Abstract
The Chlamydia pneumoniae CopN protein is a member of the YopN/TyeA/InvE/MxiC family of secreted proteins that function to regulate the secretion of type III secretion system (T3SS) translocator and effector proteins. In this study, the Scc1 (CP0432) and Scc4 (CP0033) proteins of C. pneumoniae AR-39 were demonstrated to function together as a type III secretion chaperone that binds to an N-terminal region of CopN. The Scc1/Scc4 chaperone promoted the efficient secretion of CopN via a heterologous T3SS, whereas, the Scc3 chaperone, which binds to a C-terminal region of CopN, reduced CopN secretion.
INTRODUCTION
Numerous Gram-negative bacterial pathogens utilize type III secretion systems (T3SSs) to inject effector proteins into their host's cells in order to manipulate signaling pathways that normally function to limit bacterial growth and/or dissemination (19). Effector proteins are transported across the bacterial membranes by a multicomponent type III secretion (T3S) apparatus or injectisome (4, 29). The injectisome consists of a base structure that spans both bacterial membranes and a needle structure that extends 40 to 60 nm from the bacterial cell surface. The passage of effector proteins across the eukaryotic membrane is enabled by a pore-forming translocon complex. Translocon assembly, as well as effector protein secretion and injection, is triggered by contact between a bacterium and a eukaryotic cell; therefore, the T3S process has been termed “contact dependent” (33).
The regulation of the T3S process is complex and manifested at the levels of both substrate selection and T3S apparatus activation (13, 14). Following the assembly of a secretion-competent base structure, substrates required for the assembly of the internal rod-like and external needle-like structures are selectively secreted (26). Upon completion of the needle/rod assembly, a substrate specificity switch is triggered, allowing the secretion of translocon components and/or translocon and effector proteins (1). In many T3SSs, the needle tip complex, in combination with other translocon components, plays a role in preventing the spurious release of effector proteins prior to contact with a eukaryotic cell (13). A separate protein complex, exemplified by the YopN/SycN/YscB/TyeA complex of the Yersinia sp. plasmid-encoded T3SS, also plays an essential role in preventing the premature release of effector proteins (7, 11). Upon contact with a eukaryotic cell, translocon assembly is completed and effector protein secretion and injection are triggered.
In the Yersinia spp., the YopN/SycN/YscB/TyeA complex is required to prevent effector protein secretion prior to contact with a eukaryotic cell in vivo and in the presence of extracellular calcium in vitro. The 293-residue secreted and injected YopN protein interacts with the cytosolic SycN/YscB chaperone at its N terminus and with the cytosolic TyeA protein at its C terminus (12, 22). The SycN/YscB chaperone is required for stable expression of YopN and regulation of Yop secretion, as well as for the efficient secretion and translocation of YopN. TyeA, on the other hand, functions with YopN to directly regulate effector protein secretion (7, 11). In addition, TyeA functions to inhibit YopN translocation upon contact with a eukaryotic cell. Previous studies suggest that the cytosolic YopN/SycN/YscB/TyeA complex prevents effector protein secretion from a cytosolic location, possibly via direct interactions with the T3S apparatus (16, 30).
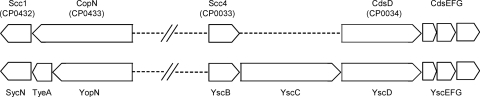
Proteins homologous to YopN and TyeA can be found in essentially all nonflagellar T3SSs; however, in the majority of these proteins, domains homologous to YopN and TyeA are present in a single protein, with sequences homologous to TyeA located in the C-terminal portion of the protein (31). In contrast, proteins homologous to the Yersinia SycN and YscB proteins have been identified only in closely related T3SSs, such as those of Pseudomonas aeruginosa, Photorhabdus luminescens, Vibrio parahaemolyticus, and Aeromonas spp. (38). In general, T3SSs that express separate proteins homologous to YopN and TyeA also encode chaperones homologous to SycN and YscB. On the contrary, most T3SSs that express a single protein with domains homologous to YopN and TyeA do not encode chaperones homologous to SycN and YscB. Indeed, no T3S chaperones have been identified for MxiC of Shigella flexneri (5) or InvE of Salmonella enterica (28); however, recently, CesL of enteropathogenic Escherichia coli was shown to function as a T3S chaperone for SepL, a YopN/TyeA family protein with domains homologous to both YopN and TyeA (41). Another possible exception to this rule is the CopN protein of Chlamydia spp. CopN is a secreted and translocated protein that is required for Chlamydia pneumoniae intracellular growth (21). CopN, like MxiC, InvE, and SepL, contains domains homologous to both YopN and TyeA (17). CopN has previously been shown to interact with a tetratricopeptide repeat-containing class II chaperone termed Scc3 (specific chlamydial chaperone 3); however, Scc3 binds to a C-terminal region of CopN (35). Interestingly, the copN gene in Chlamydia trachomatis is located adjacent to a gene that encodes a class I T3S chaperone (Scc1) whose product was found to interact with another predicted class I chaperone (CT663) in a yeast 2-hybrid screen (36). Although Scc1 and CT663 share no significant amino acid sequence homology with SycN and YscB, their gene locations (Fig. 1) and interaction are reminiscent of the Yersinia SycN and YscB chaperones.
Fig. 1.
Genetic map of C. pneumoniae chromosomal regions and Y. pestis plasmid pCD1 regions encoding CopN- and YopN-specific chaperones. The C-terminal region of CopN is homologous to TyeA of Y. pestis. The locations of the genes encoding Scc1 and Scc4 of C. pneumoniae are similar to those of sycN and yscB in Y. pestis. The C. pneumoniae YscC homolog CdsC is located downstream of the CdsEFG-encoding genes.
