Abstract
Streptococcus pneumoniae strains lacking the enzyme dihydrolipoamide dehydrogenase (DLDH) show markedly reduced ability to grow on raffinose and stachyose as sole carbon sources. Import of these sugars occurs through the previously characterized raffinose ATP-binding cassette (ABC) transport system, encoded by the raf operon, that lacks the necessary ATP-binding protein. In this study, we identified the raffinose ATP-binding protein RafK and showed that it was directly involved in raffinose and stachyose import. RafK carries a C-terminal regulatory domain present in a subset of ATP-binding proteins that has been involved in both direct regulation of transporter activity (inducer exclusion) and transcription of transporter genes. Pneumococci lacking RafK showed a 50- to 80-fold reduction in expression of the raf operon genes aga (alpha-galactosidase) and rafEFG (raffinose substrate binding and permease genes), and both glucose and sucrose inhibited raffinose uptake through inducer exclusion. Like RafK, the presence of DLDH also activated the expression of raf operon genes, as DLDH-negative pneumococci showed a significantly decreased expression of aga and rafEFG, but DLDH did not regulate rafK or the putative regulatory genes rafR and rafS. DLDH also bound directly to RafK both in vitro and in vivo, indicating the possibility that DLDH regulates raffinose transport by a direct interaction with the regulatory domain of the transporter. Finally, although not as attenuated as DLDH-negative bacteria, pneumococci lacking RafK were significantly outcompeted by wild-type bacteria in colonization experiments of murine lung and nasopharynx, indicating a role for raffinose and stachyose transport in vivo.
INTRODUCTION
Dihydrolipoamide dehydrogenases (DLDH) are enzymes classically involved in the reoxidation of dihydrolipoamide during conversion of 2-oxo acids (such as pyruvate) in several multienzyme complexes in the central metabolism (34, 52). However, previous work from our and another laboratory have shown that DLDH is not involved in metabolizing 2-oxo acids in the pneumococcus, indicating that DLDH must serve other functions (41, 42). This is supported by evidence that organisms that lack 2-oxo acid dehydrogenases still express a DLDH protein (9, 10). One such function was suggested by Richarme in the 1980s when he showed that Escherichia coli strains that fail to make lipoic acid or strains treated with an inhibitor that mainly inhibits DLDH function resulted in reduced import of galactose, maltose, and ribose through ATP-binding cassette (ABC) transporters (35). We then showed that inactivation of DLDH in the pneumococcus results in an inability of the bacteria to import and utilize galactose and the alpha-galactoside sugars raffinose and stachyose and that a lack of DLDH is associated with an almost complete attenuation of this strain in animal infection experiments (41).
Transport of carbohydrates and other energy sources is important for many aspects of bacterial life and therefore highly regulated. Fitness in the bacterial host environment is intricately tied to accessibility of available energy sources and cofactors. In both Gram-positive and Gram-negative organisms, two types of transport systems are responsible for uptake of energy sources. Phosphoenolpyruvate (PEP)-sugar phosphotransferase systems (PTS) are the family of transporters generally responsible for uptake of easily utilizable carbon sources and are pivotal in the regulation of other catabolic systems, including ABC transporters, through carbon catabolite repression (CCR) and inducer exclusion (15, 38, 43, 51). ABC transporters are involved in importing alternate sources of energy and metal ions but are also involved in protein secretion, cell signaling, adhesion, and invasion, as well as antibiotic resistance, and inactivation of these systems is often associated with a decreased fitness in the host environment (28). This is especially true for the pneumococcus, which relies heavily on ABC transporters due to the lack of biosynthetic genes in the genome (7, 13, 16, 46).
This study focuses on the effect of DLDH on the raffinose transport system. DLDH-negative bacteria fail to grow with raffinose as the sole carbon source, but the mechanism of regulation has not been determined (41). In pneumococci, raffinose is transported through the raffinose ABC transporter encoded mainly by the raf operon. This operon has been characterized in some detail previously and contains all the genes necessary for raffinose transport and utilization except the ATP-binding protein of the transporter, which has not been characterized (36). The system is similar in function to the well-characterized multiple-sugar metabolism (MSM) system in Streptococcus mutans (37, 45) but shows a narrower range of substrate transport and transports only raffinose and stachyose (36). The expression of the raf operon is induced by raffinose in the medium and undergoes carbon catabolite repression in the presence of sucrose through an unknown mechanism that does not involve the catabolite control protein A (CcpA) (36, 49).
