Abstract
Although Streptococcus parauberis is known as a bacterial pathogen associated with bovine udder mastitis, it has recently become one of the major causative agents of olive flounder (Paralichthys olivaceus) streptococcosis in northeast Asia, causing massive mortality resulting in severe economic losses. S. parauberis contains two serotypes, and it is likely that capsular polysaccharide antigens serve to differentiate the serotypes. In the present study, the complete genome sequence of S. parauberis (serotype I) was determined using the GS-FLX system to investigate its phylogeny, virulence factors, and antigenic proteins. S. parauberis possesses a single chromosome of 2,143,887 bp containing 1,868 predicted coding sequences (CDSs), with an average GC content of 35.6%. Whole-genome dot plot analysis and phylogenetic analysis of a 60-kDa chaperonin-encoding gene and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-encoding gene showed that the strain was evolutionarily closely related to Streptococcus uberis. S. parauberis antigenic proteins were analyzed using an immunoproteomic technique. Twenty-one antigenic protein spots were identified in S. parauberis, by reaction with an antiserum obtained from S. parauberis-challenged olive flounder. This work provides the foundation needed to understand more clearly the relationship between pathogen and host and develops new approaches toward prophylactic and therapeutic strategies to deal with streptococcosis in fish. The work also provides a better understanding of the physiology and evolution of a significant representative of the Streptococcaceae.
INTRODUCTION
Streptococcosis of cultured fish contributes to major economic losses in the aquaculture industries of Israel (34), Italy (43), South Korea (86), Japan (4), and the United States (79). The major etiological species include Streptococcus parauberis, Streptococcus iniae, Streptococcus difficilis, Lactococcus garvieae, Lactococcus piscium, and Vagococcus salmoninarum (8, 72).
S. parauberis is a coccoid, nonmotile, alpha-hemolytic, Gram-positive bacterium of the Streptococcaceae family. S. parauberis and S. uberis are common agents of bovine mastitis (98). The two species are closely related and can be classified as pyogenic streptococci by phylogenic analysis, using the sequences of several genes, such as that of 16S rRNA and those encoding superoxide dismutase A and chaperonin 60, to this end (3, 12).
In aquaculture, S. parauberis was first reported in cultured turbot (Scophthalmus maximus) (28) and was recently determined to be the dominant etiological agent of streptococcosis, capable of inducing chronic infections exhibiting clinical symptoms, such as hemorrhagic septicemia, exophthalmia, meningitis, skin lesions, and cachexia. In olive flounder (Paralichthys olivaceus), the dominant aquaculture species in South Korea, streptococcosis results in continuous mortality over a period of several weeks, and the causative bacterium is antibiotic resistant (76) in South Korea (74) and Japan (55).
Genome sequences of many Streptococcus species are now available, facilitating the conduct of comparative evolutionary studies, including analysis of positive selection pressures and mobile gene transfer. Such work has been performed using data on important human and agricultural Streptococcus pathogens, such as S. pyogenes (39), S. pneumoniae (52), S. agalactiae (44), S. thermophilus (70), S. suis (49), S. sanguinis (101), S. gordonii (96), S. equi (50), and S. mutans (2). However, the present work is the first complete genome analysis of a fish-pathogenic Streptococcus sp. To date, genetic information on the roles played by habitat adaptation, virulence determinants, invasion, and multidrug resistance in the molecular pathogenesis of S. parauberis remains scanty. Thus, it is very important to derive a complete genome sequence to aid in the development of vaccines and methods for preventing fish streptococcosis.
In the present study, the complete genome sequence of S. parauberis KCTC11537BP isolated from diseased flounder was obtained and compared with those of related pathogenic and nonpathogenic streptococci. The identification of S. parauberis antigenic proteins was also performed using an immunoproteomic technique.
MATERIALS AND METHODS
Bacterial strain.
Streptococcus parauberis KCTC11537BP was isolated from the spleen of diseased olive flounder collected from an aquaculture farm in Jeju Island, South Korea, in 2006 (74). S. parauberis KCTC11537BP was cultured on tryptone soya agar (TSA; Oxoid Ltd., Cambridge, United Kingdom) or in tryptone soya broth (TSB; Oxoid Ltd.) containing 2% (wt/vol) sodium chloride, at 25°C for 24 h. Bacteria were stored in TSB containing 10% (vol/vol) glycerol at −70°C.
Preparation of genomic DNA.
S. parauberis was cultured in TSB at 25°C for 24 h, and genomic DNA was extracted using the Qiagen Genomic-tip 500/G kit (Qiagen, Hilden, Germany) and the genomic DNA buffer set (Qiagen), according to the manufacturer's instructions.
Whole-genome sequencing.
The complete genome of S. parauberis was sequenced by Takara Bio, Inc. (Otsu, Japan), using a Roche GS-FLX system (71). A total of 192,571 reads of 75,170,197 bp were obtained, resulting in a 34-fold coverage of the genome and producing 181 contigs, with an average length of 29,746 bp. The fosmid library was constructed using an EpiFOS fosmid library kit (Epicenter Biotechnologies, Madison, WI), according to the manufacturer's protocol. All contigs were assembled using fosmid end-sequencing data, which are valuable when dealing with large contigs and confirm the accuracy of assembling. To this end, 576 fosmid clones were randomly picked, and 1,146 sequences of a total length of 1,021,933 bp were read. These sequences were assembled using Phred/Phrap/Consed analysis software (35, 36, 45). Gaps between contigs were filled in by direct PCR sequencing using primers annealing to the ends of neighboring contigs. To verify and determine assembled sequences, 18 primer sets were constructed to cover the entire chromosomal DNA of strain KCTC11537BP at unique flanking sequences (data not shown). PCR amplification of DNA fragments approximately 2.9 to 3.2 kb in length was achieved using LA Taq polymerase (Takara Bio, Inc.), according to the manufacturer's instructions.
The determination of potential protein-coding sequences and functional categorization were performed using three programs: Glimmer (27), CRITICA (9), and the CLC main workbench (CLC bio, Aarhus, Denmark). tRNA genes were identified by tRNAScan-SE (68), whereas rRNA genes were predicted by comparison of the genome sequence to that in the rRNA database (41, 100). Annotated genes were identified by NCBI BLAST searching and were also compared to coding sequences (CDSs) of the S. uberis genome (GenBank accession no. NC_0120041), which were used as a reference. The acceptable initiation codons were ATG, TTG, and GTG in Glimmer and CRITICA and ATG, TTG, and CTG upon NCBI BLAST searching.
Comparative genome analyses, including dot blotting, construction of a genome rearrangement map, and preparation of Venn diagrams showing graphical analytical results, were conducted using the in silico molecular cloning tool, the genomic edition, version 4.1.21 (In Silico Biology Co., Ltd., Yokohama, Japan).
Phylogenetic analyses.