In this study, we demonstrate that C. pneumoniae AR-39 encodes a T3S chaperone composed of Scc1 (CP0432) and Scc4 (CP0033) that binds to an N-terminal region of CopN. Furthermore, we demonstrate a specific effect of the Scc1/Scc4 chaperone complex, but not the individual chaperones, on CopN secretion using a heterologous Yersinia T3SS.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Yersinia pestis KIM strains (Table 1) used in these studies are avirulent and are excluded from the National Select Agent Registry due to deletion of the 102-kb pgm locus (39). In addition, the Y. pestis KIM strains used in CopN secretion assays carried a deletion of the yopE and sycE loci (3) and were cured of the plasminogen activator (Pla) protease-encoding pPCP1 plasmid (40). Experiments with attenuated Y. pestis strains were reviewed and approved by the Institutional Biosafety Committee at the University of Miami. Y. pestis strains were routinely grown in heart infusion broth (HIB) or on tryptose blood agar (TBA) base plates (BD-Difco) at 27°C. For secretion assays, Y. pestis strains were grown in TMH medium in the presence or absence of 2.5 mM CaCl2 as described previously (23). E. coli DH5α and BL21(DE3) were grown in HIB or Luria-Bertani (LB) medium. When appropriate, antibiotics were routinely used at the following concentrations: ampicillin, 50 μg/ml; streptomycin, 50 μg/ml; kanamycin, 25 μg/ml; trimethoprim, 25 μg/ml; chloramphenicol, 20 μg/ml.
Table 1.
Bacterial strains used in this study
| Strain | Description | Source |
|---|---|---|
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA argF)U169 endA1 recA1 hsdR17 deoR supE44 thi-1 gyr96 relA1 | 6 |
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | 37 |
| Y. pestis | ||
| KIM8-3002.P1 | Smr pCD1 (ΔyscB)/pMT1 | 23 |
| KIM8-3002.P108 | Smr pCD1 (ΔyopN-tyeA-sycN-yscB)/pMT1 | This study |
| KIM8 Δ4 | pCD1 (ΔsycE-yopE::dhfr)/pMT1 | 3 |
| KIM8-3002.P109 | Smr pCD1 (ΔyopN-tyeA-sycN-yscB ΔsycE-yopE::dhfr)/pMT1 | This study |
Construction of the Y. pestis yopN tyeA sycN yscB deletion strain.
Lambda Red-mediated recombination was used to delete the coding sequences for yopN, tyeA, and sycN from a ΔyscB mutant Y. pestis strain (KIM8-3002.P1) (23) using a previously described modification (24) of the procedure of Datsenko and Wanner (10). The oligonucleotide primers used to amplify the kanamycin cassette from plasmid pKD4 were YopN-SycN-P1 and YopN-SycN-P2 (see Table S1 in the supplemental material). The resultant PCR product was gel purified and electroporated into Y. pestis KIM8-3002.P1 carrying plasmid pKD46, and kanamycin-resistant ΔyopN-tyeA-sycN-yscB deletion mutants were selected for by plating on TBA plates containing 25 μg/ml kanamycin and confirmed by PCR analyses. Plasmid pCP20 (8), which encodes the FLP recombinase, was electroporated into the ΔyopN-tyeA-sycN-yscB deletion mutant strain to remove the FLP recognition sequence-flanked kan cassette. Plasmids pCP20 and pKD46, which carry temperature-sensitive origins of replication, were cured by overnight growth at 39°C. The presence of the deletion and the absence of pCP20 and pKD46 were confirmed by PCR analyses and by agarose gel electrophoresis of plasmids isolated by the method of Kado and Liu (27). The resultant Pgm− pPCP1− (Pla−) ΔyopN-tyeA-sycN-yscB deletion mutant, designated KIM8-3002.P108, was used for a second round of Lambda Red-mediated recombination to remove the coding sequences for SycE and YopE. A ca.1-kb fragment was amplified from plasmid pCD1-Δ4 of Y. pestis KIM8 Δ4 (3) using oligonucleotide primers YopE-dhfr-F and YopE-dhfr-R. Plasmid pCD1-Δ4 carries a deletion of pCD1 sequences from position 42,958 to position 47,891 (sycE and yopE coding sequences) and an insertion of dhfr. The resultant purified PCR product was electroporated into KIM8-3002.P108 carrying plasmid pKD46, and trimethoprim-resistant Pgm− pPCP1− (Pla−) ΔyopN-tyeA-sycN-yscB ΔsycE-yopE deletion mutants were selected for on TBA plates containing 25 μg/ml trimethoprim. Plasmid pKD46 was cured by overnight growth at 39°C, and the presence of the ΔyopN-tyeA-sycN-yscB and ΔsycE-yopE deletions, as well as the absence of plasmid pKD46, was confirmed by PCR analyses and by the method of Kado and Liu (27). The resultant Pgm− pPCP1− (Pla−) ΔyopN-tyeA-sycN-yscB ΔsycE-yopE deletion strain, designated KIM8-3002.P109, and the parent KIM8 Δ4 strain were used for CopN, Scc1 (CP0432), Scc3 (CP0832), and Scc4 (CP0033) expression and secretion studies.
Construction of plasmid pMAL-CopN/Scc1/Scc4.
