| SL131 | TR530 ΔacrF::Kn | This study |
| SL138 | MC4100 λRS88[mdtA-lacZ] | This study |
| SL142 | SL102 (pCA24N) | This study |
| SL143 | SL102 (pCA-mdtA) | This study |
| SL150 | SL138 ΔmdtA::Kn | This study |
| TR1162 | TR530 ΔbaeR | This study |
| Plasmid | ||
| pUC19 | Ampr cloning vector; used for β-lactamase production in the study | Invitrogen |
| pRS415 | bla-T14-EcoRI-SmaI-BamHI-lacZ+ | 34 |
| pCA24N | Vector control for the ASKA library | 11 |
| pCA-mdtA | pCA24N containing the mdtA gene behind the pT5-lac promoter (ASKA library) | 11 |
| Phage | ||
| λRS88 | bla′-lacZsc | 34 |
MIC experiments.
MIC experiments were done in 96-well polystyrene plates. Overnight cultures were grown in triplicate in LB, subcultured (2 μl per well) into 200 μl of LB in a 96-well plate containing serial dilutions of the compound to be tested, and grown for 24 h. A Perkin Elmer Victor2 1420 plate reader was used to take readings of the optical density at 600 nm (OD600) every hour for 8 h, with a final reading at 24 h. The same plate reader was used for all plate readings in the other methods described below. The MIC was determined as the lowest concentration of compound tested at which growth was inhibited (OD600 maximum after 24 h, 0.1).
Construction of a single-copy mdtA-lacZ promoter fusion.
The mdtA-lacZ promoter fusion (strain SL138) was made as described previously using the transcriptional fusion vector pRS415 and the λRS88 phage for the transfer of a single-copy fusion to the chromosome (34). Restriction enzyme-tagged primers (Eco-mdtA-fwd, 5′-CGCGAATTCAATATTTGCTGGCAGGATCG-3′, and Bam-mdtA-rev, 5′-CGAGGATCCGGCGATAACCACCACGATTA-3′) were used to amplify 501 bp of the mdtABCD-baeSR promoter region, including the predicted BaeR box binding site and CpxR binding site. The PCR product was purified (MP Biomedicals GeneClean III kit), restriction digested with EcoRI and BamHI (Invitrogen), and cloned into the EcoRI-BamHI-digested pRS415 vector, which contains a promoterless lacZ gene. λRS88 was used to move the promoter fusion onto the chromosome of MC4100 at the λatt site in single copy as described previously (34).
β-Galactosidase assays.
β-Galactosidase assays were based on the 96-well plate assay described previously (36). Briefly, 2-ml overnight cultures in LB plus appropriate antibiotics were subcultured (1/50) into fresh medium of the same kind and grown for approximately 3 h (early log phase), induced with the chemical of interest, and grown for an additional 2 h (final OD600, ∼0.7). For complementation experiments with mdtA, the pCA-mdtA plasmid from the ASKA collection lacking the green fluorescent protein (GFP) tag was used with the pCA24N vector control (11). For these experiments, pCA-mdtA was induced with 0.1 mM IPTG (isopropyl-β-d-thiogalactoside; Invitrogen) for 2 h at the same time as chemical induction. The cultures were centrifuged, and the cells were resuspended in 2 ml of freshly prepared 1× Z buffer (10 ml 10× Z buffer [10× Z buffer is 600 mM Na2HPO4 · 7H2O, 400 mM NaH2PO4 · H2O, 100 mM KCl, 10 mM MgSO4 · 7H2O], 90 ml distilled water [dH2O], 270 μl β-mercaptoethanol). The OD600 was read in 96-well polystyrene plates using a plate reader. Cells were lysed using chloroform and 0.1% SDS, and cellular debris was precipitated; then, the lysed cell mixture was diluted in 1× Z buffer in 96-well plates (50 μl lysed cell mixture, 150 μl 1× Z buffer), and 50 μl 10-mg/ml ONPG (o-nitrophenyl-β-d-galactopyranoside) (Sigma) was added. The A420 was read 20 times over approximately 30 min in the plate reader, and Miller units were calculated. Experiments were done in triplicate.
Biofilm assays.
E. coli K-12 strain 2K1056 was used for all biofilm assays because MC4100 does not form biofilms (26). Individual colonies were inoculated into 200 μl fresh LB medium without antibiotics in a 96-well polystyrene plate in triplicate. The plates were grown static at room temperature (approximately 22°C) for 48 h, and then the liquid culture was discarded and the biofilm was rinsed once with distilled water. The biofilms were stained with 125 μl 1% crystal violet for 15 min, rinsed thoroughly with distilled water, and dried. The crystal violet was dissolved off the wells with 200 μl of an 80:20 ethanol-acetone solution by gentle shaking for 5 min; 80-μl aliquots were transferred to a fresh plate, and the A600 was read in a plate reader.
RESULTS
Sodium tungstate and flavonoids are novel inducers of the BaeSR pathway.