Phylogenetic relationships among Streptococcus species were analyzed in terms of two housekeeping genes: the GAPDH gene (gap) and the 60-kDa chaperonin gene (cpn). The gap sequences from 17 streptococci, including those of S. iniae (GenBank accession no. AF4219032.1), S. uberis 0140J (YP_002562909.1), S. agalactiae NEM319 (NP_736245.1), S. suis BM407 (YP_003027928.1), S. thermophilus LMG18311 (YP_140202.1), S. gordonii strain Challis substrain CH1 (ABV11012.1), S. dysgalactiae (ACX85248.1), S. equinus (BAF02541.1), S. mutans UA159 (AE014133.1), S. equi subsp. equi 4047 (YF_002745743.1), S. dysgalactiae subsp. equisimilis GGS_124 (YF_002997632.1), S. equi subsp. zooepidemicus (YF_002745200.1), S. pyogenes MGAS315 (NP_664005.1), S. pneumoniae TCH8431/19A (YF_003723450.1), S. oralis ATCC 35037 (ZP_06612207.1), S. sanguinis SK36 (YP_001036026.1), and S. parasanguinis ATCC 15912 (ZP_06899803.1), were used to construct the gap phylogenetic tree. The cpn tree was formed from 13 streptococcal sequences, including those of S. uberis 0140J (GenBank accession no. CAR43696.1), S. agalactiae COH1 (ZP_00785873.1), S. suis BM407 (YP_003027923.1), S. thermophilus LMG18311 (YP_138711.1), S. gordonii strain Challis substrain CH1 (YP_001451151.1), S. equinus (ABX57775.1), S. mutans UA159 (NP_722255.1), S. equi subsp. equi 4047 (YP_002745626.1), S. dysgalactiae subsp. equisimilis GGS_124 (YP_002997735.1), S. equi subsp. zooepidemicus (YP_002743690.1), S. pyogenes MGAS5005 (AAZ52379.1), S. pneumoniae TIGR4 (NP_346336.1), and S. sanguinis SK36 (YP_001034236.1). These gene sequences were aligned using ClustalW (93) after trimming to remove ambiguously aligned regions. The phylogenetic trees were calculated using the maximum likelihood method of MEGA 4.0 (90), employing 1,000 bootstrap replications.
Whole-cell lysates.
Samples to be subjected to two-dimensional electrophoresis (2-DE) were prepared using both chemical and mechanical extraction to ensure high yield and optimum solubility of whole-cell proteins (46, 60). First, S. parauberis KCTC11537BP cultured in TSB was harvested by centrifugation at 4,500 × g for 15 min at 4°C. The pellet was washed three times with phosphate-buffered saline (PBS; 3 mM KCl, 137 mM NaCl, 1.5 mM KH2PO4, and 8 mM Na2HPO4; pH 7.4), and the pellet was stored at −20°C until use. For chemical extraction, bacterial pellets were resuspended in 100-μl amounts of lysis buffer A (12 mM Tris, 5% [vol/vol] glycerol, 0.4% [wt/vol] SDS, and 200 mM dithiothreitol [DTT]). For mechanical extraction, 400-μl amounts of lysis buffer B [2 M thiourea, 7 M urea, 40 mM Tris, 1% (wt/vol) DTT, 4% (wt/vol) 3-[(3-cholamidopropyl) dimethyl-ammonium]-1-propanesulfonate (CHAPS), and 0.5% (vol/vol) IPG-buffer (pH 4 to 7)] were added to each bacterial mixture, which was next placed on an ice slurry and sonicated for a total of 15 min (10 s on, and 10 s off) using an XL-2020 sonicator (Misonix Inc., Farmingdale, NY). Disrupted bacterial cells were boiled at 100°C for 10 min. Lysates were purified and concentrated using a 2-DE cleanup kit (Amersham Bioscience [GE Healthcare], Uppsala, Sweden), according to the manufacturer's directions. Protein concentrations were estimated by the Bradford method using bovine serum albumin (BSA) as a standard.
IEF and SDS-PAGE.
Isoelectric focusing (IEF) was performed using the IPGphor system (Amersham Bioscience) employing immobilized pH gradient (IPG) strips (Immobiline DryStrip; pH 4 to 7; 13 cm in length; Amersham Bioscience) using a previously reported method (87). The protein loading concentration for whole-cell lysates was adjusted to 120 μg ml−1 with rehydration buffer [9 M urea, 2% (wt/vol) CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate), 0.4% (wt/vol) DTT, 0.5% (vol/vol) IPG buffer, and 0.002% (wt/vol) bromophenol blue]. Samples were loaded onto IPG strips and focused at 86.1 kVh at 20°C using an automated system operating as follows: rehydration for 12 h (6 h at 30 V followed by 6 h at 60 V); focusing for 17 h (2 h at 200 V, 1 h at 500 V, 1 h at 1,000 V, 1 h at 2,000 V, 2 h at 4,000 V, and 10 h at 8,000 V). After IEF, IPG strips were equilibrated in 10 mg ml−1 DTT in equilibration buffer (6 M urea, 2% [wt/vol] SDS, 30% [vol/vol] glycerol, 0.002% [wt/vol] bromophenol blue, and 50 mM Tris-HCl; pH 8.8) for 15 min and next with 25 mg ml−1 of iodoacetamide in the same buffer, for another 15 min. Equilibrated IPG strips were placed onto 12.5% (wt/vol) SDS-polyacrylamide gels (18 cm by 16 cm by 0.1 cm), sealed with 0.5% (wt/vol) low-melting-point agarose (Sigma-Aldrich, St. Louis, MO), and electrophoresed at 10 mA for 15 min followed by 20 mA until the dye reached the bottom of the gel.
Visualization.
Electrophoretic gels were silver stained as previously described (87). Briefly, each 2-DE gel was fixed in solution A (50% [vol/vol] methanol, 12% [vol/vol] acetic acid, and 0.05% [vol/vol] of 37% [vol/vol] formaldehyde) for 1 h, washed twice with 50% (vol/vol) ethanol, and sensitized with a dedicated solution (0.01% [wt/vol] sodium thiosulfate; Sigma-Aldrich) for 1 min. Sensitized gels were rinsed three times with double-distilled water (DW) (DDW) and next incubated with solution B (0.1% [wt/vol] silver nitrate and 0.1% [vol/vol] of 37% [vol/vol] formaldehyde) for 30 min. Gels were next washed twice with DDW for 30 s prior to treatment with developing solution (12% [wt/vol] sodium carbonate and 0.05% [vol/vol] of 37% [vol/vol] formaldehyde) until the desired level of staining was attained. The developing solution was removed, and the reaction was stopped by the addition of solution A without formaldehyde. Stained gels were stored in 50% (vol/vol) methanol at 4°C prior to analysis. Gel images were digitized using an Epson Perfection V700 Photoimage scanner (Seiko Epson Corp., Nagano, Japan).
Production of olive flounder anti-S. parauberis KCTC11537BP serum.
The olive flounder-specific antiserum against S. parauberis KCTC11537BP was produced in a previous study (56). The flounder was intraperitoneally injected with approximately 106 CFU of S. parauberis KCTC11537BP. After 2 weeks, blood was collected and a serum supernatant was obtained by centrifugation at 1,120 × g for 15 min at 4°C. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Gyeongsang National University, Jinju, Republic of Korea.
Immunoblotting.
Two-dimensional electrophoresis was performed as described above, and gels were either stained with silver nitrate for visualization of protein spots or transferred to a 0.45-μm-pore-sized polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) for detection of antigens in whole-cell lysates. Each membrane was blocked using 5% (wt/vol) skim milk powder in PBS-T (PBS with 0.05% [vol/vol] Tween 20), for 1 h at room temperature (RT), and next washed three times with PBS-T. Membranes were incubated with flounder anti-S. parauberis KCTC11537BP serum for 1.5 h at RT, washed three times with PBS-T, and incubated separately with an anti-olive flounder IgM monoclonal antibody (85) for 1.5 h at RT. Antigenic spots from whole-cell lysates were visualized by incubation with goat anti-mouse-horse radish peroxidase (HRP) (Jackson ImmunoResearch Laboratories Inc., West Grove, PA; 1:4,000) for 1 h at RT. Membranes were next washed five times with PBS-T for 15 min, developed using an enhanced chemiluminescent (ECL) kit (GE Healthcare), and exposed to X-ray film for visualization of antigenic proteins. Images of stained gels and immunoblotted membranes were obtained and digitalized using the Epson Perfection scanner.
In-gel digestion.