Plasmid pMAL-CopN/Scc1/Scc4 was constructed through multiple steps using the PCR-ligation-PCR technique (2). A DNA fragment encoding 406 bp of malE and the TEV protease recognition site of plasmid pKm1255 (gift from David Waugh) was amplified using oligonucleotide primers MBP-BlpI-F and TEV-R. A second DNA fragment encoding CopN (starting at codon 2) was amplified from C. pneumoniae AR-39 chromosomal DNA using oligonucleotide primers CopN-F and CopN-BamHI-R. The two PCR products were ligated together and reamplified using primers MBP-BlpI-F and CopN-BamHI-R. The resultant PCR product was digested with BlpI and BamHI and inserted into BlpI- and BamHI-digested pMAL-c2X (New England BioLabs). The resulting construct, encoding MBP-CopN, was termed pMAL-CopN.
The gene for Scc1 was amplified from C. pneumoniae AR-39 chromosomal DNA using primers Scc1-RBS-BamHI-F and Scc1-RBS-R. Likewise, the gene for Scc4 (CP0033) was amplified using primers Scc4-RBS-F and Scc4-HindIII-6xHIS-R. The Scc1-RBS-BamHI-F and Scc4-RBS-F primers were designed to insert sequences encoding a strong ribosome-binding site (RBS) upstream of the scc1 and scc4 ATG start codons. The DNA fragments encoding Scc1 and Scc4 were ligated together and reamplified using primers Scc1-RBS-BamHI-F and Scc4-HindIII-6xHIS-R. The resulting DNA fragment was digested with BamHI and HindIII and inserted into BamHI- and HindIII-digested pMAL-CopN, generating plasmid pMAL-CopN/Scc1/Scc4. The pMAL-CopN/Scc1/Scc4 plasmid encodes a maltose-binding protein (MBP)-CopN hybrid protein with a TEV protease site located between MBP and CopN. In addition, the plasmid encodes Scc1 and Scc4 with a C-terminal 6-histidine tag.
Construction of plasmids pBAD18-Scc1, pBAD18-Scc1/Scc4, pBAD33-Scc3, and pFLAG-Scc4.
The scc1 gene was amplified from plasmid pMAL-CopN/Scc1/Scc4 DNA using oligonucleotide primers Scc1-XbaI-F and Scc1-HindIII-R. The DNA fragment encoding both Scc1 and Scc4 was also amplified from pMAL-CopN/Scc1/Scc4 DNA using oligonucleotide primers Scc1-XbaI-F and Scc4-HindIII-R. The resultant DNA fragments were digested with XbaI and HindIII and inserted into XbaI- and HindIII-digested pBAD18 (20), generating plasmids pBAD18-Scc1 and pBAD18-Scc1/Scc4. Plasmid pFLAG-CTC was used for construction of pFLAG-Scc4. The scc4 gene was amplified from C. pneumoniae AR-39 chromosomal DNA using primers Scc4-HindIII-F and Scc4-BamHI-R. The resultant DNA fragment was digested with HindIII and KpnI and inserted into HindIII- and KpnI-digested pFLAG-CTC (Sigma Aldrich), generating plasmid pFLAG-Scc4. Plasmid pBAD33-Scc3 expresses Scc3 under the control of the PBAD promoter of plasmid pBAD33 (20). The DNA fragment encoding Scc3 was amplified from C. pneumoniae AR-39 chromosomal DNA with primers Scc3-KpnI-F and Scc3-XhoI-R. The resultant PCR product was digested with KpnI and XhoI and inserted into KpnI- and SalI-digested pBAD33, generating plasmid pBAD33-Scc3.
Construction of plasmids pCopN, pCopN/Scc1, pCopN/Scc4, pCopN/Scc1/Scc4, and pScc3.
Plasmids pCopN, pCopN/Scc1, and pCopN/Scc1/Scc4 carry the copN gene alone, with the scc1 gene, or with both the scc1 and scc4 genes under the control of the PBAD promoter of plasmid pBAD24 (20). DNA fragments encoding CopN, CopN, and Scc1 or CopN, Scc1, and Scc4 were amplified from pMAL-CopN/Scc1/Scc4 using primer CopN-MfeI-F paired with primer CopN-HindIII-R (copN), Scc1-KpnI-R (copN and scc1), or Scc4-HindIII-R2 (copN, scc1, and scc4). The resultant DNA fragments were digested with MfeI and HindIII (or KpnI) and inserted into EcoRI- and HindIII (or KpnI)-digested pBAD24, generating plasmids pCopN, pCopN/Scc1, and pCopN/Scc1/Scc4. Plasmid pCopN/Scc4 was generated by the PCR-ligation-PCR technique using primers CopN-MfeI-F and CopN-R to amplify copN and primers Scc4-RBS-F and Scc4-HindIII-R2 to amplify scc4. The resultant PCR products were ligated together and reamplified with primers CopN-MfeI-F and Scc4-HindIII-R2. The resultant DNA fragment was digested with MfeI and HindIII and inserted into EcoRI- and HindIII-digested pBAD24. The scc3 gene was amplified from the C. pneumoniae AR-39 genomic DNA using primers Scc3-F and Scc3-R. The fragment was cloned into a Gateway compatible pBAD33-based vector using standard Gateway cloning techniques (Invitrogen Life Technologies) to yield pScc3.
Construction of plasmids pGST-CopN, pGST-CopN1-320, pGST-CopN1-220, pGST-CopN1-100, pGST-CopN101-399, pGST-CopN201-399, and pGST-CopN301-399.