A phenotypic microarray showed that a baeSR mutant is more sensitive to growth in the presence of several compounds: myricetin, sodium tungstate, gallic acid, and nickel chloride (43). Based on this observation, we predicted that perhaps the BaeSR pathway is involved in normal cellular resistance to these compounds and therefore that these compounds may also induce the Bae response. Additionally, previous work on the MdtABC efflux pump found that it might confer resistance to novobiocin, at least in the absence of the major efflux pump AcrAB (22). Finally, a recent study in Salmonella enterica serovar Typhimurium showed that 0.5 mM zinc is able to induce the BaeSR pathway (24). Using a spy-lacZ transcriptional reporter, we tested these different compounds for the ability to induce the BaeSR pathway. MICs were determined for each compound, and induction was tested at a concentration below the MIC.
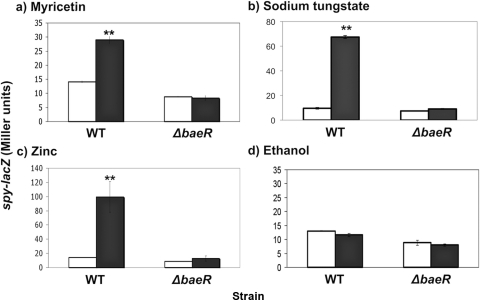
Nickel chloride (250 μg/ml) failed to induce the pathway, and gallic acid (200 μg/ml) induced it less than 2-fold, but myricetin (10 μg/ml) and sodium tungstate (5 mM) both resulted in significantly higher spy-lacZ levels than in uninduced cells (approximately 2- to 3-fold and 6- to 7-fold, respectively; P < 0.01) (Fig. 1a and b and data not shown). In either a ΔbaeR::Kn or a ΔbaeS::Kn mutant, induction of the spy-lacZ reporter by myricetin and sodium tungstate is lost (Fig. 1a and b and data not shown). This induction partially correlates with the sensitivity shown by the phenotypic microarray, where the baeSR mutant exhibited greater sensitivity to sodium tungstate and myricetin than to nickel chloride (43). However, the phenotypic microarray also showed that the sensitivity to gallic acid was greater than to myricetin, yet gallic acid did not induce the BaeSR response more than myricetin (Fig. 1a and data not shown) (43). The concentration range used to test sensitivity to gallic acid in the phenotypic microarray (the range was from 3,683 μg/ml to 136 μg/ml; Biolog Inc.) is generally much higher than our tested concentration of 200 μg/ml and could partially account for this discrepancy; structural differences could also play a role, as discussed below. A solvent control for myricetin showed that ethanol alone cannot induce the Bae response at the same concentration as that used under the myricetin conditions or at a higher concentration (Fig. 1d and data not shown). When 0.5 mM and 1 mM zinc were tested, we saw induction of the spy-lacZ reporter upon exposure to 1 mM zinc, but not 0.5 mM zinc (Fig. 1c and data not shown). Novobiocin resulted in less than 2-fold induction of the spy-lacZ reporter (data not shown).
Fig. 1.
The Bae pathway is specifically induced by myricetin, sodium tungstate, and zinc. β-Galactosidase assays using a spy-lacZ transcriptional reporter were done in both a wild-type (TR530) and a ΔbaeR::Kn (SL100) background. This was done for 10 μg/ml myricetin (a), 5 mM sodium tungstate (b), 1 mM zinc (c), and 0.76% ethanol (d). Induction was done for 2 h after the cultures reached mid-log phase (final OD600 = ∼0.7). **, P < 0.001 compared to uninduced cells. The error bars indicate standard deviations.