In this paper we have identified and characterized RafK, the raffinose transporter ATP-binding protein, located separately from the raf operon on the chromosome, and have characterized the interaction of DLDH with RafK and its effect on the expression and function of the raffinose transporter. RafK carries a regulatory domain similar to that of the maltose ATP-binding protein MalK in E. coli that is also regulated by DLDH (5, 25, 35). We show here that DLDH binds to RafK and to its regulatory domain and suggest that DLDH regulates raffinose transport both by interfering with expression of the raf operon and by directly interacting with RafK. The mechanism by which the DLDH mutant shows such a severe attenuation in vivo may make a viable target for future antibacterial drug development.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used and produced in this study are presented in Table 1. Streptococcus pneumoniae strain D39 (1) was used throughout the study as the parental strain for all mutations. Pneumococci were routinely grown at 37°C in Todd-Hewitt broth (Difco Laboratories, Detroit, MI) supplemented with 0.5% yeast extract (THY) or on tryptic soy agar (TSA) supplemented with 5% sheep blood, as appropriate. E. coli strain XL-1 Blue (Stratagene/Agilent Technologies, Santa Clara, CA) was used for cloning of recombinant protein expression and mutation constructs, and M15 (Qiagen, Valencia, CA) was used for protein expression. Frozen stocks of bacterial strains were made by adding 20% glycerol to bacterial cultures grown to an optical density at 600 nm (OD600) of ∼0.650, followed by freezing at −80°C. The following antibiotic concentrations were used for selection: ampicillin (Amp) was used at 100 μg/ml in E. coli; kanamycin (Kan) was used at 500 and 50 μg/ml for pneumococci and E. coli, respectively; erythromycin (Erm) was used at 0.3 μg/ml; and streptomycin (Sm) was used at 100 μg/ml for pneumococci. Antibiotics were obtained from Sigma-Aldrich, St. Louis, MO.
Table 1.
Strains and plasmids used in these studies
| Strain or plasmid | Characteristics | Reference |
|---|---|---|
| Strains | ||
| D39 | Wild-type, type 2 encapsulated | 1 |
| D39-Smr | D39, streptomycin resistant | This study |
| D39-C0832:1 | D39 dldh::pSH0832, DLDH negative | 41 |
| D39-P2A1 | D39 dldh-lplA intergenic::pSH08P | 41 |
| CP1296 | Rx derivative, cbp3::kan-rpsL+ | 44 |
| D39-ΔRafK | D39,ΔrafK::kan-rpsL+, RafK negative | This study |
| D39-rRafK | D39, allelic repair rafK, RafK expressing | This study |
| D39-ΔRafK-Janus | D39 ΔrafK::kan-rpsL+, RafK negative | This study |
| D39-rRafKΔlipErm | D39 allelic repair rafKΔlip, Ermr | This study |
| D39-ΔRafE | D39 rafE::pRT-ΔrafE, RafE-negative | This study |
| Plasmids | ||
| pJY4164 | Ermr, ori E. coli | 54 |
| pQE30 | Expression vector, Ampr | Qiagen |
| pAH001 | pQE30::dldh (full length) | 17 |
| pRT-JanusBglII | pGEM-T Easy::kan-rpsL+ with BglII ends | This study |
| pRT-ΔrafK | pGEM-T Easy::rafK deletion overlap PCR | This study |
| pRT-ΔrafK-Janus | pGEM-T Easy::rafK deletion with kan-rpsL+, inserted | This study |
| pRT-rRafKΔlipErm | pGEM-T Easy::rafKΔlip (amino acids 1 to 251 + Erm cassette amplified from pJY4164) | This study |
| pRT-ΔrafE | pJY4164::rafE internal fragment | This study |
| pRT-RafK | pQE30::rafK (full length) | This study |
| pRT-RafKΔlip | pQE30::rafKΔlip (amino acids 1 to 251) | This study |
| pRT-RafKLipo | pQE30::rafK lipoyl domain only (amino acids 246 to 377) | This study |
Construction of S. pneumoniae RafK mutants.
The Janus cassette was amplified from CP1296 chromosomal DNA (44) using primers Janus-pGEM-F and Janus-pGEM-R (for primer information, see Table S1 in the supplemental material) carrying BglII sites on both ends of the cassette, and the amplicon was ligated into the pGEM-T Easy vector (Promega, Madison, WI), resulting in plasmid pRT-JanusBglII. Approximately 800 base pairs upstream and downstream of the rafK gene (27, 44) were amplified by PCR using primer pairs RafK-UF-F/RafK-UFD-R2 and RafK-LFD-F2/RafK-LF-R with purified D39 chromosomal DNA as a template. The two fragments were fused by overlap PCR, in which a BglII site was engineered between the fragments, and the overlap product was ligated into the pGEM-T Easy vector (Promega, Madison, WI), resulting in plasmid pRT-ΔrafK. This vector was linearized with BglII, treated with calf intestinal alkaline phosphatase (Invitrogen, Carlsbad, CA), and ligated with the Janus cassette that had been excised from pRT-JanusBglII, resulting in plasmid pRT-ΔrafK-Janus. pRT-ΔrafK-Janus was cut with EcoRI to release the Janus cassette flanked by the up- and downstream fragments, and the linearized product was purified and transformed into streptomycin-resistant D39 bacteria and plated onto selection plates containing 500 μg/ml kanamycin. This resulted in strain D39-ΔRafK-Janus. This strain was used to perform allele insertions and a full deletion of the gene.