Plasmid pET42b (Novagen) was used for the construction of glutathione S-transferase (GST)-CopN expression vectors. DNA fragments encoding CopN1-399, CopN1-320, CopN1-220, or CopN1-100 were amplified from C. pneumoniae AR-39 chromosomal DNA using primer CopN-PshA-F paired with primer CopN-399-HindIII-R (CopN1-399), CopN-320-HindIII-R (CopN1-320), CopN-220-HindIII-R (CopN1-220), or CopN-100-HindIII-R (CopN1-100). The resultant DNA fragments were digested with PshAI and HindIII and inserted into PshAI- and HindIII-digested pET42b, generating plasmids pGST-CopN, pGST-CopN1-320, pGST-CopN1-220, and pGST-CopN1-100. DNA fragments encoding CopN101-399, CopN201-399, or CopN101-399 were amplified from plasmid pMAL-CopN/Scc1/Scc4 using primer CopN-399-HindIII-R paired with primer CopN-101-EcoRV-F (CopN101-399), CopN-201-EcoRV-F (CopN201-399), or CopN-301-EcoRV-F (CopN301-399). The resultant DNA fragments were digested with EcoRV and HindIII and inserted into PshAI- and HindIII-digested pET42b, generating plasmids pGST-CopN101-399, pGST-CopN201-399, and pGST-CopN301-399.
Construction of pGAD-CopN, pGAD-Scc1, pGAD-Scc3, pGAD-Scc4, pGBT-CopN, pGBT-Scc1, pGBT-Scc3, and pGBT-Scc4.
DNA fragments encoding CopN, Scc1, Scc3, and Scc4 were amplified from C. pneumoniae AR-39 chromosomal DNA using primers CopN-MfeI-F, CopN-NsiI-R, Scc1-MfeI-F, Scc1-PstI-R, Scc3-EcoRI-F, Scc3-NsiI-R, Scc4-EcoRI-F, and Scc4-PstI-R. The resultant DNA fragments were digested with MfeI or EcoRI and PstI or NsiI and inserted into EcoRI- and PstI-digested pGAD424 and pGBT9, generating plasmids pGAD-CopN, pGAD-Scc1, pGAD-Scc3, pGAD-Scc4, pGBT-CopN, pGBT-Scc1, pGBT-Scc3, and pGBT-Scc4.
Yeast two-hybrid assays.
Yeast two-hybrid assays were performed using methods recommended by the commercial supplier (Matchmaker Yeast Two-Hybrid System; Clontech). Plasmids were transformed into Saccharomyces cerevisiae SFY596 according to the manufacturer's instructions and plated on appropriate synthetic defined medium (SD) plates. Colony lift assays for detection of β-galactosidase activity were performed essentially as described elsewhere (Clontech).
Secretion assays.
Y. pestis KIM8 Δ4 or KIM8-P109 carrying plasmids pCopN, pCopN/Scc1, pCopN/Scc4, pCopN/Scc1/Scc4, and pCopN with Scc3 or pCopN/Scc1/Scc4 with pScc3 was grown overnight at 27°C in TMH medium with the appropriate antibiotics. The next day, overnight cultures were used to inoculate fresh TMH cultures, with or without 2.5 mM CaCl2, to an optical density at 620 nm (OD620) of 0.2. Cultures were grown for 1 h at 27°C and then for 5 h at 37°C. Bacterial cell pellets and culture supernatants were separated by centrifugation at 12,200 × g for 10 min at room temperature (RT). Culture supernatant proteins were precipitated on ice overnight with 10% (vol/vol) trichloroacetic acid and collected by centrifugation at 21,920 × g for 15 min at 4°C. Volumes of cellular fractions corresponding to equal numbers of bacteria were mixed 1:1 with 2× electrophoresis sample buffer and analyzed by SDS-PAGE and immunoblotting with antibodies specific for C. pneumoniae CopN, Scc1, Scc3, or Scc4 and Y. pestis YopM or H-NS.
GST pulldown assays.
E. coli BL21(DE3) was transformed with protein expression vector pET42b, pGST-CopN, pGST-CopN1-320, pGST-CopN1-220, pGST-CopN101-399, pGST-CopN201-399, pGST-CopN301-399, pBAD18-Scc1, pBAD18-Scc1/Scc4, pBAD33-Scc3, or pFLAG-Scc4. Overnight cultures were inoculated into 35 ml HIB to an OD620 of 0.2 and incubated with shaking at 37°C to an OD620 of 0.4 to 0.6. The production of recombinant proteins was initiated with the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) or 0.2% l-arabinose for 2 h at 37°C. Cultures were harvested by centrifugation at 6,000 × g for 20 min at 4°C, and the resulting pellets were washed with 200 ml of cold phosphate-buffered saline (140 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4). Washed pellets were resuspended in 10 ml ice-cold GST binding buffer (5 mM Na2PO4, 1.5 mM KH2PO4, 140 mM NaCl, 3 mM KCl, pH 7.4) and lysed by passage through a chilled French pressure cell at 20,000 lb/in2. Detergent (0.1% [final concentration] NP-40) was added, and the lysates were centrifuged at 6,000 × g for 20 min at 4°C to remove unlysed cells. Appropriate combinations of the lysates (2 ml of each lysate) were mixed and incubated for 5 min at RT prior to loading onto a 1-ml bed volume column of glutathione agarose beads (GST-Bind Resin; Novagen). The column was washed with 10 ml of GST binding buffer containing 0.1% NP-40 and eluted with 1 ml GST binding buffer containing 20 mM reduced glutathione. Initial lysates and eluted samples were analyzed by SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting.
Volumes of cellular fractions corresponding to equal numbers of bacteria or equal amounts of protein were mixed 1:1 (vol/vol) with 2× electrophoresis sample buffer and analyzed by SDS-PAGE and immunoblotting essentially as previously described (23). CopN, Scc1, Scc3, and Scc4 were detected with affinity-purified antipeptide antibodies raised in rabbits against peptides corresponding to residues 385 to 399 of CopN, 31 to 45 of Scc1, 158 to 172 of Scc3, and 32 to 46 of Scc4 (ProteinTech Group). Y. pestis proteins were visualized as previously described, using polyclonal antiserum specific for YopM and H-NS. Band intensities were quantitated using an Alpha Innotech 5500 imaging system (Cell Biosciences).