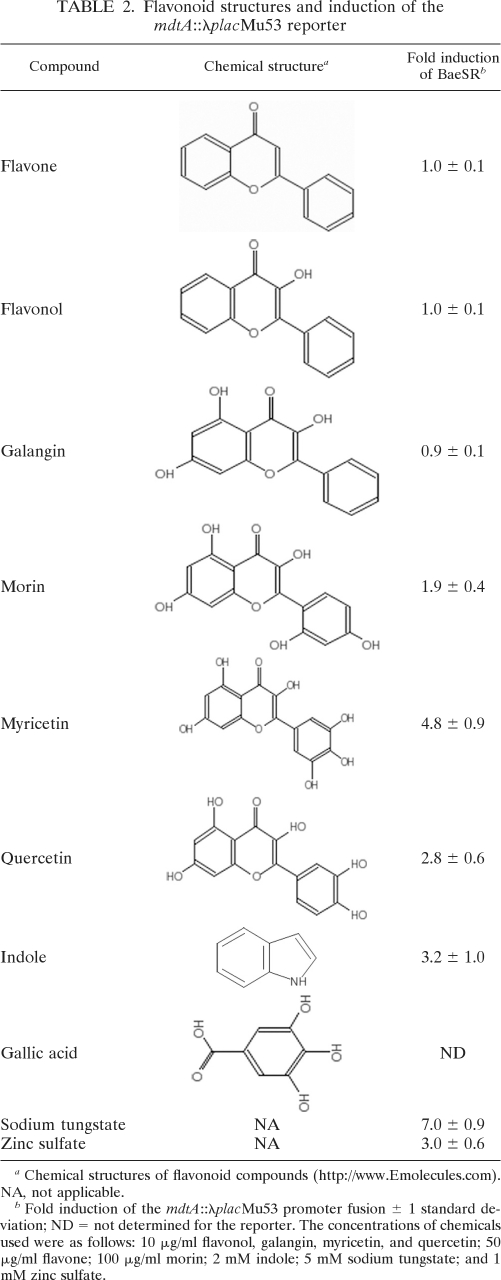
Myricetin is part of a class of plant secondary metabolites known as flavonoids. Interestingly, flavonoids are closely related to condensed tannins, which were previously shown to be more toxic to a strain lacking BaeSR (44). To further test the ability of flavonoids to induce the BaeSR pathway, a number of structurally related compounds were chosen for testing. The ability of myricetin, quercetin, galangin, morin, flavone, and flavonol to induce expression of an mdtA-lacZ reporter gene located at the native mdtA locus (mdtA::λplacMu53) was tested (Table 2). Quercetin induced the BaeSR pathway similarly to myricetin, and morin induced it approximately 2-fold, but galangin, flavone, or flavonol exposure did not result in BaeSR pathway activation (Table 2). Differences in the abilities of certain compounds of this class to induce the Bae response may be related to differences in the numbers and arrangements of their hydroxyl groups, especially on the B ring, where they have been shown to affect flavonoid reactivity (Table 2) (30). Gallic acid, which was shown here to weakly induce BaeSR, also contains three hydroxyl groups (Table 2); although the single ring structure of gallic acid is much smaller than the ring structure of the flavonoids, the hydroxylated ring structure could explain its ability to (weakly) induce BaeSR. Altogether, these data demonstrate that the Bae response is strongly induced by flavonoids bearing hydroxylated B rings, together with some transition metals.
Table 2.
Flavonoid structures and induction of the mdtA::λplacMu53 reporter
Chemical structures of flavonoid compounds (http://www.Emolecules.com). NA, not applicable.
Fold induction of the mdtA::λplacMu53 promoter fusion ± 1 standard deviation; ND = not determined for the reporter. The concentrations of chemicals used were as follows: 10 μg/ml flavonol, galangin, myricetin, and quercetin; 50 μg/ml flavone; 100 μg/ml morin; 2 mM indole; 5 mM sodium tungstate; and 1 mM zinc sulfate.
Finally, to test the specificities of these inducing compounds for the BaeSR pathway, we tested the induction of several transcriptional lacZ reporters fused to CpxR-regulated genes (cpxP, degP, and dsbA). Since the Cpx response is known to respond to other BaeSR-inducing cues, such as indole, PapG, and spheroplasting, and CpxR is known to bind to the mdtABCD-baeSR and acrD promoters, we needed to ensure that these inducing cues do not activate the Cpx pathway. We observed that none of these lacZ fusions were significantly induced in the presence of 5 mM sodium tungstate (1.3-, 1.2-, and 1.1-fold, respectively) (data not shown) or 10 μg/ml quercetin (0.9-, 0.8-, and 0.9-fold, respectively) (data not shown). The degP promoter is also regulated by the σE response (5), so it does not appear that the σE response is induced by sodium tungstate and the flavonoids, either.
A ΔmdtA::Kn efflux pump mutant is more sensitive to induction by sodium tungstate and flavonoids.
The first reporter our laboratory used to test myricetin and sodium tungstate induction of the Bae pathway was an mdtA::λplacMu53 strain (CO4), in which the λplacMu53 element is inserted in the mdtA gene. Myricetin (10 μg/ml) induces this reporter approximately 5-fold, and sodium tungstate (5 mM) induces it approximately 6- to 7-fold (Table 2). Curiously, our spy-lacZ promoter fusion at the λRS88 site is induced only 2-fold by myricetin (Fig. 1a). We hypothesized that perhaps the diminished induction of the spy-lacZ reporter was because there was a reduced concentration of inducer present due to the action of the intact MdtABC efflux pump in this strain compared to the strain carrying the λplacMu53 element in the mdtA gene, where the efflux pump would be inactive. To test this idea, we moved the ΔmdtA::Kn mutation into the strain carrying the λRS88[spy-lacZ] reporter (SL102), and reexamined spy-lacZ induction.