To delete the gene, D39-ΔRafK-Janus was transformed with linearized product from vector pRT-ΔrafK carrying the flanking sequences fused together. The transformed pneumococci were selected on streptomycin plates and named D39-ΔRafK. To reinsert the original gene, rafK with upper and lower flanking sequences was amplified using primers RafK-UF-F and RafK-LF-R by PCR from D39 genomic DNA, the PCR product was used to transform D39-ΔRafK-Janus, and the transformants were selected on plates containing raffinose as the sole carbon source and named D39-rRafK. Finally, D39-ΔRafK-Janus was also used to insert a variant rafK allele lacking the C-terminal regulatory domain. This was done by first PCR amplifying a truncated version of RafK (RafKΔlip, encoding amino acids 1 to 251) using primers RafK-UF-F and RafK-dlip-UFD-R and fusing it first with an erythromycin cassette (amplified from plasmid pJY4164 with primers RafK-dlip-erm-F and RafK-dlip-erm-R) directly downstream of the truncated gene and then with the downstream flanking sequence amplified with primers RafK-dlip-LF-F and RafK-LF-R by overlap PCR. This PCR product was cloned into pGEM-T Easy, resulting in plasmid pRT-rRafKΔlipErm. The construct was released from the plasmid with EcoRI and transformed into D39-ΔRafK-Janus, and transformants were selected on erythromycin plates. These were named D39-rRafKΔlipErm.
Mutants carrying the Janus cassette, deletion strains, and strains carrying reinserted allele variants were verified by PCR and by DNA sequencing using primers involved in the cloning process as well as primers RafK-UU-F and RafK-LL-R at the Roswell Park Biopolymer Sequencing Facility, Roswell Park Cancer Institute, Buffalo, NY, and further confirmed by separating whole-cell lysates on an SDS-PAGE gel, followed by a Western blotting procedure, detecting the presence of RafK with anti-RafK antibodies generated as described below. Whole-cell lysates were prepared by growing cells to late log phase (OD600, ∼0.700), washing them in 1× phosphate-buffered saline (PBS), and resuspending them in 500 μl 100 mM potassium phosphate buffer (pH 7.4) containing 100 μg/ml lysozyme and 20 units/ml DNase I, followed by incubation for 2 h at 37°C. Insoluble debris was removed by centrifugation at 15,000 × g for 10 min. Blot and protein gel pictures were minimally processed with the Auto-Levels adjustment in Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA).
To verify that the deletion of rafK (SPD_1409) did not produce polar effects on downstream genes, we compared the expression of the downstream gene SPD_1408 by quantitative reverse transcriptase PCR (qRT-PCR) on cDNA produced from strains D39 and D39-ΔRafK grown to an OD600 of 0.6 using primers SPD_1408-rRT-F and SPD_1408-rRT (for primer information, see Table S1 in the supplemental material). The SPD_1408 expression was normalized to levels of the housekeeping gene cyclophilin D (SPD_1367 in the D39 genome) (27) as described in detail below. The expression levels were not significantly different between the strains.
Mutational inactivation of the rafE gene.
An internal fragment of the rafE open reading frame (ORF) (nucleotides 6 to 534 of the SPD_1677 open reading frame) was amplified from D39 chromosomal DNA by PCR using the rafE-pJY-F and rafE-pJY-R primer pair with EcoRI and XbaI restriction sites added to the 5′ ends of the forward and reverse primers, respectively (see Table S1 in the supplemental material). The PCR amplicons were digested with EcoRI and XbaI, ligated into EcoRI–XbaI-digested plasmid pJY4164 (53), and transformed into E. coli XL1-Blue. Erythromycin-resistant clones were screened by PCR and verified by restriction digest of pure plasmid for insert of the appropriate size. Two independent clones, each harboring each rafE insert, were verified by sequencing, and one of them, designated pRT-ΔrafE, was used for insertion-duplication mutagenesis.
The resulting strain, D39-ΔRafE, was verified by sequencing over the insertion points in the chromosome. As the mutants carry the complete pJY4164 plasmid disrupting the rafE gene, we expected this insertion to cause polar effects on the downstream permease genes rafF and rafG. This was confirmed by comparing the expression of the rafG gene by qRT-PCR on cDNA produced from strains D39 and D39-ΔRafE1 grown to an OD600 of 0.5 using primers RafG-qRT-F and RafG-qRT-F. The rafG expression was normalized to levels of the housekeeping gene cyclophilin D (SPD_1367 in the D39 genome) (27) as described in detail below. The expression levels were 287 times lower in the rafE mutant strain, confirming that little to no transporter was expressed in this strain.
Construction of RafK recombinant protein.