Protein spots were identified by peptide mass finger printing (PMF), using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) as previously described (87). In brief, protein spots of interest were excised from silver-stained gels and subjected to in-gel digestion with 12.5 ng/ml porcine trypsin (Promega, Madison, WI), at 37°C overnight (approximately 16 h). The supernatant was collected and extracted twice with an equal volume of 5% (vol/vol) formic acid in acetonitrile, and extracts were pooled and dried in a vacuum centrifuge. Dried tryptic peptides were redissolved in 1 ml of sample solution (93:5:2 [vol/vol/vol] ratio of DW, acetonitrile, and trifluoroacetic acid [TFA]), and targeting to MALDI plates was achieved using a solution-phase nitrocellulose-mediated method. Alpha-cyano-4-hydroxycinnamic acid (40 mg/ml) and nitrocellulose (20 mg/ml) were prepared separately as solutions in acetone and were mixed with isopropanol at a ratio of 2:1:1 (vol/vol/vol). Internal standards, des-Arg-bradykinin (monoisotopic mass of 904.4681) and angiotensin I (1296.6853) (both from Sigma-Aldrich) were added to each mixture to generate a matrix solution. One-microliter amounts of matrix solution were spotted onto target circles on a MALDI plate, dried, and analyzed using a Voyager-DE STR MALDI-TOF mass spectrometer (PerSeptive Biosystems, Framingham, MA). Proteins were identified by comparing the mass spectra obtained to those in the National Center for Biotechnology Information (NCBI) and SwissProt protein sequence databases, using the Prospector (http://prospector.ucsf.edu) and Mascot (http://www.matrixscience.com) tools.
RESULTS AND DISCUSSION
General features of the genome.
The genome of S. parauberis strain KCTC11537BP (GenBank accession no. CP002471) consists of a single circular chromosome of 2,143,887 bp (Fig. 1), which places the genome size in the middle of the 1.8-to-2.3-Mb range of streptococcal genomes sequenced to date. The average GC content of 35.6% is significantly lower than those of S. pyogenes (38.5%) (39) and S. pneumoniae (39.7%) (92). This average also differs when the nucleotide content of coding sequences is considered; in such sequences, G and A (17.4 and 31.9% of the total) are relatively more abundant than are C and T (18.2 and 32.5%).
Fig. 1.
Circular representation of the S. parauberis KCTC11537BP genome. The outer circle indicates predicted coding regions on the forward DNA strand. The next circle (moving inward) indicates predicted coding regions on the reverse DNA strand. The next circle shows GC content (%) of the S. parauberis genome. The final circle shows GC skew, calculated as (G − C)/(G + C). Numbers on the outsides of circles indicate locations in the S. parauberis genome.
The likely origin of replication of the S. parauberis KCTC11537BP chromosome was identified by several features required for action of the product of the dnaA (STP_0001) gene, which interacts with repetitive nonpalindromic nonamer sequences, located within the oriC region as determined by GC skew analysis, and by the similarity of the origin to those of other genomic sequences (18, 58, 69). The replication termination site appears to be localized near 0.9 Mb, according to GC skew analysis, and a coding bias within the two genomic strands is evident in the outer strand from 0 Mb to 0.9 Mb and on the inner strand from 0.9 Mb to 2.1 Mb. Also, several relevant genes are in the 0.9-Mb region, including parC and parE, which together encode the topoisomerase IV subunit involved in chromosome partitioning (1). The S. parauberis KCTC11537BP genome showed an asymmetric pattern distinct from those of other streptococci upon GC skew analysis. A previous report suggested that such asymmetry is caused by mutational bias, resulting from dissimilar rates of mutation within the two strands, rather than selective bias (66).
A total of 2,641 CDSs were annotated in the S. parauberis KCTC11537BP genome, using CRITICA (2,189 CDSs), Glimmer (2,256 CDSs), and the CLC main workbench (2,056 CDSs). After detailed analysis, 1,868 genes were selected on the basis of significant conservation among annotated CDSs. Approximately 45.2% of annotated genes were transcribed in the positive direction and 54.8% negatively, with respect to the direction of DNA replication. The S. parauberis genome encodes 15 rRNAs and 54 tRNAs (Table 1).
Table 1.
Overall features of the genome of S. parauberis KCTC11537BP
| Parameter | Value |
|---|---|
| No. of circular chromosomes | 1 |
| Size (bp) | 2,143,887 |
| GC content (%) | 35.6 |
| No. of rRNA genes | 15 |
| No. of tRNA genes | 54 |
| No. of annotated genes | |
| CRITICA | 2,189 |
| Glimmer | 2,256 |
| NCBI (manually) | 2,056 |
| No. of significant CDSs | 1,868 |
The Clusters of Orthologous Groups (COGs) program is very useful for compilation of the annotated gene data of a complete genome, to easily describe and systematically deduce the functions of protein families (91). The 1,868 predicted genes of S. parauberis were sorted, with respect to COG classification (Table 2). A total of 444 (23.8%) genes were associated with information storage and processing, 311 (16.6%) with cellular processes and signaling, and 714 (38.3%) with metabolism. A total of 399 (21.3%) residual genes, which could not be categorized into COG classes, have poorly characterized functions and features (Table 2).
Table 2.
Distribution of proteins among functional categories
| Category | Code | Functional classification | Annotated gene |
|
|---|---|---|---|---|
| No. | % | |||
| Information storage and processing | ||||
| J | Translation, ribosomal structure, and biogenesis | 158 | 8.5 | |
| A | RNA processing and modification | 0 | 0 | |
| K | Transcription | 166 | 8.9 | |
| L | Replication, recombination, and repair | 118 | 6.3 | |
| B | Chromatin structure and dynamics | 2 | 0.1 | |
| Cellular processes and signaling | ||||
| D | Cell cycle control, cell division, and chromosome partitioning | 18 | 0.9 | |
| Y | Nuclear structure | 0 | 0 | |
| V | Defense mechanisms | 50 | 2.7 | |
| T | Signal transduction mechanisms | 48 | 2.6 | |
| M | Cell wall/membrane/envelope biogenesis | 105 | 5.6 | |
| N | Cell motility | 13 | 0.7 | |
| Z | Cytoskeleton | 0 | 0 | |
| W | Extracellular structures | 0 | 0 | |
| U | Intracellular trafficking, secretion, and vesicular transport | 16 | 0.8 | |
| O | Posttranslational modification, protein turnover, and chaperones | 61 | 3.3 | |
| Metabolism | ||||
| C | Energy production and conversion | 89 | 4.8 | |
| G | Carbohydrate transport and metabolism | 201 | 10.8 | |
| E | Amino acid transport and metabolism | 146 | 7.8 | |
| F | Nucleotide transport and metabolism | 76 | 4.1 | |
| H | Coenzyme transport and metabolism | 50 | 2.7 | |
| I | Lipid transport and metabolism | 61 | 3.3 | |
| P | Inorganic ion transport and metabolism | 81 | 4.3 | |
| Q | Secondary metabolite biosynthesis, transport, and catabolism | 10 | 0.5 | |
| Poorly characterized | ||||
| R | General functional prediction only | 226 | 12.1 | |
| S | Function unknown | 173 | 9.2 | |
| X | Not annotated | 0 | 0 | |
Comparison of the genome sequence with those of other Streptococcus spp.
S. parauberis appears to be biochemically and serologically indistinguishable from the closely related species S. uberis (98). In the present study, alignment of the entire S. parauberis genome revealed extensive genomic rearrangement in strain KCTC11537BP relative to the genomes of S. uberis NC_012004, S. pyogenes NC_003485, S. equi subsp. zooepidemicus NC_011134, S. mutans NC_004350, S. agalactiae NC_007432, and S. thermophilus NC_006449, upon dot blot analysis using in silico molecular cloning, genomic edition, version 4.1.21, software. Such analysis showed that S. parauberis KCTC11537BP shares higher homology with S. uberis than with other Streptococcus spp. (Fig. 2) but exhibits variation in two regions of the genome (approximately 0.1 to 0.4 Mb and 1.4 to 1.9 Mb). These regions may have been changed by internal mutation, which could explain the derivation of S. parauberis from S. uberis. Approximately 77.6% of annotated genes were conserved, upon high-query coverage, between S. parauberis KCTC11537BP and S. uberis. Thus, S. parauberis KCTC11537BP isolated from olive flounder shares genomic features with a bovine mastitis strain.