RESULTS
To confirm the previously identified interaction of Scc1 (CP0432) with Scc4 (CP0033; CT663 in C. trachomatis) (36), as well as that of CopN (CP0433) with Scc3 (CP0832) (35), the copN, scc1, scc4, and scc3 genes from C. pneumoniae AR-39 were amplified (see Table S1 in the supplemental material) and inserted into the pGAD424 and pGBT9 yeast two-hybrid vectors. Yeast two-hybrid studies confirmed that CopN (CP0433) interacts with Scc3 (CP0832) and that Scc1 (CP0432) interacts with Scc4 (CP0033) as previously reported (35, 36); however, no interaction of Scc1 or Scc4 with CopN was detected (data not shown). These findings indicate that C. pneumoniae Scc1 and Scc4 interact directly and could function as a heterodimeric CopN-specific chaperone similar to the Yersinia sp. YopN-specific SycN/YscB chaperone.
The C. pneumoniae Scc1/Scc4 chaperone and Scc3 chaperone bind to CopN.
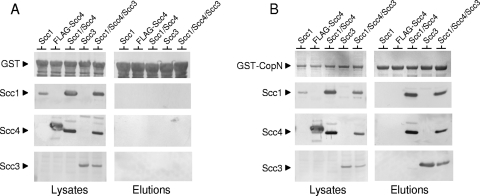
To determine if the Scc1/Scc4 complex interacts with CopN, GST pulldown studies were conducted using GST and GST-CopN. Whole-cell lysates from E. coli BL21(DE3) expressing GST or GST-CopN were mixed with lysates expressing Scc1, FLAG-tagged Scc4, Scc3, Scc1, and Scc4 or Scc1, Scc4, and Scc3. Scc4 without a FLAG tag was unstable and poorly expressed in the absence of Scc1. Lysates were combined, incubated for 5 min at RT to allow complex formation, and applied to glutathione-Sepharose columns. The columns were washed and eluted with 20 mM reduced glutathione, and initial lysates along with elutions were analyzed by SDS-PAGE and immunoblotting (Fig. 2). No interaction of the individual Scc1 or Scc4 protein with GST-CopN was detected; however, Scc3 efficiently bound to and coeluted with GST-CopN as previously reported (35). Interestingly, Scc1 and Scc4 coeluted with GST-CopN when lysates containing both Scc1 and Scc4 or all three chaperone-like proteins (Scc1, Scc4, and Scc3) were used in pulldown experiments. No interaction of Scc1, Scc4, or Scc3 with GST alone was detected (Fig. 2A). These results demonstrate that a complex composed of Scc1 and Scc4, but not the individual proteins, directly interacts with CopN, suggesting that Scc1 and Scc4 form a heterodimeric chaperone similar to the Yersinia sp. SycN/YscB chaperone.
Fig. 2.
The Scc1/Scc4 chaperone and the Scc3 chaperone directly interact with CopN. E. coli BL21(DE3) lysates expressing GST (A) or GST-CopN (B) were combined with lysates expressing Scc1, FLAG-Scc4, Scc1/Scc4, Scc3, or Scc1/Scc4/Scc3. GST, GST-CopN, and interacting proteins were purified using glutathione agarose beads. Initial lysates and elutions were analyzed by SDS-PAGE and immunoblotting with antisera specific for GST, Scc1, Scc3, and Scc4.
Localization of the Scc1/Scc4 and Scc3 binding sites on CopN.
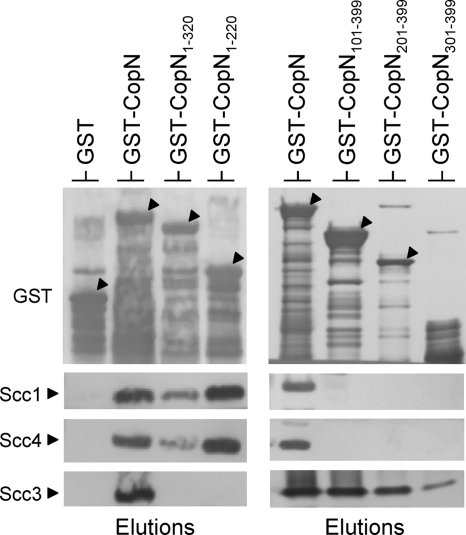
The majority of class 1 T3S chaperones interact with an N-terminal region of their cognate substrate termed the chaperone-binding domain (CBD) (15). The CBD of YopN consists of YopN residues 32 to 77 (34). To begin to locate the binding site on CopN for the Scc1/Scc4 complex, deletions in pGST-CopN were constructed that removed the coding sequence for residues 321 to 399 (pGST-CopN1-320), residues 221 to 399 (pGST-CopN1-220), residues 101 to 399 (pGST-CopN1-100), residues 1 to 100 (pGST-CopN101-399), residues 1 to 200 (pGST-CopN201-399), and residues 1 to 300 (pGST-CopN301-399). The resulting constructs were moved into E. coli BL21(DE3) for pulldown experiments. The pGST-CopN1-100 construct failed to express a stable CopN product and was not used in subsequent studies. BL21(DE3) lysates containing GST, GST-CopN, GST-CopN1-320, GST-CopN1-220, GST-CopN101-399, GST-CopN201-399, or GST-CopN301-399 were combined with lysates containing Scc1, Scc4, and Scc3 and analyzed as described for Fig. 2. As expected, Scc1, Scc4, and Scc3 all coeluted with full-length GST-CopN but not with GST alone (Fig. 3). Removal of the CopN C-terminal 79 (GST-CopN1-320) or 179 (GST-CopN1-220) residues abolished the binding of Scc3 to CopN but had no effect on the binding of Scc1 and Scc4. In contrast, deletion of the CopN N-terminal 100, 200, or 300 residues eliminated the binding of Scc1 and Scc4 to CopN but had little or no effect on the interaction of Scc3 with CopN. These results indicate that the Scc1/Scc4 complex binds to an N-terminal region of CopN, whereas Scc3 binds to a C-terminal region of CopN as previously reported (35).