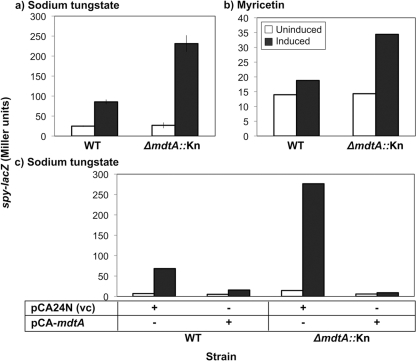
In support of our hypothesis, elimination of the Mdt efflux pump led to a large increase in spy-lacZ induction compared to the wild-type counterpart (Fig. 2). For sodium tungstate, induction increased from 6- or 7-fold to 8- or 9-fold, for myricetin from approximately 2-fold to 3-fold, and for quercetin from 2-fold to 3-fold (Fig. 2a and b and data not shown). These differences are significantly different from the fold inductions observed in a WT (mdtA+) strain background (P < 0.05 for myricetin and P < 0.001 for sodium tungstate; Student's t test). However, the same increased induction was not observed for indole. This hyperinducible phenotype was confirmed by comparing β-galactosidase activity produced in wild-type and ΔmdtA::Kn strains that carried an mdtA-lacZ reporter gene, λRS88[mdtA-lacZ], on a lambda phage integrated at a site distant from the mdtABCD locus (data not shown).
Fig. 2.
A ΔmdtA::Kn mutant is more sensitive to sodium tungstate induction than the wild type. (a and b) β-Galactosidase assays were done with WT (TR530) and ΔmdtA::Kn (SL102) strains that were uninduced or induced by 5 mM sodium tungstate (a) or 10 μg/ml myricetin (b). (c) Complementation of ΔmdtA::Kn with pCA-mdtA (SL143) or the vector control (vc) pCA24N (SL142). Overexpression was induced with 0.1 mM IPTG either with or without 5 mM sodium tungstate. The error bars indicate standard deviations.
The above observations suggest that the newly identified inducers of the Bae response are in fact substrates of the MdtABC efflux pump and that they accumulate to higher levels, and therefore cause greater Bae response induction, in strains lacking the MdtABC pump. To confirm our observations, complementation experiments were carried out, and the effects of mutating a gene downstream of the Mdt efflux pump were examined to ensure that the effects we observed were not due to polar effects on expression of the baeSR genes. These experiments were performed in the spy-lacZ reporter strain and repeated in the mdtA-lacZ background, and the same results were obtained in each (Fig. 2c and data not shown). Overexpression of the mdtA gene from an inducible plasmid, pCA-mdtA, abolished the hyperinducible phenotype of the mdtA-null strain and in fact reduced the amount of induction even in WT cells (Fig. 2c). Additionally, induction of the Bae response in a ΔmdtD::Kn mutant was examined, and no differences from the WT were detectable (data not shown). MdtD is encoded in the mdtABCD-baeSR operon, but it is a predicted MFS-type efflux pump and is not thought to function with MdtABC (2, 22). Since mutating mdtD did not produce the same phenotype as the ΔmdtA::Kn mutant, the ΔmdtA::Kn mutant phenotype is not due to polar effects on baeSR expression. Next, we examined mutations in two other BaeR regulon members, spy and acrD, for their induction phenotypes. No differences from the WT were observed (data not shown). Based on these results, we concluded that the ΔmdtA::Kn hyperinducible phenotype is specific to loss of mdtA and is not a general phenomenon observed when BaeR regulon members are mutated.
These results suggest that the MdtABC efflux pump plays an important role in the cell's response to Bae-inducing compounds, especially to sodium tungstate, and may in fact be the downstream target in the Bae regulon that accounts for the increase in sensitivity seen in the baeSR mutant that Zhou and colleagues analyzed in their phenotypic microarray (43). The BaeR-dependent specificity of induction of the Bae regulon by these compounds, the small Bae regulon, and the similar chemical structures shared by many of the inducers further lend support to the hypothesis that these compounds are specific substrates of the MdtABC-TolC efflux pump and that efflux of such inducing compounds is the mechanism by which BaeSR maintains envelope homeostasis. If indeed these compounds are specific substrates of the MdtABC pump, this could also account for the low basal levels of expression of the mdtABCD-baeSR operon, since it would be required only in the presence of very specific inducing cues.
Cellular indole does not affect BaeSR pathway activity.
Indole is produced during biogenesis and breakdown of tryptophan, suggesting the possibility that the Bae response might function to protect cells from this normal by-product of metabolism. We have observed induction of BaeSR by indole at a 2 mM concentration. Previous reports have detected maximum indole concentrations in stationary-phase cell culture supernatants of approximately 340 μM (39). The requirement for relatively high concentrations of indole to induce the BaeSR pathway suggests that the Bae response works to alleviate envelope stress caused by excess indole rather than as part of a response to cellular levels of indole produced during metabolism. Indeed, we observed induction of the BaeSR pathway by 2 mM, but not by 1 mM or 500 μM, indole using our spy-lacZ reporter (data not shown).