Fig. 2.
Dot blot analysis of the genome sequence of S. parauberis KCTC11537BP and six other species of Streptococcus (S. uberis NC_012004, S. pyogenes NC_003485, S. equi subsp. zooepidemicus NC_011134, S. mutans NC_004350, S. agalactiae NC_007432, and S. thermophilus NC_006449).
The genus Streptococcus may be taxonomically subdivided into closely related groups, such as pyogenic and oral strains. Previously, S. parauberis and S. uberis were classified as “other” streptococci (57). However, overviews of taxonomic and nomenclature changes (38, 47) have suggested that S. parauberis and S. uberis should be grouped as pyogenic streptococci. In the present study, in an effort to form a more accurate understanding of relationships among streptococci, the sequences of two genes (those of gap and cpn) of 13 and 17 Streptococcus strains, respectively, were downloaded from NCBI and used in phylogenetic analysis. A close relationship between S. parauberis KCTC11537BP and S. uberis and an association of both strains with pyogenic streptococci were confirmed by unrooted tree analysis of gap and cpn gene sequence similarities in both species and in various other streptococci (Fig. 3).
Fig. 3.
Phylogenetic relationship of the S. parauberis KCTC11537BP gap gene with those of 17 other streptococci (A), and that of the cpn gene with those of 13 other streptococci (B). Trees were calculated using the maximum-likelihood method, with 1,000 bootstrap replications.
Regulation and signaling.
Bacteria are affected by developments in their surroundings, or materials produced by metabolic processes, to which they must mount appropriate responses. In most instances, a response involves transcriptional activation of genes, the products of which deal specifically with a given molecular signal. Gene expression is tightly controlled by a repertoire of transcriptional regulators, particularly the so-called sigma factors (81). A sigma factor is a prokaryotic transcription initiation factor that enables specific binding of RNA polymerase to gene promoters. The number of sigma factors present in any genome varies considerably; 18 factors may be expressed by Bacillus subtilis (58), whereas only 2 relevant sequences are present in the genome of S. parauberis KCTC11537BP. These include the RNA polymerase sigma factor RpoD (STP_0520), a representative of a transcription factor class recognizing a large number of promoters that control expression of housekeeping genes. Another example is the alternative sigma factor, also termed the extracytoplasmic function (ECF) sigma factor 54 modulation protein, RpoN (STP_1227). An anti-sigma factor has also been identified; this is the molecular chaperone STP_0201, which has the ability to respond to environmental or host stimuli, driving expression of relevant functional genes. However, sigma factors characteristic of S. pyogenes and S. pneumoniae (39, 59) were not found in the present work.
Sequencing of S. parauberis KCTC11537BP revealed the presence of GTP pyrophosphokinase (STP_1498) and ppGpp synthesis (STP_1534) genes, which together encode a bifunctional enzyme involved in synthesis and hydrolysis of ppGpp during amino acid starvation. This universal nucleotide is produced in response to circumstances that cause growth arrest (17). Recent findings suggest that ppGpp regulates the differential binding abilities of sigma factors to core RNA polymerase (54), and ppGpp appears to be required for the function of many alternative sigma factors (see Table S1 in the supplemental material).
In bacteria expressing many two-component control systems, such as Escherichia coli or Bacillus subtilis, it appears that the systems arose by gene duplication from one or more ancestral prototypes and acquired the capacity to respond to new input signals and to deliver novel output promoter specificities (48). For example, the B. subtilis two-component families originated from a single progenitor pair, as confirmed by the conservation of sequences around the active histidine sites of the kinases and the active site residues of the regulators, the structure of the output domain, and the relative chromosomal order of kinase and response regulator genes (37). Such regulatory systems adapt to environmental changes, gradually becoming a family of proteins widely distributed among many bacterial genera (88). Analysis of the S. parauberis KCTC11537BP genome revealed 17 sensor histidine kinases and 11 response regulators (see Table S2 in the supplemental material). S. parauberis has a larger number of two-component systems than does S. pneumoniae or Lactobacillus lactis. This suggests that these systems may play important roles in adaptation to various niches in different hosts.
Metabolic pathways.
The S. parauberis KCTC11537BP genome encodes two energy metabolism-related proteins, subunits of the cytochrome d ubiquinol oxidase (STP_1626 and STP_1627). This operon (STP_1624 to STP_1628) shows high similarity to a sequence encoding components of the S. agalactiae respiratory chain (102). Compared to those of S. agalactiae, the pathways are incomplete, as genes required for biosynthesis of quinine (required for reduction of oxygen) and heme (a cytochrome oxidase cofactor) are not encoded. The presence of two distinct metabolic routes toward energy production, fermentation, and respiration endows S. parauberis KCTC11537BP with a metabolic versatility that may promote survival in diverse niches. The ability to respire aerobically may be important for the spread and dissemination of the bacterium, although the requirements for exogenous heme and quinine suggest that such an ability will be strongly linked to environmental conditions dictated either by the host or niche microbiota.
Carbohydrate metabolism is key for the survival of S. parauberis KCTC11537BP, and current knowledge of sugar metabolism by this organism, combined with genomic data, suggest that S. parauberis KCTC11537BP is more capable of metabolizing a wider variety of carbohydrates than is any other previously sequenced Gram-positive organism. Genes involved in the transport and metabolism of glucose, fructose, sucrose, lactose, galactose, mannose, cellobiose, maltose/maltodextrin, ribulose, and possibly sorbitol (see Table S3 in the supplemental material) are evident in the genome. Forty-four loci of the S. parauberis genome may express components of phosphotransferase systems (PTSs) that may be partially aligned with (or divergent from) reported pathways. In comparison, the nonpathogenic bacterium S. thermophilus has seven PTSs, four of which contain pseudogenes (14). In a previous study, Ward et al. (97) found that S. uberis 0140J contained five CDSs, for which no orthologous matches were apparent in other streptococci. In S. parauberis KCTC11537BP, four CDSs matched those of S. uberis; these include genes encoding mannitol-specific PTSs (STP_0827 and STP_0829), ribulose-phosphate 3-epimerase (STP_1669), and mannitol-1-phosphate 5-dehydrogenase (STP_0826). In comparison with those of other streptococci, the S. parauberis KCTC11537BP and S. uberis genomes contain a distinct inventory of genes encoding carbohydrate degradation and utilization. Such diversity of sugar transport and utilization genes affords the capacity for survival in complex host and environmental niches.
ATP-binding cassette (ABC) systems are universally distributed among living organisms and play roles in many different aspects of bacterial physiology. ABC transporters are best known as essential for the import of particular nutrients and the export of toxic molecules but can also mediate transport of many other physiological substrates (26). The S. parauberis KCTC11537BP genome encodes 82 such transporters (see Table S4 in the supplemental material); such genes account for almost 4% of all S. parauberis KCTC11537BP open reading frames (ORFs). Thirty-two ABC transporters are input and output systems, with binding and permease functions, whereas 8 function within membranes. ABC transporters exhibit specificities for different substrates—amino acids, oligopeptides, polysaccharides, osmoprotectants (glycine and betaine), and metal ions—and function in the recognition and processing of DNA lesions. S. parauberis KCTC11537BP synthesizes glutamine and also possesses five predicted glutamine ABC transporters, emphasizing the importance of this amino acid as a principal source of nitrogen and a substrate for amino acid biosynthesis.
The S. parauberis KCTC11537BP genome encodes a polyphosphate kinase (STP_ 1474) not found in any other Streptococcus sp. except S. uberis. This gene is normally highly conserved in Gram-negative bacteria (95); the product catalyzes the transfer of the terminal phosphate of ATP to form a long-chain polyphosphate (polyP), in a freely reversible reaction (64). In previous studies, E. coli mutants lacking the polyphosphate kinase were shown to be deficient in response to stress (nutritional or osmotic) and failed to survive in stationary phase (7, 24, 83). In Vibrio cholerae, the polyphosphate store has been shown to enhance the ability of the species to overcome environmental stresses (low pH, excess salinity, or the presence of H2O2) in minimal medium with a low phosphate content (53). The presence of a polyphosphate kinase gene in the S. parauberis KCTC11537BP genome suggests that the organism can possibly be equipped to tolerate a rather low-phosphate environment, such as can be found in coastal waters.