Fig. 3.
Localization of the binding sites for the Scc1/Scc4 chaperone and the Scc3 chaperone on CopN. E. coli BL21(DE3) lysates expressing GST, GST-CopN, GST-CopN1-320, GST-CopN1-220, GST-CopN101-399, GST-CopN201-399, or GST-CopN301-399 were combined with lysates expressing Scc1, Scc3, and Scc4. GST-CopN hybrid proteins and interacting proteins were purified using glutathione agarose beads. Elutions were analyzed by SDS-PAGE and immunoblotting with antisera specific for GST, Scc1, Scc3, and Scc4.
The Scc1/Scc4 chaperone promotes efficient CopN secretion via the Yersinia sp. T3SS.
T3S chaperones have been shown to have a variety of activities that promote the secretion of their cognate substrate or substrates. These include preventing the degradation and/or aggregation of their substrates, maintaining substrates in a partially unfolded secretion-competent state, and/or directly targeting substrates to the T3S apparatus (15). Previous studies have demonstrated that CopN can be secreted via the Yersinia sp. plasmid-encoded T3SS (17); therefore, the effects of Scc1, Scc4, and Scc3 on CopN expression and secretion was determined by expressing various combinations of these proteins in avirulent Y. pestis KIM8 Δ4, which expresses a functional calcium-regulated T3SS.
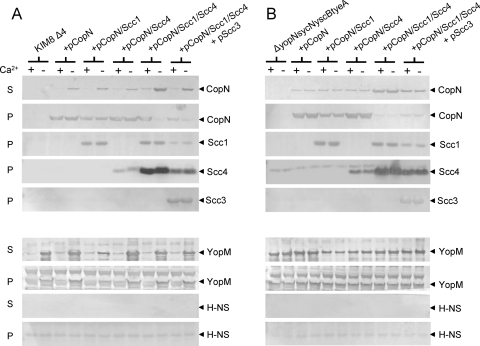
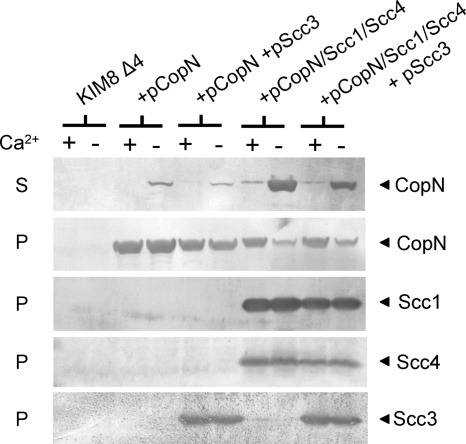
As demonstrated previously (17), CopN expressed in the absence of all other chlamydial proteins is secreted by the Yersinia T3SS at low levels in the absence of calcium (21% of total CopN secreted) but not in the presence of calcium, confirming that CopN is a T3S substrate (Fig. 4A). Coexpression of the individual Scc1 or Scc4 protein with CopN had no effect on CopN secretion; in contrast, coexpression of both Scc1 and Scc4 dramatically increased CopN secretion (91% of total CopN secreted), indicating that Scc1 and Scc4 function together as a T3S chaperone that specifically promotes efficient secretion of CopN. Expression of Scc3 in the presence of Scc1 and Scc4 resulted in a slight decrease in the expression of Scc1 and Scc4 and in a modest reduction in CopN secretion (63% of total CopN secreted), suggesting that Scc3 may have a direct or indirect inhibitory effect on CopN export.
Fig. 4.
Expression and secretion of CopN in the presence or absence of Scc1, Scc4, and Scc3. Y. pestis KIM8 Δ4 (parent) (A) and KIM8-P109 ΔyopN-sycN-yscB-tyeA) (B) alone or carrying plasmids pCopN, pCopN/Scc1, CopN/Scc4, and pCopN/Scc1/Scc4 or plasmids pCopN/Scc1/Scc4 and pScc3 were cultured in TMH medium with (+) and without (−) 2.5 mM calcium for 5 h at 37°C. Secreted (S) and/or cell pellet (P) proteins were analyzed by SDS-PAGE and immunoblotting with antisera specific for CopN, Scc1, Scc4, Scc3, YopM, or H-NS.
To determine if the inhibitory effect of Scc3 on CopN secretion was dependent upon the Scc1/Scc4 chaperone, secretion of CopN was measured in both the presence and the absence of the Scc1/Scc4 and Scc3 chaperones (Fig. 5). As expected, only low levels of CopN were secreted in the absence of Scc1 and Scc4; however, even less CopN was secreted in the presence of the Scc3 chaperone, suggesting that Scc3 functions to limit CopN secretion independently of the Scc1/Scc4 chaperone.
Fig. 5.
Expression and secretion of CopN in the presence or absence of Scc3. Y. pestis KIM8 Δ4 carrying plasmid pCopN or pCopN/Scc1/Scc4 with and without plasmid pScc3 was cultured in TMH medium with (+) and without (−) 2.5 mM calcium for 5 h at 37°C. Secreted (S) and/or cell pellet (P) proteins were analyzed by SDS-PAGE and immunoblotting with antisera specific for CopN, Scc1, Scc4, and Scc3.