The full gene sequence for rafK (primer pair RafK-pQE-F and RafK-pQE-R), as well as two truncated variants covering the N-terminal ATPase domain only (primer pair RafK-pQE-F and RafK-dlip-R) or the C-terminal regulatory lipoyl domain only (primer pair RafK-lipo-F and RafK-pQE-R), was PCR amplified from D39 DNA and inserted into the pQE30 vector (Qiagen, Valencia, CA), resulting in plasmids pRT-RafK (full length), pRT-RafKΔlip (RafK lacking the regulatory domain), and pRT-RafKLipo (regulatory domain alone), respectively. The correct sequence was verified by DNA sequencing. Each plasmid was transformed into E. coli strain M15. For protein expression and purification, 2 to 400 ml of cells were grown to an OD600 of 0.650 and induced at 37°C for 4 h with 1 mM IPTG. Cells were pelleted by centrifugation at 6,000 × g for 15 min and resuspended in 4 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 0.05% Tween 20, pH 8.0). Cells were then lysed by sonication and centrifuged at 15,000 × g at 4°C for 10 min to remove insoluble debris. Lysates were incubated with Ni-NTA agarose (Invitrogen, Carlsbad, CA). Beads and protein were washed three times with 5 ml of wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 0.05% Tween 20, pH 8.0), and His-tagged proteins were eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, 0.05% Tween 20, pH 8.0). Purified RafK protein were assayed for ATPase activity using the ATP luciferase assay (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and used immediately in binding assays, as ATPase activity and binding were significantly reduced after 2 days in elution buffer at 4°C. Protein concentration was quantified using a BioTek Synergy 2 plate reader with the Take3 microdrop addition (BioTek, Winooski, VT).
The production of an expression vector for DLDH has already been described (17). Induction and expression of DLDH was done as described for RafK, with the exception that DLDH was precipitated in 100% ammonium sulfate directly after elution from the Ni-agarose and protein not used immediately was stored at 4°C with little to no loss of enzymatic activity, measured as described in reference 41.
Antibody production.
Rabbit polyclonal antibodies to RafK and DLDH were generated by Lampire Biological Laboratories, using the Express-Line service (Lampire Biological Laboratories, Pipersville, PA). Recombinant full-length RafK and DLDH were used for immunization. Antibody was purified from antiserum using protein G-Sepharose columns as per the manufacturer's instructions (GE Healthcare, Piscataway, NJ).
Mouse antibodies against DLDH were generated by injecting 20-week-old female BALB/cByJ mice (Jackson Laboratory, Bar Harbor, ME) subcutaneously with 10 μg recombinant DLDH combined with 100 μl Titermax gold adjuvant (Sigma-Aldrich, St. Louis, MO), as per manufacturer's instructions. After 2 weeks, mice were boosted twice with 10 μg recombinant DLDH at 10-day intervals and sacrificed 1 week after the last booster.
Antibodies used for immunoprecipitations were routinely adsorbed with whole bacterial cells lacking the specific antigen to which antibodies had been raised to remove antibodies that reacted with nonspecific antigens. Polyclonal Anti-RafK or anti-DLDH antiserum (100 μl) was incubated with 100 ml late-log-phase D39-ΔRafK or D39-C0832 bacteria, respectively, that were previously washed three times and resuspended in 10 ml PBS. Bacteria and antibody were incubated at 37°C for 1 h, and bacteria were removed by centrifugation at 6,000 × g for 10 min. This incubation with bacteria was repeated using a fresh 100 ml of bacterial cells washed and resuspended in 10 ml PBS. Postadsorbed and/or purified antibody concentrations were quantified using a BioTek Synergy 2 plate reader with the Take3 microdrop addition (BioTek, Winooski, VT). Antibodies were verified for reactivity by Western blotting that detected proteins of interest in cell lysates from S. pneumoniae strain D39.
RNA isolation and quantitative RT-PCR (qRT-PCR).
RNA was purified using Qiashredder columns and the RNeasy minikit, according to the manufacturer's instructions (Qiagen, Valencia, CA). Briefly, frozen stocks of bacteria were used to inoculate a 10-ml culture in THY. When the bacteria reached an OD600 of ∼0.5, 1 ml of bacteria were removed and pelleted by centrifugation at 9,000 × g for 2 min at 4°C. The pellet was resuspended in 0.5 ml of 0.9% NaCl; 1 ml of RNAprotect (Qiagen, Valencia, CA) was added, and the mixture was incubated at room temperature for 5 min. Cells were then pelleted at 9,000 × g for 2 min at room temperature. These cells were frozen at −80°C or used directly for RNA isolation. Cells were resuspended in 500 μl TE buffer (10 mM Tris, 1 mM EDTA, pH 7.5) plus 25% (wt/vol) glucose and treated with 20 μl of a 100-mg/ml lysozyme stock and 10 μl of 5,000-U/ml mutanolysin stock at 37°C for 15 min to remove cell wall (lysozyme and mutanolysin were purchased from Sigma-Aldrich, St. Louis, MO). Qiashredder columns were then used to remove unwanted cellular debris, and nucleic acids were isolated on RNeasy columns. Contaminating DNA was removed on the RNeasy columns using the Bio-Rad RNase-free DNase kit. Purity and quantitation of RNA were determined using the BioTek Synergy-2 plate reader with the Take3 microdrop addition. cDNA was synthesized from 500 ng total RNA using the Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). cDNA was amplified using SYBR green supermix with gene-specific primers (see Table S1 in the supplemental material) and quantified on a Bio-Rad iCycler according to the manufacturer's instructions. Assays were performed in duplicate or triplicate using a 96-well plate in 20-μl reaction volumes. Expression was analyzed using the standard curve method (32) and normalized to levels of cyclophilin D (SPD_1367 in the D39 genome) (27). Significance of expression levels was determined by comparison to D39 wild-type expression using Student's t test with significance being defined as P < 0.05.