Apart from stress pathways common to eubacteria, lactic acid-producing bacteria must deal with acidification of the immediate environment. The principal means of protection against acid stress in S. parauberis KCTC11537BP is likely the action of the membrane-bound, acid-stable, proton translocation F0F1 ATPase, a protein that can maintain the intracellular pH at 7.5, protecting S. mutans against an acidified environment. The genes encoding components of this ATPase are present in the streptococcal genome (11, 82). The arginine deiminase (STP_1157) pathway, in cooperation with the action of ornithine carbamoyltransferase (STP_1155) and carbamate kinase (STP_1152), is used by some species of Lactococcus, Streptococcus, and Lactobacillus to survive a decrease in pH. Several proteases possibly involved in the stress response, and other stress-related proteins are encoded by the genome; these include cold-shock proteins (STP_0511 and STP_1760) and a heat-shock protein (HSP; STP_1570).
Virulence factors.
Virulence factors of S. parauberis KCTC11537BP (see Table S5 in the supplemental material) help protect the bacterium against possible host defenses, allow it to occupy an ecological niche in the aquatic environment, and contribute to the ability of the bacterium to cause damage to the host.
There are three ways in which streptococcal proteins may be maintained at the cell surface. A protein may be covalently anchored through the C terminus to the cell wall peptidoglycan, can be tethered to the cell membrane via an N-terminal association with a lipid (a lipoprotein [LP]), or may be retained on the cell surface (or sometimes interact with the surface) via noncovalent interaction with cell surface components, such as other proteins or polysaccharides.
The major virulence factor of group A streptococci (GAS) is a cell surface antigen termed the M protein (40). M proteins are acid- and heat-stable, trypsin-labile fibrillar proteins associated with the outer surface of the cell wall. The M protein is anchored in the cell membrane, extends through the peptidoglycan layer, and projects from the surface of the bacterial cell. Strains that are rich in M protein are resistant to phagocytosis and intracellular killing by polymorphonuclear cells (84). The S. iniae M-like protein (SiM) is another prime candidate in virulence. This protein enhances adherence to fish epithelial cells, and macrophage resistance (10, 67). S. parauberis KCTC11537BP also encodes a SiM protein (STP_1567), which plays a key role in virulence.
Several genes encoding known proteins similar to streptococcal extracellular matrix-binding proteins were also identified in S. parauberis KCTC11537BP; these include metal ABC transporters (STP_1167 and STP_0295), an extracellular oligopeptide-binding protein (STP_0388), an oligopeptide transporter and associated permease proteins (STP_1402, STP_1403, and STP_1404), a glutamine ABC transporter, glutamine-binding proteins/permeases (STP_1006, STP_1007, and STP_1008) that enable cell aggregation, and a fibronectin/fibrinogen-binding protein (STP_0963). Such proteins are also expressed by S. pneumoniae (51) and S. pyogenes (21) and are required for cell internalization and interactions of potential relevance in pathogenesis (78). Genes encoding a surface protein that binds plasminogen, glyceraldehyde-3-phosphate dehydrogenase (STP_1661), and enolase (STP_0468) (25) were also found in S. parauberis KCTC11537BP.
S. parauberis KCTC11537BP can adhere to the agglutinin of internal organs, the extracellular matrix, and epithelial cell surface receptors. Ajdić et al. (2) reported in 2002 that S. mutans expressed two major types of adhesins, cell-surface proteins and sucrose-derived glucans. In S. parauberis KCTC11537BP, adhesion to internal organs, required for colonization, is achieved by the expression of surface proteins (STP_1755 and STP_0624) and surface-anchored proteins (STP_0597, STP_0900, STP_1632, and STP_1446) (75). Lipoproteins and cell wall-anchored (CWA) proteins are exposed on the surface and frequently serve as virulence factors (42, 77, 80). The lgt and lspA genes, the products of which are expected to function in lipoprotein processing, are present; these genes encode prolipoprotein diacylglyceryl transferase (STP_0381) and lipoprotein signal peptidase (STP_0559), respectively. A gene encoding sortase A (STP_0694), which is involved in the processing of CWA proteins, was also found. Interestingly, the numbers of such proteins (nine lipoproteins and six CWA proteins) are fewer compared to those of other Streptococcus species. S. sanguinis has 60 LPs and 33 CWA proteins, S. mutans 29 and 6, and S. pneumoniae 40 and 12. Such arrays of surface proteins may contribute to the ability of diverse Streptococcus spp. to colonize and interact with a host.
The GAS capsule is composed of a polymer of hyaluronic acid containing repeating units of glucuronic acid and N-acetylglucosamine (89). Synthesis of the polymer requires the products of three genes, hasA, hasB, and hasC, located in the same operon (29). The hasA gene encodes hyaluronate synthase (STP_1716) (31); hasB encodes UDP-glucose dehydrogenase (STP_1715) (30); and hasC encodes UDP-glucose pyrophosphorylase (STP_1711) (23). The expression of has genes is transcriptionally controlled, and the genes are transcribed as a single message from a promoter upstream of hasA (22). hasA and hasB of the has operon (hasABC) in S. parauberis KCTC11537BP is arranged similarly to that in GAS. However, hasC was identified downstream of hasB in a reverse orientation and separated from it by some 3 kb. It is apparently unrelated to capsule biosynthesis (97). In a previous study, capsule production is dependent only upon functional hasA and hasB, but not hasC, in GAS (6). S. parauberis KCTC11537BP encodes hasA and hasB genes that could contribute to capsule production for resistance against phagocytosis.
In S. pneumoniae and other streptococci, the spread of resistance to β-lactam antibiotics has occurred in natural populations when segments of penicillin-binding proteins (PBPs) from β-lactam-sensitive strains were replaced by homologous blocks originating from resistant strains, resulting in the formation of gene mosaics (33). Such transfers are likely mediated by natural transformation with exogenous DNA, and species boundaries may be crossed (32). The two most important PBPs associated with penicillin resistance in S. pneumoniae are encoded by pbp1A and pbp2X. The S. parauberis KCTC11537BP genome encodes four PBPs, including pbp1A and pbp2X. When the encoded proteins bind β-lactam antibiotics, cell wall construction by the bacterium is inhibited.
Antigenic protein analysis.
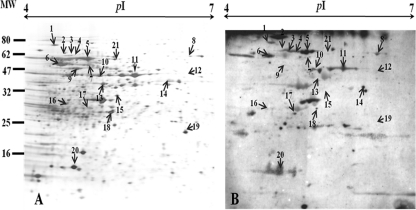
Recent achievements in genomics have facilitated the rapid interchange of biological information. Proteomics, the systematic study of proteins in eukaryotes and prokaryotes, has progressed extremely rapidly. The techniques of proteomics (high-resolution 2-DE and protein characterization) are widely used in microbiological research to analyze global protein synthesis as an indicator of gene expression. Progress in microbial proteomics has been aided by the widespread availability of whole-genome sequences for a number of bacterial groups (13, 16). Thus, proteomics has emerged as a valuable method for analyzing genome-encoded proteins and is useful for studying protein functions in cellular architecture, metabolic regulation, and disease pathology (20, 62). We identified antigenic proteins of S. parauberis KCTC11537BP using immunoproteomics. The approach involved antigen identification using a specific flounder anti-S. parauberis KCTC11537BP serum generated by an experimental S. parauberis KCTC11537BP challenge. Twenty-one antigenic proteins were identified (Fig. 4 and Table 3).
Fig. 4.