Expression of CopN with or without its cognate chaperones has no effect on the calcium-dependent regulation of the Yersinia sp. T3SS.
The YopN/SycN/YscB/TyeA complex of Yersinia spp. is required to prevent Yop effector protein secretion in the presence of calcium; therefore, it is possible that expression of CopN, Scc1, Scc4, and/or Scc3 could augment or interfere with the regulatory function of these proteins. The expression and secretion of the Yersinia YopM protein were examined to assess the effects of the chlamydial proteins on the function of the Yersinia T3SS. The only significant effect observed was a modest decrease in YopM expression and secretion in strains expressing CopN and Scc1 (Fig. 4A). The cytoplasmic H-NS protein was used as a lysis control protein. No H-NS was observed in the culture supernatant fractions, confirming that the CopN present in the culture supernatants was due to the T3S process and not cell lysis.
Although it is inefficient, recent studies have demonstrated that calcium chelation stimulates the release of chlamydial T3S substrates from purified elementary bodies (25), indicating that the activity of the chlamydial T3SS, like that of the Yersinia sp. T3SS, may be regulated, in part, by extracellular calcium. To determine if the CopN/Scc1/Scc4 or CopN/Scc1/Scc4/Scc3 complex can substitute for the YopN/SycN/YscB/TyeA complex and restore calcium-regulated Yop secretion, the various CopN-expressing constructs were moved into an avirulent Y. pestis ΔyopN-sycN-yscB-tyeA deletion strain. The ΔyopN-sycN-yscB-tyeA deletion strain expressing CopN, the CopN/Scc1/Scc4 complex, or the CopN/Scc1/Scc4/Scc3 complex secreted Yops in the presence or absence of calcium, indicating that the chlamydial CopN/Scc1/Scc4 and CopN/Scc1/Scc4/Scc3 complexes are unable to complement the regulatory function of the YopN/SycN/YscB/TyeA complex.(Fig. 4B). Importantly, the effects of the Scc1/Scc4 complex and Scc3 on CopN secretion were maintained in the ΔyopN-sycN-yscB-tyeA background. Overall, these studies demonstrate that Scc1 and Scc4 form a chaperone complex that binds to an N-terminal region of CopN and facilitates CopN secretion. In contrast, Scc3 binds to a C-terminal region of CopN and appears to have a small inhibitory affect on CopN secretion.
DISCUSSION
Members of the YopN/TyeA/InvE/MxiC family of proteins are found in essentially all nonflagellar T3SSs and function to regulate the secretion of translocator and effector proteins (13, 31). The majority of the members of this family of proteins are expressed as single proteins, with domains homologous to YopN and TyeA, and interact with no identified T3S chaperones. In contrast, the Yersinia YopN and TyeA proteins function as part of a larger cytosolic YopN/TyeA/SycN/YscB complex (34). The SycN and YscB proteins form a heterodimeric T3S chaperone that binds to an N-terminal CBD on YopN and functions to prevent YopN degradation and promote the efficient secretion of YopN (12). CopN of Chlamydia spp. is a secreted protein with domains homologous to YopN and TyeA that is encoded directly upstream of a gene encoding a predicted T3S chaperone (Scc1) (17), suggesting that CopN may represent an example of a single protein YopN/TyeA/InvE/MxiC family member that utilizes a T3S chaperone. Interestingly, previous yeast two-hybrid studies have indicated that the Scc1 chaperone does not interact with CopN but does interact with another potential T3S chaperone (CT663) (36). Notably, the locations of the genes encoding the two interacting chaperones closely matched the locations of the genes encoding SycN and YscB in the yersiniae (Fig. 1). In this study, we confirm that the C. pneumoniae Scc1 and Scc4 (CT663 homolog) proteins function together as a T3S chaperone for CopN, suggesting that CopN, like YopN, utilizes a chaperone complex for its efficient secretion.
GST and GST-CopN pulldown experiments indicated that the Scc1/Scc4 complex interacts with an N-terminal region of CopN. Furthermore, expression of CopN with both Scc1 and Scc4 but not the individual proteins facilitated efficient secretion of CopN via the Yersinia sp. T3SS. These studies represent the first direct demonstration of a T3S chaperone function for a chlamydial class I chaperone and establish a function for both Scc1 and Scc4. Interestingly, the Scc3 chaperone has been previously shown to bind to a C-terminal region of CopN (35). We confirmed this finding and demonstrated that coexpression of Scc3 with CopN alone or with CopN, Scc1, and Scc4 had no stimulatory effect on the secretion of CopN but instead appeared to reduce CopN secretion. Importantly, the C-terminal region of CopN recognized by Scc3 corresponds to the region with homology to the Yersinia TyeA protein. TyeA has been shown to specifically reduce the translocation or injection of YopN by Yersinia spp. (7, 11). The C-terminal region of CopN that corresponds to TyeA might have a similar function, and it is possible that the binding of Scc3 to this region may enhance this function. In this regard, the CopN-Scc3 interaction may be primarily regulatory in nature. This assumption is supported by the fact that expression of Scc3 had no positive effect on CopN expression, stability, or secretion. Furthermore, tetratricopeptide repeat-containing chaperones, like Scc3, have previously been shown to be involved in a variety of regulatory protein interactions aside from their interaction with their cognate substrate (9, 18, 32).