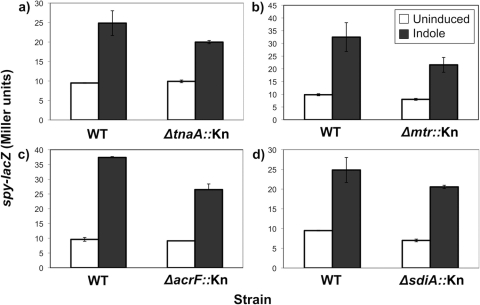
To further test whether BaeS could be involved in responding to cellular indole produced during metabolism, we tested several mutants involved in tryptophan metabolism that would be expected to alter cellular and secreted levels of indole for their effects on Bae pathway activity and the ability of the Bae pathway to be induced by excess indole. TnaA (tryptophanase) metabolizes tryptophan to indole and pyruvate and could contribute to basal levels of BaeSR pathway activity by producing indole (37). AcrE is a component of the AcrEF multidrug efflux pump that was shown to export cellular indole (10). If normal indole export contributes to basal BaeSR pathway activity, we should see a loss of BaeSR activity upon loss of the AcrEF efflux pump. Finally, Mtr is a tryptophan-indole-proton symporter, which would be necessary for BaeSR pathway activation by indole if indole had to be imported into the cell to access and subsequently activate BaeSR (41). Mutants carrying insertion mutations of each of these genes, tnaA, acrE, and mtr, were examined for their effects on basal BaeSR pathway activity and activation by addition of exogenous indole. None of these mutations had a significant effect on Bae pathway activity or induction by indole at 2 mM (Fig. 3a, b, and c) (P > 0.05). These results lend further support to the hypothesis that induction of the Bae pathway by indole is not linked to the normal physiological production of indole. They also show that exogenously added indole does not need to be imported (or exported) in order to induce the BaeSR two-component pathway.
Fig. 3.
Normal production and transport of cellular indole appear unrelated to Bae pathway activity. β-Galactosidase assays were done to compare BaeSR pathway induction by 2 mM indole in WT (TR530) and mutant strains in the cellular indole pathway. TR530 was compared to ΔtnaA::Kn (SL113) (TnaA is tryptophanase) (a), Δmtr::Kn (SL103) (Mtr is the tryptophan/indole/H+ symporter) (b), ΔacrF::Kn (SL131) (AcrF is the indole efflux pump) (c), and ΔsdiA::Kn (SL114) (SdiA is a LuxR homologue predicted to interact with indole) (d). The error bars indicate standard deviations.
Indole affects biofilm formation separately from its induction of the BaeSR pathway.
In addition to its role in cellular tryptophan production, indole has recently been strongly implicated as a cell-cell signaling molecule and linked to repression of biofilm formation through the LuxR homologue SdiA (35). SdiA is known to repress various motility and chemotaxis genes, and more recently, expression of sdiA was shown to increase in the presence of indole, while biofilm formation was repressed (40). When Lee and colleagues analyzed an sdiA mutant in the presence of glucose (indole production is catabolite repressed), biofilm formation was increased and addition of indole could no longer repress biofilm formation, suggestive of an interaction between SdiA and cellular indole to affect biofilm formation (14).
Since indole is an inducer of the BaeSR pathway and affects biofilm formation, it is pertinent to determine whether these effects are somehow linked or whether indole affects BaeSR and SdiA independently. To answer this question, we first looked at an sdiA knockout in the same assay as for the tnaA, acrE, and mtr mutants. Again, we saw no significant difference in Bae pathway activity or induction by indole, indicating that sdiA is not required for BaeSR pathway activity and gene regulation (Fig. 3d) (P > 0.05).
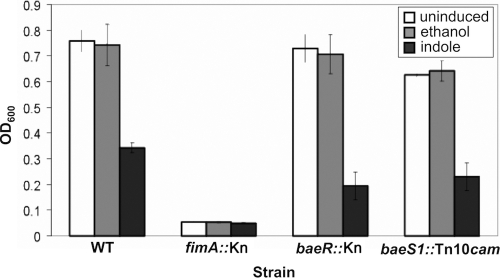
To address the potential link between Bae pathway induction by indole and the effect of indole on biofilm formation, we looked at biofilm formation in several strains: a WT strain that forms biofilms well in vitro (2K1056), a cell deficient in BaeSR activity (ΔbaeR::Kn; SL99), and a strain with a constitutively activated BaeSR pathway activity (baeS1::Tn10cam; SL119) (27). Furthermore, we examined the effects of indole addition on biofilm formation with each of these strains; 500 μM indole was used to be consistent with previous studies on the effects of indole on biofilm formation, and a fimA knockout was used as a negative control (2K1056 ΔfimA::Kn) (14).
We observed no significant changes from WT biofilm formation in either the BaeR knockout or the BaeS gain-of-function mutant (Fig. 4). Furthermore, addition of exogenous indole to the cells during biofilm growth inhibited biofilm formation at least 2-fold in each of these strains (Fig. 4). Ethanol was used as a solvent control for indole addition and did not affect biofilm formation (Fig. 4). Thus, the Bae response and its activation by indole are unrelated to biofilm formation.
Fig. 4.