The 2-DE profile of a whole-cell lysate of S. parauberis KCTC11537BP (A) and 2-DE immunoblotting using a flounder anti-S. parauberis KCTC11537BP serum (B). Arrows with numbers indicate protein spots analyzed by MALDI-TOF. MW, molecular weight (in thousands); pI, isoelectric point.
Table 3.
Identification of antigenic protein profiles using flounder anti-S. parauberis KCTC11537BP serum
| Spot no. | Protein name | Scorea | No. of queries matchedb | Sequence coverage (%)c | MW (kDa)/pI | Species | Functional classification |
|---|---|---|---|---|---|---|---|
| 1 | Chaperone protein DnaK (STP_0200) | 46 | 7 | 19 | 64.9/4.16 | Streptococcus pyogenes serotype M1 | Posttranslational modification, protein turnover, chaperones |
| 2 | 60-kDa chaperonin (STP_1756) | 83 | 12 | 32 | 57.3/4.69 | Streptococcus agalactiae serotype Ia | Posttranslational modification, protein turnover, chaperones |
| 3 | GMP synthase (STP_0712) | 70 | 14 | 27 | 58.3/4.90 | Streptococcus thermophilus LMG 18311 | Nucleotide transport and metabolism |
| 4 | Galactose-6-phosphate isomerase subunit LacB (STP_0246) | 35 | 4 | 38 | 18.9/5.51 | Staphylococcus aureus subsp. aureus Mu3 | Carbohydrate transport and metabolism |
| 5 | Elongation factor Tu (STP_0412) | 52 | 7 | 16 | 43.8/4.91 | Streptococcus pyogenes serotype M1 | Translation, ribosomal structure, and biogenesis |
| 6 | Enolase (STP_0468) | 97 | 14 | 33 | 47.1/4.63 | Streptococcus agalactiae serogroup Ia | Carbohydrate transport and metabolism |
| 7 | Arginine deiminase (STP_1157) | 117 | 13 | 33 | 46.5/5.18 | Streptococcus agalactiae serogroup Ia | Amino acid transport and metabolism |
| 8 | IMP dehydrogenase (STP_1861) | 60 | 8 | 26 | 53.0/5.66 | Streptococcus mutans NN2025 | Nucleotide transport and metabolism |
| 9 | Phosphoglycerate kinase (STP_1660) | 46 | 7 | 27 | 42.2/4.96 | Streptococcus uberis 0140J | Carbohydrate transport and metabolism |
| 10 | Ornithine carbamoyltransferase, catabolic (STP_1155) | 78 | 10 | 36 | 38.1/5.25 | Streptococcus agalactiae serogroup III | Amino acid transport and metabolism |
| 11 | Glyceraldehyde-3-phosphate dehydrogenase (STP_1661) | 72 | 9 | 27 | 35.8/5.38 | Streptococcus dysgalactiae subsp. equisimilis | Carbohydrate transport and metabolism |
| 12 | Catabolite control protein A (STP_0341) | 88 | 10 | 37 | 36.7/5.79 | Streptococcus pyogenes MGAS8232 | Transcription |
| 13 | l-lactate dehydrogenase (STP_0692) | 62 | 6 | 14 | 35.3/5.14 | Streptococcus pyogenes serotype M1 | Energy production and conversion |
| 14 | Phosphofructokinase (STP_0581) | 53 | 7 | 22 | 35.6/5.33 | Streptococcus equi subsp. equi 4047 | Carbohydrate transport and metabolism |
| 15 | 30S ribosomal protein S2 (STP_1769) | 145 | 14 | 60 | 28.6/5.22 | Streptococcus agalactiae serogroup III | Translation, ribosomal structure, and biogenesis |
| 16 | Tyrosyl-tRNA synthetase (STP_1617) | 48 | 7 | 26 | 47.5/5.46 | Streptococcus pneumoniae D39 | Translation, ribosomal structure, and biogenesis |
| 17 | 50S ribosomal protein L1 (STP_0303) | 43 | 5 | 27 | 24.4/9.22 | Streptococcus gordonii str. Challis | Translation, ribosomal structure, and biogenesis |
| 18 | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase (STP_1097) | 53 | 6 | 38 | 26.1/5.10 | Streptococcus pyogenes serotype M1 | Carbohydrate transport and metabolism |
| 19 | Uracil phosphoribosyltransferase (STP_1330) | 42 | 5 | 36 | 22.9/5.45 | Streptococcus salivarius | Nucleotide transport and metabolism |
| 20 | Phosphocarrier protein HPr (STP_1055) | 36 | 3 | 44 | 8.9/4.71 | Streptococcus equinus | Carbohydrate transport and metabolism |
| 21 | NADP-dependent glyceraldehyde-3-phosphate dehydrogenase (STP_1053) | 95 | 12 | 36 | 50.8/5.02 | Streptococcus uberis 0140J | Carbohydrate transport and metabolism |
Mascot scores are given as −10 × log10(P) values, where P is the probability that the observed match is a random event. A value greater than 70 (P < 0.05) indicates a statistically significant match.
“Queries matched” denotes matching tryptic digest fragments upon peptide mass fingerprinting.
Amino acid sequence coverage of a protein from matched tryptic digest fragments.
The 2-DE immunoproteomics data identified chaperone protein (no. 1, STP_0200), the 60-kDa chaperonin (no. 2, STP_1756), enolase (no. 6, STP_0468), and GAPDH (no. 11, STP_1661); all of these proteins are associated with virulence and antibiotic resistance (20, 94). The chaperone protein and the 60-kDa chaperonin are termed heat-shock protein 70 (HSP 70) and HSP 60. Such proteins are representative of a class of functionally related proteins, the expression of which is increased when cells are exposed to elevated temperature or other stressors (73). Both GAPDH and enolase interact with plasminogen-binding proteins that may enhance bacterial invasion or movement through normal tissue barriers (15). Two ribosomal proteins, 30S ribosomal protein S2 (no. 15, STP_1769) and 50S ribosomal protein L1 (no.17, STP_0303) were also identified; some ribosomal proteins have been reported to catalyze posttranslational modifications involving acetylation and methylation of amino acids. Such changes extend the range of protein functions. The functional groups attached include acetate, phosphate, and various lipids and carbohydrates. Also, changes in the chemical structure of an amino acid or structural changes, such as formation of a disulfide bridge, can affect protein function (5). Phosphoglycerate kinase (no. 9, STP_1660) and phosphofructokinase (no. 14, STP_0581) were also identified; these proteins provide a regulatory link between glycolytic activity and regulation of signal transduction, involving the formation of a biofilm as a protective antigen. Arginine deiminase (no. 7, STP_1157) interacts with ornithine carbamoyltransferase (no. 10, STP_1155) to serve as an important regulator of metabolic pathways in some species of Lactococcus, Streptococcus, and Lactobacillus; this system allows the bacteria to survive a decrease in pH (65).
Immunoproteomics has been used widely to identify immunogenic molecules and pathogenicity factors in various streptococcal species, such as S. pyogenes (19, 61), S. pneumoniae (63), and S. suis (99). Our immunoproteomics data are limited compared to our genomic information. However, we have identified proteins that interact with host defense systems, thus obtaining an insight into bacterial survival and virulence mechanisms.
An alliance of proteomics and genomics will yield near-complete, accurate gene catalogs of microbes, essential for study of systems biology.
Conclusion.