Carbohydrate uptake assays.
Bacteria were grown to mid-log phase (OD600, ∼0.500), centrifuged at 9,000 × g for 2 min, washed twice in cold PBS, and resuspended in an equal volume of cold PBS. Cells were kept on ice until 1 min prior to addition of radiolabeled carbohydrate. At this time point, cells were placed in a 30°C water bath. At time zero, 10 μl diluted radiolabeled carbohydrate (50 μCi/ml, diluted in PBS) was added and the cells were gently vortexed. The tube was then placed in a 30°C water bath for incubation. After 2 min (glucose) or 10 min (raffinose) of incubation, cells were captured on a 0.45-μM-pore-size HAWP filter (Millipore, Billerica, MA) and unbound radiolabeled carbohydrates were washed through the filter with 5 ml PBS. Filters were then placed in scintillation vials and radioactivity was counted in a Wallac model 1409 liquid scintillation counter. Glucose (d-[1-14C]-glucose; ARC 0120A) and raffinose ([galactose-6-3H]; ART 0229) were obtained from American Radiolabeled Chemicals, Inc., St. Louis, MO. Significance of uptake was determined by comparison with D39 wild-type uptake using Student's t test with significance being defined as P < 0.05.
To test for carbon catabolite repression of raffinose transport, bacteria were grown as described above but washed and resuspended in semidefined SH minimal medium, as previously described (41). Cells (1.5-ml aliquots) were then exposed to 130 μl of either PBS alone or PBS containing glucose, sucrose, or lactose to a final concentration of 20 mM. Bacteria were incubated for 15 min at room temperature before raffinose uptake was assessed. Bacteria were also used to assess alpha-galactosidase activity as described below.
Alpha-galactosidase activity.
Bacterial cells (2 ml) incubated in SH medium with PBS alone or PBS with glucose, lactose, or sucrose (to a final concentration of 20 mM) were pelleted by centrifugation and placed at −80°C for 1 h. After the pellet was thawed on ice, the cells were resuspended in 2 ml PBS, and 200 μl of the resuspended cells were pelleted at 9,000 × g for 2 min, resuspended in 100 μl of the lysis buffer (100 mM NaHPO4-KPO4, pH 7.5, 0.25% Triton X-100), and incubated for 5 min at 37°C to facilitate lysis. A small volume of lysate (2 μl) was used to determine protein concentration in a BioTek Synergy 2 plate reader with the Take3 microdrop addition (BioTek, Winooski, VT). Lysate (20 μl) was added into 980 μl substrate buffer (1 mM MgCl2, 4.5 mM β-mercaptoethanol, 90 μg/ml p-nitrophenyl-α-d-galactopyranoside, 100 mM potassium phosphate, pH 7.5), and activity was determined spectrophotometrically at 405 nm over 40 min in the BioTek Synergy 2 plate reader. Activity was determined as U/mg bacterial lysate, where 1 U corresponds to the conversion of 1 mM substrate per minute at 25°C.
Single-carbon-source growth assays.
Cells from a frozen stock were inoculated into 10 ml THY, grown to early log phase (OD600, ∼0.300), washed twice in sterile PBS, and resuspended in 4 ml of SH medium containing either no carbohydrate source or 20 mM (each) glucose, raffinose, stachyose, or isomaltose. Growth was monitored by measuring the optical density at 600 nm over 48 h. Carbohydrates were purchased from Sigma (Sigma-Aldrich, St. Louis, MO).
Cell fractionation.
Pneumococci from a frozen stock were inoculated into 100 ml THY, grown to mid-log phase (OD600, ∼0.500), washed twice in sterile PBS, and resuspended in 5 ml of protoplast buffer (20% [wt/vol] sucrose, 5 mM Tris-HCl, 2.5 mM MgCl2, pH 7.4). Then, 250 units of mutanolysin and 100 μg/ml lysozyme were added. Cells were incubated overnight at room temperature, pelleted at 5,000 × g for 10 min to remove cell wall supernatant, washed once in protoplast buffer, and then lysed in 3.5 ml of deionized water. Nonlysed cells were pelleted at 5,000 × g for 10 min. The lysed cells were centrifuged at 100,000 × g for 90 min to pellet membrane. All fractions were then mixed with 200 μl LDS sample buffer and run out on precast 4 to 12% SDS-PAGE gels (Invitrogen, Carlsbad, CA). Fractions were verified using antibodies against proteins associated mainly with one fraction. The cell wall fraction was verified using antibodies against PspA (31), a choline-binding protein that is associated to the cell wall. Antibodies to DLDH were used as the membrane control, as DLDH is most often found associated with the membrane (17, 41). Streptavidin-HRP was used as a cytosol protein control, as it binds to biotin carboxyl carrier protein accB, also known as BCCP (SPD_0386), which is associated mainly with cytosol (17). All antibodies were diluted 1:10,000 and incubated with blots at room temperature for 30 min. Goat anti-rabbit-horseradish peroxidase (HRP) (DLDH) and goat anti-mouse-HRP (PspA) conjugated antibodies were used as secondary reagents (Invitrogen, Carlsbad, CA), diluted 1:5,000 in 10 ml of PBS and incubated with blots at room temperature for 30 min. The blots were detected using ECL detection reagent (GE Healthcare, Piscataway, NJ) after exposure on film. Controls for cell fractions are shown in Fig. S1 in the supplemental material.