The effect of indole on biofilm formation is independent of the Bae pathway. Biofilms were grown using the WT strain 2K1056 and the derivatives shown for 48 h at room temperature in 96-well polystyrene plates, followed by crystal violet staining and absorbance (A600) readings to detect the amount of biofilm present. ΔfimA::Kn is a biofilm-deficient negative control, ΔbaeR::Kn is a BaeSR pathway-null mutant, and baeS1::Tn10cam is a BaeSR pathway gain-of-function mutant. Biofilms were grown with no additions, a 0.5% ethanol solvent control, or 500 μM indole. The error bars indicate standard deviations.
DISCUSSION
Previous characterization of the BaeSR system led to the conclusions that BaeSR is a classical two-component signal transduction pathway, that it can be grouped with the other five known envelope stress responses, and that it is induced by stressors, such as spheroplasting, PapG overexpression, indole, and tannin exposure (44). Furthermore, mutations in BaeSR resulted in sensitivity to several compounds (43).
In this study, we identified several novel inducers of the BaeSR two-component system in E. coli. Addition of subinhibitory concentrations of myricetin, quercetin, morin, sodium tungstate, or zinc to E. coli cultures resulted in greater than 2-fold induction of at least two reporters of Bae pathway activity, and this induction was lost in a baeR mutant strain (Fig. 1 and data not shown). The molecular nature of the inducing cue is harder to elucidate. Previous work with indole and the flavonoids myricetin and quercetin demonstrated that they cause membrane damage (7). Interestingly, studies on quercetin and the related catechins have also shown that they can associate with liposomes and form degradation products in the presence of copper(II) and oxygen, which could result in hydrogen peroxide and free-radical formation (18). Thus, we think it likely that oxidative stress is a component of the inducing signal. This hypothesis is supported by the finding that Bae response induction by flavonoids is correlated with the number of hydroxyl groups found on the B rings of these compounds, which has been shown to alter their reactivity (30), and the finding that some metals (zinc and tungstate) that are known to cause oxidative stress also induce the Bae response (Table 2 and Fig. 1). When we tested hydrogen peroxide, cumene peroxide, and iron(II)sulfate, however, they did not induce spy-lacZ (data not shown), so it is not likely that oxidative stress alone serves as the inducing signal. We think it likely that BaeS senses a specific stress, perhaps oxidation of a particular molecule that is generated in the presence of certain metals and potentially oxidative compounds, such as sodium tungstate, zinc, and the flavonoids. One hypothesis could be that oxidative stress or membrane damage results in the oxidation or liberation of certain inducing compounds in the membrane, thereby activating BaeS. Alternatively, it may be that metals such as tungstate induce the Bae response in a manner that is distinct from that of the flavonoids. Further work is needed to address the nature of the BaeS-inducing signal(s).
We also investigated the cellular role of the Bae pathway in E. coli. Evidence that this role is very specific can be found in our analysis of a mutant in the efflux pump that shares the operon with the baeSR genes. The overexpression phenotypes of the MdtABC efflux pump have previously been characterized in strains lacking the major efflux pump AcrAB-TolC, and these studies all conclude that overexpressing MdtABC increases resistance to deoxycholate and novobiocin (2, 22). The increased resistance to novobiocin and deoxycholate conferred by the MdtABC efflux pump is seen only in an acrAB mutant, however, and results from our laboratory have shown that novobiocin does not strongly induce the spy-lacZ reporter (<2-fold induction) (data not shown). Thus, the sensing and efflux of these compounds is not likely a primary function of the Bae response. In fact, until now, no phenotype for a mutant of the MdtABC efflux pump had been identified. A ΔmdtA::Kn-null mutation abolishes pump activity, since MdtA is the membrane fusion protein and is required for proper pump assembly (22). When we characterized this mutant with respect to Bae pathway activity, we found high sensitivity of induction upon exposure to sodium tungstate relative to a wild-type strain. We found similar results with the inducers myricetin and quercetin, but not indole. Interestingly, of these compounds, indole is the weakest inducer and has also been shown to be effluxed from the cell using a different efflux pump, AcrEF (10).
Wild-type sensitivity could be completely restored to the ΔmdtA::Kn mutant by exogenous addition of the mdtA gene on a plasmid. Additionally, a downstream mutation in the mdtD gene did not mimic the mdtA-null phenotype, which confirms that the mutation does not exert its effects through polar effects on transcription of the downstream baeSR genes, and further, it supports an independent role for the mdtD gene product. Consistent with our observations, the deoxycholate and novobiocin resistance phenotypes characterized previously are dependent on the expression of the mdtABC genes, but not mdtD (2, 22). MdtD is a predicted MFS-type efflux pump, and these data fit with MdtD having a separate function from the MdtABC pump. There is some possible evolutionary support for a separate function of MdtD in some species, as well. We searched for predicted mdtABCD-baeSR operon homologues using the NCBI Gene database (http://www.ncbi.nlm.nih.gov/gene) and found that in several closely related organisms the operon is predicted to be identical to that of E. coli (Salmonella enterica, Shigella spp., Yersinia spp., Citrobacter rodentium, Cronobacter turicensis, and Photorhabdus asymbiotica). In other cases mdtD appears to be replaced by mdtE (Klebsiella pneumoniae, Serratia proteamaculans, and Enterobacter cloacae). In one instance, the operon is missing mdtD entirely (mdtABC-baeSR in Proteus mirabilis), and in other microbes, baeSR are not part of the operon and mdtD is missing (mdtABC in Pseudomonas fluorescens, Burkholderia spp., Desulfovibrio magneticus, Bradyrhizobium sp., and Pantoea ananatis). This is certainly not comprehensive, but it does indicate that mdtABC, mdtD, and baeSR did not always evolve together.