The complete genome of S. parauberis KCTC11537BP is midsized and has a lower GC content than have other streptococci. This first analysis of a Gram-positive bacterium revealed numerous biological, virulence, and pathogenetic factors, reflecting adaptation of the organism to become an obligate and versatile fish pathogen. As genomic analysis shows, S. parauberis KCTC11537BP could possibly possess the ability to regulate the metabolism of more carbohydrates than other Streptococcus species do and to synthesize all the amino acids and regulatory factors required for survival in a hostile environment. Sequencing of the complete genomes of bacterial pathogens has stimulated research on novel vaccine candidates. Simultaneously, such work greatly aids in the development of transcriptomics and proteomics. The present study applied immunoproteomics, combining the use of a specific antiserum with the precision of mass spectrometry, to define the immunoreactive antigenic pattern of an in vivo host-pathogen interaction. Such fundamental data provide an essential basis for the development of new prophylactic and therapeutic strategies to counter fish streptococcal infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the World Class University Program (R32-10253) funded by the Korean Ministry of Education, Science, and Technology of South Korea.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Adams D. E., Shekhtman E. M., Zechiedrich E. L., Schmid M. B., Cozzarelli N. R. 1992. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell 71:277–288 [DOI] [PubMed] [Google Scholar]
- 2. Ajdić D., et al. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434–14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alber J., El-Sayed A., Lämmler C., Hassan A. A., Zschöck M. 2004. Polymerase chain reaction mediated identification of Streptococcus uberis and Streptococcus parauberis using species-specific sequences of the genes encoding superoxide dismutase A and chaperonin 60*. J. Vet. Med. B Infect. Dis. Vet. Public Health 51:180–184 [DOI] [PubMed] [Google Scholar]
- 4. Aoki T., Takami K., Kaito T. 1990. Drug resistance in a non-hemolytic Streptococcus sp. isolated from cultured yellowtail Seriola quinqueradiata. Dis. Aquat. Organ. 8:171–177 [Google Scholar]
- 5. Arnold R. J., Reilly J. P. 1999. Observation of Escherichia coli ribosomal proteins and their posttranslational modifications by mass spectrometry. Anal. Biochem. 269:105–112 [DOI] [PubMed] [Google Scholar]
- 6. Ashbaugh C. D., Alberti S., Wessles M. R. 1998. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J. Bacteriol. 180:4955–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ault-Riché D., Fraley C. D., Tzeng C. M., Kornberg A. 1998. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180:1841–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Austin B., Austin D. A. 1999. Bacterial pathogens: diseases of farmed and wild fish, p. 13–15, 3rd ed. Springer-Praxis, Praxis Publishing, Ltd., Chichester, United Kingdom [Google Scholar]
- 9. Badger J. H., Olsen G. J. 1999. CRITICA: coding region identification tool invoking comparative analysis. Mol. Biol. Evol. 16:512–524 [DOI] [PubMed] [Google Scholar]
- 10. Baiano F. C. F., Tumbol R. A., Umapathy A., Hurvitz A. C. 2008. Identification and molecular characterisation of a fibrinogen binding protein from Streptococcus iniae. BMC Microbiol. 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bender G. R., Sutton S. V., Marquis R. E. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentley R. W., Leigh J. A., Collins M. D. 1991. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 41:487–494 [DOI] [PubMed] [Google Scholar]
- 13. Blackstock W. P., Weir M. P. 1999. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 17:121–127 [DOI] [PubMed] [Google Scholar]
- 14. Bolotin A., et al. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 22:1554–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle M. D., Lottenberg R. 1997. Plasminogen activation by invasive human pathogens. Thromb. Haemost. 77:1–10 [PubMed] [Google Scholar]
- 16. Cash P. 2000. Proteomics in medical microbiology. Electrophoresis 21:1187–1201 [DOI] [PubMed] [Google Scholar]
- 17. Cashel M., Gentry D. R., Hernandez V. J., Vinella D. 1996. The stringent response, p. 1458–1496 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2 American Society for Microbiology, Washington, DC [Google Scholar]
- 18. Christensen B. B., Atlung T., Hansen F. G. 1999. DnaA boxes are important elements in setting the initiation mass of Escherichia coli. J. Bacteriol. 181:2683–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole J. N., et al. 2005. Surface analyses and immune reactivities of major cell wall-associated proteins of group A streptococcus. Infect. Immun. 73:3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordwell S. J., Nouwens A. S., Walsh B. J. 2001. Comparative proteomics of bacterial pathogens. Proteomics 1:461–472 [DOI] [PubMed] [Google Scholar]
- 21. Courtney H. S., Li Y., Dale J. B., Hasty D. L. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 62:3937–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crater D. L., van de Rijn I. 1995. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J. Biol. Chem. 270:18452–18458 [DOI] [PubMed] [Google Scholar]
- 23. Crater D. L., Dougherty B. A., van de Rijn I. 1995. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose pyrophosphorylase activity. J. Biol. Chem. 270:28676–28680 [DOI] [PubMed] [Google Scholar]
- 24. Crooke E., Akiyama M., Rao N. N., Kornberg A. 1994. Genetically altered levels of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 269:6290–6295 [PubMed] [Google Scholar]
- 25. Cunningham M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davidson A. L., Dassa E., Orelle C., Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72:317–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delcher A. L., Harmon D., Kasif S., White O., Salzberg S. L. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doménech A., et al. 1996. Streptococcosis in cultured turbot (Schophtalmus maximus) associated with Streptococcus parauberis. J. Fish Dis. 19:33–38 [Google Scholar]
- 29. Dougherty B. A., van de Rijn I. 1992. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. J. Exp. Med. 175:1291–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dougherty B. A., van de Rijn I. 1993. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity. J. Biol. Chem. 268:7118–7124 [PubMed] [Google Scholar]
- 31. Dougherty B. A., van de Rijn I. 1994. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J. Biol. Chem. 269:169–175 [PubMed] [Google Scholar]
- 32. Dowson C. G., et al. 1990. Penicillin-resistant viridans streptococci have obtained altered penicillin-binding protein genes from penicillin-resistant strains of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 87:5858–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dowson C. G., Coffey T. J., Kell C., Whiley R. A. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635–643 [DOI] [PubMed] [Google Scholar]
- 34. Eldar A., et al. 1995. Streptococcus shiloi, the name for an agent causing septicemic infection in fish, is a junior synonym of Streptococcus iniae. Int. J. Syst. Bacteriol. 45:840–842 [DOI] [PubMed] [Google Scholar]
- 35. Ewing B., Hillier L., Wendl M. C., Green P. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8:175–185 [DOI] [PubMed] [Google Scholar]
- 36. Ewing B., Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8:186–194 [PubMed] [Google Scholar]
- 37. Fabret C., Feher V. A., Hoch J. A. 1999. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J. Bacteriol. 181:1975–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Facklam R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15:613–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferretti J. J., et al. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 98:4658–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischetti V. A. 1991. Streptococcal M protein. Sci. Am. 264:58–65 [DOI] [PubMed] [Google Scholar]
- 41. Gardner P. P., et al. 2009. Rfam: updates to the RNA families database. Nucleic Acids Res. 37:D136–D140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ge X., Kitten T., Munro C. L., Conrad D. H., Xu P. 2010. Pooled protein immunization for identification of cell surface antigens in Streptococcus sanguinis. PLoS One. 5:e11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghittino C., Prearo M. 1992. Report of streptococcosis in rainbow trout (Oncorhynchus mykiss) in Italy: preliminary note. Boll. Soc. Ital. Patol. Ittica 8:4–11 [Google Scholar]
- 44. Glaser P., et al. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499–1513 [DOI] [PubMed] [Google Scholar]
- 45. Gordon D., Abajian C., Green P. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:195–202 [DOI] [PubMed] [Google Scholar]
- 46. Görg A., Weiss W., Dunn M. J. 2004. Current two-dimensional electrophoresis technology for proteomics. Proteomics 4:3665–3685 [DOI] [PubMed] [Google Scholar]