Finally, expression of CopN, Scc1, and Scc4 with and without Scc3 in a Yersinia ΔyopN-sycN-yscB-tyeA deletion strain failed to restore any calcium-dependent regulation of Yop secretion. This is not surprising, as these proteins share only limited amino acid sequence homology; however, homologs of CopN, including YopN and TyeA, have a role in controlling the secretion of translocators and effector proteins in all T3SSs where their function has been investigated. Therefore, CopN and its interacting Scc1, Scc4, and Scc3 chaperones likely function in a similar manner to coordinate the secretion of chlamydial translocator and effector proteins.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI050552 from the National Institutes of Health and a Grant-in-Aid (AHA0051373B) from the American Heart Association.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 13 May 2011.
REFERENCES
- 1. Agrain C., et al. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 56:54–67 [DOI] [PubMed] [Google Scholar]
- 2. Ali S. A., Steinkasserer A. 1995. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. Biotechniques 18:746–750 [PubMed] [Google Scholar]
- 3. Bartra S. S., Jackson M. W., Ross J. A., Plano G. V. 2006. Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid. Infect. Immun. 74:1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blocker A., et al. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botteaux A., Sory M. P., Biskri L., Parsot C., Allaoui A. 2009. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71:449–460 [DOI] [PubMed] [Google Scholar]
- 6. Cambau E., Bordon F., Collatz E., Gutmann L. 1993. Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid. Antimicrob. Agents Chemother. 37:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng L. W., Kay O., Schneewind O. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherepanov P. P., Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14 [DOI] [PubMed] [Google Scholar]
- 9. Darwin K. H., Miller V. L. 2000. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol. Microbiol. 35:949–960 [DOI] [PubMed] [Google Scholar]
- 10. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Day J. B., Ferracci F., Plano G. V. 2003. Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47:807–823 [DOI] [PubMed] [Google Scholar]
- 12. Day J. B., Plano G. V. 1998. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol. Microbiol. 30:777–788 [DOI] [PubMed] [Google Scholar]
- 13. Deane J. E., Abrusci P., Johnson S., Lea S. M. 2010. Timing is everything: the regulation of type III secretion. Cell. Mol. Life Sci. 67:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edqvist P. J., et al. 2003. YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J. Bacteriol. 185:2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldman M. F., Cornelis G. R. 2003. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219:151–158 [DOI] [PubMed] [Google Scholar]
- 16. Ferracci F., Schubot F. D., Waugh D. S., Plano G. V. 2005. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57:970–987 [DOI] [PubMed] [Google Scholar]
- 17. Fields K. A., Hackstadt T. 2000. Evidence for the secretion of Chlamydia trachomatis CopN by a type III secretion mechanism. Mol. Microbiol. 38:1048–1060 [DOI] [PubMed] [Google Scholar]
- 18. Francis M. S., Lloyd S. A., Wolf-Watz H. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075–1093 [DOI] [PubMed] [Google Scholar]
- 19. Galán J. E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573 [DOI] [PubMed] [Google Scholar]
- 20. Guzman L. M., Belin D., Carson M. J., Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang J., Lesser C. F., Lory S. 2008. The essential role of the CopN protein in Chlamydia pneumoniae intracellular growth. Nature 456:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iriarte M., et al. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson M. W., Day J. B., Plano G. V. 1998. YscB of Yersinia pestis functions as a specific chaperone for YopN. J. Bacteriol. 180:4912–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson M. W., Silva-Herzog E., Plano G. V. 2004. The ATP-dependent ClpXP and Lon proteases regulate expression of the Yersinia pestis type III secretion system via regulated proteolysis of YmoA, a small histone-like protein. Mol. Microbiol. 54:1364–1378 [DOI] [PubMed] [Google Scholar]
- 25. Jamison W. P., Hackstadt T. 2008. Induction of type III secretion by cell-free Chlamydia trachomatis elementary bodies. Microb. Pathog. 45:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Journet L., Agrain C., Broz P., Cornelis G. R. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302:1757–1760 [DOI] [PubMed] [Google Scholar]
- 27. Kado C. I., Liu S. T. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kubori T., Galán J. E. 2002. Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184:4699–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubori T., et al. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602–605 [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Argudo I., Blocker A. J. 2010. The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol. Microbiol. 78:1365–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pallen M. J., Beatson S. A., Bailey C. M. 2005. Bioinformatics analysis of the locus for enterocyte effacement provides novel insights into type-III secretion. BMC Microbiol. 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pallen M. J., Francis M. S., Futterer K. 2003. Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 223:53–60 [DOI] [PubMed] [Google Scholar]
- 33. Rosqvist R., Magnusson K. E., Wolf-Watz H. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schubot F. D., et al. 2005. Three-dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. J. Mol. Biol. 346:1147–1161 [DOI] [PubMed] [Google Scholar]
- 35. Slepenkin A., de la Maza L. M., Peterson E. M. 2005. Interaction between components of the type III secretion system of Chlamydiaceae. J. Bacteriol. 187:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spaeth K. E., Chen Y. S., Valdivia R. H. 2009. The Chlamydia type III secretion system C-ring engages a chaperone-effector protein complex. PLoS Pathog. 5:e1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Studier F. W., Moffatt B. A. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113–130 [DOI] [PubMed] [Google Scholar]
- 38. Troisfontaines P., Cornelis G. R. 2005. Type III secretion: more systems than you think. Physiology (Bethesda) 20:326–339 [DOI] [PubMed] [Google Scholar]
- 39. Une T., Brubaker R. R. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Welkos S. L., Friedlander A. M., Davis K. J. 1997. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb. Pathog. 23:211–223 [DOI] [PubMed] [Google Scholar]
- 41. Younis R., et al. 2010. SepL resembles an aberrant effector in binding to a class 1 type III secretion chaperone and carrying an N-terminal secretion signal. J. Bacteriol. 192:6093–6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.