Cumulatively, these data support the hypothesis that the primary role of the Bae response is to detect specific envelope-damaging compounds that can be effluxed by the MdtABC pump. This conclusion is in agreement with multiple microarray studies that have failed to identify an extensive Bae regulon (3, 25). Interestingly, however, the Mdt pump is not required to sense the presence of these compounds, since the Bae pathway can be strongly upregulated in its absence. This observation indicates that BaeS senses some altered aspect of envelope physiology caused by the inducing compounds, or the compounds themselves, rather than the activity of the MdtABC efflux pump.
An additional goal of this study was to investigate the connection of Bae pathway induction to the physiological role of indole in the cell. Much work with indole in E. coli has led to the recent conclusion that indole is a cell-cell signaling molecule (14). The negative effect of indole on biofilm formation and its putative interaction with the LuxR homologue SdiA to affect this are particularly interesting (16, 39). Indole is one of the first signals shown to induce the Bae pathway, so we wanted to determine whether the Bae pathway plays a role in the regulation of biofilm formation, perhaps under stressful conditions in the environment. This study shows through mutational analyses that disrupting normal cellular production of indole does not affect Bae pathway activity. Furthermore, biofilm assays show that disruptions in normal Bae pathway activity, whether via inhibition (deletion of baeR) or enhanced pathway activity (a baeS1::Tn10cam gain-of-function mutant), do not affect the ability of indole to repress biofilm formation, and these mutations do not themselves affect the ability of E. coli to form a biofilm under the conditions tested. The additional observations that induction of the Bae pathway by indole requires high, near-toxic concentrations of indole while the effect of indole on biofilm formation occurs at more relevant physiological concentrations allow us to conclude that the Bae pathway is not involved in the cellular functions of indole related to SdiA and biofilm formation. Additionally, indole is known to induce other stress responses at high concentrations, suggesting it is a general envelope stress signal that disrupts multiple aspects of envelope physiology (27).
The purpose of this study was to clarify the inducing cues and cellular role of the Bae pathway in E. coli. We identified several compounds that strongly induce the Bae pathway, namely, sodium tungstate, zinc, and the flavonoids myricetin, quercetin, and morin. It is interesting that flavonoids and indole are known plant secondary metabolites, so it is easy to imagine that they would be present in the mammalian diet and intestine, where E. coli is commonly found. Other studies on flavonoids and metals led us to hypothesize that oxidative stress and membrane damage may play a role in the induction mechanism; our data indicate that a specific signal associated with these stresses is required, however, because several oxidative compounds on their own fail to induce the pathway. The MdtABC efflux pump is a known member of the small BaeR regulon; we found that a ΔmdtA::Kn-null mutant is highly sensitive to many of these inducing cues and that exogenous addition of MdtA to this mutant restores normal induction. These data indicate a major role for the MdtABC efflux pump in the Bae pathway and support the hypothesis that compounds that induce the Bae pathway are substrates of MdtABC. Finally, our work on the weak inducer indole showed that the Bae pathway is not involved in the normal physiological roles of indole in the cell or with the recently identified role of indole in biofilm formation signaling. We believe that the central role of the Bae pathway is to respond to specific envelope-damaging MdtABC substrates, to upregulate the MdtABC efflux pump, and thereby to restore envelope homeostasis by ridding the cell of these compounds.
ACKNOWLEDGMENTS
This study was supported by a Post-Graduate Scholarship (S.K.D.L.) and Operating Grants from the National Sciences and Engineering Research Council of Canada and a Senior Scholar Award from the Alberta Heritage Foundation for Medical Research (T.L.R.).
Footnotes
Published ahead of print on 22 April 2011.