Immunoprecipitations.
Cell lysates were prepared by growing one liter of bacteria in THY with or without 20 mM raffinose to an OD600 of 0.8. Cells were centrifuged at 8,000 × g for 30 min at 4°C and washed once in 25 ml of cold PBS. Cells were centrifuged again at 10,000 × g for 20 min and resuspended in 10 ml of 100 mM potassium phosphate buffer, pH 7.4. To these cells were added 1 mg/ml lysozyme, 170 μg/ml phenylmethylsulfonyl fluoride (PMSF), and 100 μM leupeptin. The cells were incubated for 1.5 h at 37°C and then cooled on ice before being sonicated eight times for 8 s each time using a Branson Sonifier 450. Insoluble cell debris and any remaining whole cells were pelleted at 15,000 × g for 15 min at 4°C.
Protein G dynabeads (500 μl [30 mg]) (Invitrogen, Carlsbad, CA) were washed with PBS plus 0.02% Tween 20 (PBS-T), and ∼200 μg mouse polyclonal anti-DLDH antibody or rabbit polyclonal anti-RafK antibody was bound as per the manufacturer's instructions. Cell lysates were incubated with protein G–antibody-coated beads for at least 15 min at room temperature. Dynabeads and associated antibodies and proteins were washed three times with 1 ml of PBS-T, and then the antibodies and proteins were eluted off the beads by using 150 μl of 1× LDS sample buffer with reducing agent (Invitrogen, Carlsbad, CA) and boiling for 10 min. Eluates from immunoprecipitations and associated proteins from whole-cell lysates or recombinant proteins were run out on 4 to 12% SDS-PAGE gels and blotted with anti-RafK antibody and anti-DLDH antibody.
Mouse pneumonia and nasopharyngeal colonization model.
The animal experiments were performed essentially as described earlier (3, 6, 41). Frozen stocks of bacteria were thawed, washed once, and resuspended in 1.5 ml sterile, cold PBS. Bacteria were plated on TSA blood agar plates to verify quantities in stocks. Bacteria (1 × 107 to 2 × 107 CFU) in a 40-μl volume were aspirated into the nares of isoflurane-anesthetized CBA/CaHN-Btkxid/J mice (Jackson Laboratory, Bar Harbor, ME). In competition experiments, wild-type and mutant pneumococci were mixed in a 1:1 ratio. Mice were monitored and sacrificed at 24 h postinoculation. Lungs were removed by opening the thoracic cavity and excising the lung lobes. Nasopharyngeal tissue was harvested by defleshing the nose and skull of the mouse, cutting each maxillary bone and the skull bone between the eyes, removing the bone, and removing the tissue present in the nasal conchae with forceps. Harvested tissue was homogenized, and the homogenate was serially diluted on TSA–5% blood agar plates. For competition experiments, an equal volume of homogenate was also plated on TSA–5% blood agar plates with 500 μg/ml kanamycin to detect the mutants only. Data were plotted using Prism for Mac OS X version 5.0b (Graphpad Software Inc., La Jolla, CA). Statistical significance for noncompetitive assays was determined using Student's t test, with significance defined as P < 0.05. Statistical significance for competitive assays were determined by comparing paired groups using the Wilcoxon signed-rank test, with significance defined as P < 0.05. The competitive index (CI) was calculated using the ratio of mutant to wild-type CFU divided by the mutant to wild-type input CFU count.
RESULTS
Inactivation of the pneumococcal raffinose ATP-binding protein RafK.