- 47. Hardie J. M., Whiley R. A. 1997. Classification and overview of the genera Streptococcus and Enterococcus. Soc. Appl. Bacteriol. Symp. Ser. 26:1S–11S [PubMed] [Google Scholar]
- 48. Hoch J. A. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3:165–170 [DOI] [PubMed] [Google Scholar]
- 49. Holden M. T., et al. 2009. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:e6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holden M. T., et al. 2009. Genomic evidence for the evolution of Streptococcus equi: host restriction, increased virulence, and genetic exchange with human pathogens. PLoS. Pathog. 5:e1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holmes A. R., et al. 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 41:1395–1408 [DOI] [PubMed] [Google Scholar]
- 52. Hoskins J., et al. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jahid I. K., Silva A. J., Benitez J. A. 2006. Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl. Environ. Microbiol. 72:7043–7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jishage M., Kvint K., Shingler V., Nyström T. 2002. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 16:1260–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kanai K., et al. 2009. Serological differentiation of Streptococcus parauberis strains isolated from cultured Japanese flounder in Japan. Fish Pathol. 44:33–39 [Google Scholar]
- 56. Kang S. H., et al. 2006. Efficacy of protein A-HRP in an immunological study of black rockfish (Sebastes schlegeli Higendorf) humoral immune responses. Fish Shellfish Immunol. 20:295–304 [DOI] [PubMed] [Google Scholar]
- 57. Kilpper-Bälz R., Schleifer K. H. 1987. Streptococcus suis sp. nov., nom. rev. Int. J. Syst. Bacteriol. 37:160–162 [Google Scholar]
- 58. Kunst F., et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249–256 [DOI] [PubMed] [Google Scholar]
- 59. Lee M. S., Morrison D. A. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lehner I., Niehof M., Borlak J. 2003. An optimized method for the isolation and identification of membrane proteins. Electrophoresis 24:1795–1808 [DOI] [PubMed] [Google Scholar]
- 61. Lei B., Mackie S., Lukomski S., Musser J. M. 2000. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect. Immun. 68:6807–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin D., Tabb D. L., Yates J. R., III 2003. Large-scale protein identification using mass spectrometry. Biochim. Biophys. Acta 1646:1–10 [DOI] [PubMed] [Google Scholar]
- 63. Ling E., et al. 2004. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 138:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Littauer U. Z., Kornberg A. 1957. Reversible synthesis of polyribonucleotides with an enzyme from Escherichia coli. J. Biol. Chem. 226:1077–1092 [PubMed] [Google Scholar]
- 65. Liu Y., Dong Y., Chen Y. Y., Burne R. A. 2008. Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl. Environ. Microbiol. 74:5023–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lobry J. R. 1996. Asymmetric substitution patterns in the two DNA strands of bacteria. Mol. Biol. Evol. 13:660–665 [DOI] [PubMed] [Google Scholar]
- 67. Locke J. B., Aziz R. K., Vicknair M. R., Nizet V., Buchanan A. C. 2008. Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS One 3:e2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lowe T. M., Eddy S. R. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mackiewicz P., Zakrzewska-Czerwinska J., Zawilak A., Dudek M. R., Cebrat S. 2004. Where does bacterial replication start? Rules for predicting the oriC region. Nucleic Acids Res. 32:3781–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Makarova K., et al. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Margulies M., et al. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mata A. I., et al. 2004. Multiplex PCR assay for detection of bacterial pathogens associated with warm-Water streptococcosis in fish. Appl. Environ. Microbiol. 70:3183–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Morimoto R. I., Kline M. P., Bimston D. N., Cotto J. J. 1997. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 32:17–29 [PubMed] [Google Scholar]
- 74. Nho S. W., et al. 2009. Phenotypic characteristics of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus). FEMS Microbiol. Lett. 293:20–27 [DOI] [PubMed] [Google Scholar]
- 75. Nobbs A. H., Lamont R. J., Jenkinson H. F. 2009. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73:407–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Park Y. K., et al. 2009. Antibiotic susceptibility and resistance of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus). Vet. Microbiol. 136:76–81 [DOI] [PubMed] [Google Scholar]
- 77. Parsons H. K., Metcalf S. C., Tomlin K., Read R. C., Dockrell D. H. 2007. Invasive pneumococcal disease and the potential for prevention by vaccination in the United Kingdom. J. Infect. 54:435–438 [DOI] [PubMed] [Google Scholar]
- 78. Peacock S. J., Foster T. J., Cameron B. J., Berendt A. R. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477–3486 [DOI] [PubMed] [Google Scholar]
- 79. Perera R. P., Johnson S. K., Collins M. D., Lewis D. H. 1994. Streptococcus iniae associated with mortality of Tilapia nilotica × T. aurea hybrids. J. Aquat. Anim. Health 6:335–340 [Google Scholar]
- 80. Piard J. C., et al. 1997. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J. Bacteriol. 179:3068–6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Potvin E., Sanschagrin F., Levesque R. C. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 32:38–55 [DOI] [PubMed] [Google Scholar]
- 82. Quivey R. G., Kuhnert W. L., Hahn K. 2001. Genetics of acid adaptation in oral streptococci. Crit. Rev. Oral Biol. Med. 12:301–314 [DOI] [PubMed] [Google Scholar]
- 83. Rao N. N., Kornberg A. 1996. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J. Bacteriol. 178:1394–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robinson J. H., Kehoe M. A. 1992. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol. Today 13:362–367 [DOI] [PubMed] [Google Scholar]
- 85. Shin G. W., et al. 2006. Production of monoclonal antibodies against serum immunoglobulins of black rockfish (Sebastes schlegeli Higendorf). J. Vet. Sci. 7:293–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shin G. W., et al. 2006. Discrimination of streptococcosis agents in olive flounder (Paralichthys olivaceus). Bull. Eur. Assoc. Fish Pathol. 26:68–79 [Google Scholar]
- 87. Shin G. W., et al. 2006. Partial two-dimensional gel electrophoresis (2-DE) maps of Streptococcus iniae ATCC29178 and Lactococcus garvieae KG9408. Dis. Aquat. Organ. 70:71–79 [DOI] [PubMed] [Google Scholar]
- 88. Stock A. M., Robinson V. L., Goudreau P. N. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215 [DOI] [PubMed] [Google Scholar]
- 89. Stoolmiller A. C., Dorfman A. 1969. The biosynthesis of hyaluronic acid by Streptococcus. J. Biol. Chem. 244:236–246 [PubMed] [Google Scholar]
- 90. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 91. Tatusov R. L., Koonin E. V., Lipman D. J. 1997. A genomic perspective on protein families. Science 278:631–637 [DOI] [PubMed] [Google Scholar]
- 92. Tettelin H., et al. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506 [DOI] [PubMed] [Google Scholar]
- 93. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thongboonkerd V., et al. 2002. Fluoride exposure attenuates expression of Streptococcus pyogenes virulence factors. J. Biol. Chem. 277:16599–16605 [DOI] [PubMed] [Google Scholar]
- 95. Tzeng C. M., Kornberg A. 1998. Polyphosphate kinase is highly conserved in many bacterial pathogens. Mol. Microbiol. 29:381–382 [DOI] [PubMed] [Google Scholar]
- 96. Vickerman M. M., Iobst S., Jesionowski A. M., Gill S. R. 2007. Genome-wide transcriptional changes in Streptococcus gordonii in response to competence signaling peptide. J. Bacteriol. 189:7799–7807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ward P. N., et al. 2009. Evidence for niche adaptation in the genome of the bovine pathogen Streptococcus uberis. BMC Genomics 10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williams A. M., Collins M. D. 1990. Molecular taxonomic studies on Streptococcus uberis types I and II. Description of Streptococcus parauberis sp. nov. J. Appl. Bacteriol. 68:485–490 [DOI] [PubMed] [Google Scholar]
- 99. Wu Z., Zhang W., Lu C. 2008. Immunoproteomic assay of surface proteins of Streptococcus suis serotype 9. FEMS Immunol. Med. Microbiol. 53:52–59 [DOI] [PubMed] [Google Scholar]
- 100. Wuyts J., Perrière G., Van De Peer Y. 2004. The European ribosomal RNA database. Nucleic Acids Res. 32:D101–D103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu P., et al. 2007. Genome of the opportunistic pathogen Streptococcus sanguinis. J. Bacteriol. 189:3166–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamamoto Y., et al. 2005. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol. Microbiol. 56:525–534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.