REFERENCES
- 1. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baranova N., Nikaido H. 2002. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 184:4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bury-Moné S., et al. 2009. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5:e1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casadaban M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555 [DOI] [PubMed] [Google Scholar]
- 5. Danese P. N., Snyder W. B., Cosma C. L., Davis L. J., Silhavy T. J. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387. [DOI] [PubMed] [Google Scholar]
- 6. Domka J., Lee J., Wood T. K. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 72:2449–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garbe T. R., Kobayashi M., Yukawa H. 2000. Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch. Microbiol. 173:78–82 [DOI] [PubMed] [Google Scholar]
- 8. Hirakawa H., Inazumi Y., Masaki T., Hirata T., Yamaguchi A. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:1113–1126 [DOI] [PubMed] [Google Scholar]
- 9. Jones C. H., Danese P. N., Pinkner J. S., Silhavy T. J., Hultgren S. J. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawamura-Sato K., et al. 1999. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol. Lett. 179:345–352 [DOI] [PubMed] [Google Scholar]
- 11. Kitagawa M., et al. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12:291–299 [DOI] [PubMed] [Google Scholar]
- 12. Kleerebezem M., Crielaard W., Tommassen J. 1996. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the proton motive force under stress conditions. EMBO J. 15:162–171 [PMC free article] [PubMed] [Google Scholar]
- 13. Laubacher M. E., Ades S. E. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 190:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J., Jayaraman A., Wood T. K. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J., Bansal T., Jayaraman A., Bentley W. E., Wood T. K. 2007. Enterohemorrhagic Escherichia coli biofilms are inhibited by 7-hydroxyindole and stimulated by isatin. Appl. Environ. Microbiol. 73:4100–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee J., Maeda T., Hong S. H., Wood T. K. 2009. Reconfiguring the quorum-sensing regulator SdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl. Environ. Microbiol. 75:1703–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luirink J., von Heijne G., Houben E., de Gier J. W. 2005. Biogenesis of inner membrane proteins in Escherichia coli. Annu. Rev. Microbiol. 59:329–355 [DOI] [PubMed] [Google Scholar]
- 18. Makris D. P., Rossiter J. T. 2000. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 48:3830–3838 [DOI] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20. McBroom A. J., Johnson A. P., Vemulapalli S., Kuehn M. J. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 188:5385–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McBroom A. J., Kuehn M. J. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagakubo S., Nishino K., Hirata T., Yamaguchi A. 2002. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 184:4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagasawa S., Ishige K., Mizuno T. 1993. Novel members of the two-component signal transduction genes in Escherichia coli. J. Biochem. 114:350–357 [DOI] [PubMed] [Google Scholar]
- 24. Nishino K., Nikaido E., Yamaguchi A. 2007. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:9066–9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishino K., Honda T., Yamaguchi A. 2005. Genome-wide analyses of Escherichia coli gene expression responsive to the BaeSR two-component regulatory system. J. Bacteriol. 187:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pratt L. A., Kolter R. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285–293 [DOI] [PubMed] [Google Scholar]
- 27. Raffa R. G., Raivio T. L. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599–1611 [DOI] [PubMed] [Google Scholar]
- 28. Raivio T. L. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119–1128 [DOI] [PubMed] [Google Scholar]
- 29. Raivio T. L., Laird M. W., Joly J. C., Silhavy T. J. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the cpx envelope stress response. Mol. Microbiol. 37:1186–1197 [DOI] [PubMed] [Google Scholar]
- 30. Ratty A. K., Das N. P. 1988. Effects of flavonoids on nonenzymatic lipid peroxidation: structure-activity relationship. Biochem. Med. Metab. Biol. 39:69–79 [DOI] [PubMed] [Google Scholar]
- 31. Ruiz N., Silhavy T. J. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122–126 [DOI] [PubMed] [Google Scholar]
- 32. Ruiz N., Kahne D., Silhavy T. J. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57–66 [DOI] [PubMed] [Google Scholar]
- 33. Sailer F. C., Meberg B. M., Young K. D. 2003. Beta-lactam induction of colanic acid gene expression in Escherichia coli. FEMS Microbiol. Lett. 226:245–249 [DOI] [PubMed] [Google Scholar]
- 34. Simons R. W., Houman F., Kleckner N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96 [DOI] [PubMed] [Google Scholar]
- 35. Sitnikov D. M., Schineller J. B., Baldwin T. O. 1996. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc. Natl. Acad. Sci. U. S. A. 93:336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slauch J. M., Silhavy T. J. 1991. Cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 173:4039–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Snell E. E. 1975. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 42:287–333 [DOI] [PubMed] [Google Scholar]
- 38. Torres-Cabassa A. S., Gottesman S. 1987. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang D., Ding X., Rather P. N. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 183:4210–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei Y., Lee J. M., Smulski D. R., LaRossa R. A. 2001. Global impact of sdiA amplification revealed by comprehensive gene expression profiling of Escherichia coli. J. Bacteriol. 183:2265–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yanofsky C., Horn V., Gollnick P. 1991. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J. Bacteriol. 173:6009–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X. S., García-Contreras R., Wood T. K. 2007. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J. Bacteriol. 189:3051–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou L., Lei X. H., Bochner B. R., Wanner B. L. 2003. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. J. Bacteriol. 185:4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zoetendal E. G., Smith A. H., Sundset M. A., Mackie R. I. 2008. The BaeSR two-component regulatory system mediates resistance to condensed tannins in Escherichia coli. Appl. Environ. Microbiol. 74:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]