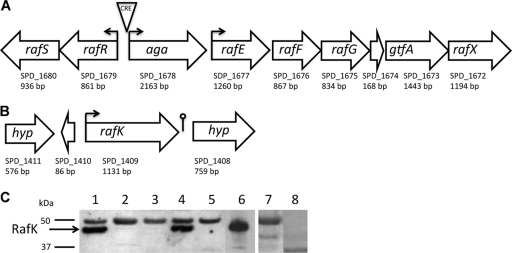
The raffinose transport system in the pneumococcus consists of the transcriptional regulators RafR and RafS, α-galactosidase (Aga), a substrate-binding protein (RafE), the permeases RafF and RafG, a sucrose phosphorylase (GtfA), and a protein of unknown function (RafX) (Fig. 1 A) (36). The gene for the ATP-binding protein of this ABC transport system has not been characterized, although it has been putatively identified based on homology to the MsmK protein of Streptococcus mutans (18, 19). The gene (open reading frame SPD_1409 in the D39 genome sequence) was found independently located in the genome with a distinct ribosome-binding site (predicted by RBSFinder) and terminator (predicted by TransTerm) (Fig. 1B) and was verified as described below to encode the only ATP-binding protein responsible for raffinose transport. We therefore designated it rafK. The gene directly upstream of rafK encodes a putative tRNA and the downstream gene a hypothetical protein without identifiable functional domains, and thus they do not appear to be related to carbohydrate utilization or transport. The expression of the downstream gene was unaffected by mutation of the rafK gene as verified by qRT-PCR. The RafK predicted protein had a typical ATPase core as well as a large C-terminal regulatory domain with homology to that of the maltose ABC transport protein MalK in E. coli (5, 39). This regulatory domain includes a fold similar to the attachment domain for dihydrolipoamide in acetyltransferases of 2-oxo acid dehydrogenases, the substrate of DLDH, which we will refer to here as the regulatory lipoyl domain.
Fig. 1.
Raffinose transport system. (A) The raf locus in S. pneumoniae D39. The large boxed arrows represent ORFs. Below, the corresponding gene numbers are listed as well as the size of the gene in base pairs (bp). Small arrows indicate putative promoter sites and orientation of corresponding transcripts. CRE, catabolite response element. (B) The rafK locus in S. pneumoniae D39. The rafK gene has its own putative promoter with a predicted ribosomal binding site, and a predicted terminator is located immediately after the gene. (C) Western blot of bacterial lysates using anti-RafK rabbit polyclonal antibodies (lanes 1 to 6), rabbit preimmune serum (lane 7), or anti-lipoic acid rabbit polyclonal antibodies (lane 8). Lanes: 1, D39 lysate (parental wild-type); 2, D39-ΔRafK-Janus lysate (RafK Janus mutant); 3, D39-ΔRafK lysate (RafK deletion mutant); 4, D39-rRafK lysate (allelic repair of RafK Janus mutant with full-length RafK); 5, D39-rRafKΔlipErm lysate (allelic repair of RafK Janus mutant with RafK lacking the lipoyl domain); 6, recombinantly expressed RafK protein; 7, D39 lysate probed with preimmune serum; 8, D39 lysate lysate probed with rabbit anti-lipoic acid antibodies. The RafK-specific band is indicated in the figure with an arrow. The anti-RafK polyclonal serum also contained unspecific reactivity against a 50-kDa protein that was present also in preimmune sera (lane 7) and could not be adsorbed away.
RafK was cloned into the pQE30 expression vector, and recombinant protein was expressed and purified in E. coli and used to generate polyclonal rabbit antibodies. The polyclonal antiserum generated from recombinant RafK detected its antigen (Fig. 1C, lane 6) as well as protein expressed in the D39 wild-type strain (Fig. 1C, lane 1). A knockout mutant for rafK (D39-ΔRafK-Janus) was created in pneumococci using the Janus system presented by Sung et al. (44), in which the ORF was replaced with a cassette containing the kanamycin resistance cassette, and verified using PCR and sequencing. This mutant had no expression of RafK in a Western blot detected by polyclonal antibody produced against the recombinant protein (Fig. 1C, lane 2), similar to the complete deletion mutant D39-ΔRafK (Fig. 1C, lane 3). Lysate from a strain where the wild-type rafK allele was reinserted back into the same locus expressed amounts of RafK equal to those of wild-type bacteria (Fig. 1C, lane 4). In order to assess whether the regulatory domain played a critical role in transport of substrate through the ABC transporter, a truncated gene product lacking the regulatory domain was reinserted in the original rafK locus, resulting in strain D39-RafKΔlipErm. However, the mutant containing the gene without the lipoyl domain was found to have no detectable expression of the truncated protein (Fig. 1C, lane 5).
Though the regulatory domain of RafK has a lipoyl-like fold, it does not contain the specific motif associated with an attached lipoic acid. This was verified in Western blotting experiments with D39 cell lysates using a rabbit α-lipoic acid antibody (Abcam, Cambridge, MA) that failed to detect RafK (Fig. 1C, lane 8).
Characterization of the raffinose ATP-binding protein RafK.
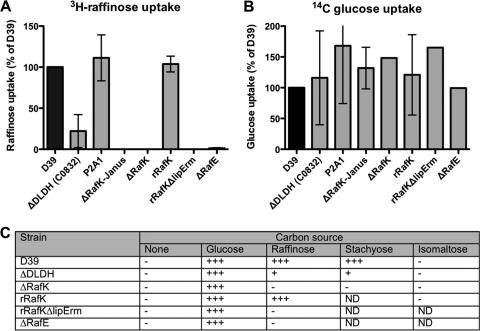
To verify the specific role of RafK as the ATP-binding protein in the raffinose transport system, uptake of radiolabeled raffinose was measured in pneumococci lacking RafK (D39-ΔRafK) and the strains in which full-length (D39-rRafK) and truncated (D39-rRafKΔlipErm) rafK genes were reinserted in the rafK locus, and uptake was compared with that of the D39 parent strain. RafK-negative bacteria were found to have no detectable transport of raffinose compared to that of the wild-type D39 parental strain (Fig. 2 A). The strain with reinserted full-length RafK transported raffinose to the same degree as the parent strain, whereas the strain complemented with RafK lacking the regulatory domain did not transport any raffinose, which was not surprising, as it did not express any RafK protein (Fig. 2A; Fig. 1C, lane 5). We also verified that the raf operon expressed the other part of the transporter. Inactivation of rafE by insertion duplication mutagenesis caused polar effects on rafF and rafG expression and resulted in a strain (D39-ΔRafE) that was found to have essentially undetectable raffinose transport (Fig. 2A). All strains took up radiolabeled glucose to the same degree as the D39 strain (Fig. 2B).
Fig. 2.
RafK mutant growth and uptake characterization. (A and B) Uptake of radiolabeled raffinose (A) and glucose (B) by wild-type D39 pneumococci and mutants of raffinose transport. Uptake was measured as the percentage of radioactivity for each strain compared to that of the D39 parental strain. Bars represent results of two or more experiments, and error bars represent standard deviations. (C) Growth of wild-type and mutant strains in minimal medium with addition of single carbon sources. Bacterial cells with altered transport activity for raffinose were seeded into semidefined minimal medium containing no carbohydrate source, or 20 mM glucose, raffinose, stachyose, or isomaltose. Growth was monitored by measuring optical density at 600 nm over 48 h. Growth was recorded as follows: −, no detectable growth; +, growth reaching an OD600 between 0.1 and 0.3 after 48 h; ++, growth reaching an OD600 between 0.3 and 0.6 within 48 h; +++, growth reaching an OD600 of ≥0.6 within 48 h.
Similarly, strains lacking RafK or RafEFG expression failed to grow in a minimal medium with raffinose or stachyose as single carbon sources (Fig. 2C). The strain complemented with full-length RafK grew equally as well on raffinose and stachyose as the wild-type strain (Fig. 2C), whereas bacteria complemented with a truncated version of RafK lacking the regulatory domain were unable to grow on raffinose as a sole carbon source (Fig. 2C), which was attributed to the fact that the strain showed no expression of truncated protein (Fig. 1C, lane 5). These data support the hypothesis that RafK is the sole ATP-binding protein responsible for raffinose transport and that the other part of the transporter is provided by the raf operon previously characterized in the pneumococcus. Finally, none of the strains grew on isomaltose, which confirmed that the raffinose transport system, albeit highly homologous to the MSM transport system in S. mutans, has a narrower range of substrates (Fig. 2C).
Role of RafK and RafEFG in the regulation of raffinose transport.
The raffinose transport system is divided into four potential operons based on putative promoters identified by Rosenow (Fig. 1A and reference 36) and by us (Fig. 1B). The aga gene has its own putative promoter, and the rafEFG genes have a putative promoter downstream of the aga gene, suggesting that levels of the raffinose transporter might be regulated differentially from those of aga. The rafR and rafS genes have their own putative promoter and are expressed in the opposite direction from aga. Of these three promoters, the aga promoter contains a catabolite response element (CRE) motif, but aga expression does not involve CcpA, as CcpA-negative bacteria express aga to the same degree as wild-type bacteria (36, 49). aga expression is induced by raffinose and repressed by sucrose as measured by α-galactosidase activity (36). The regulation of the rafK locus has not been characterized.
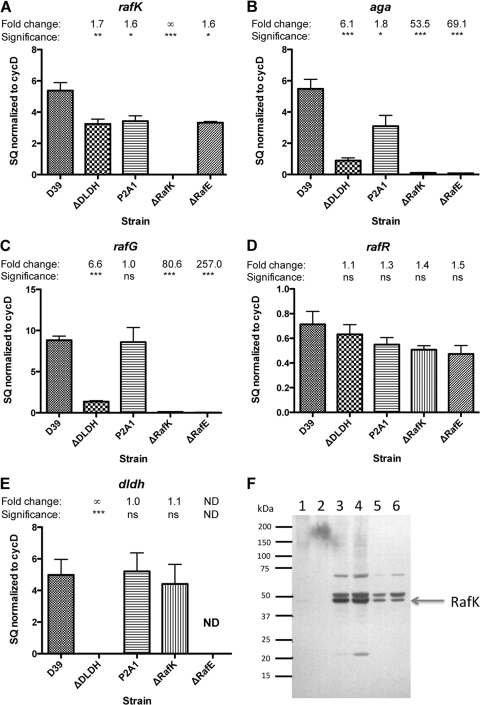
To study the role of RafK and the raffinose permeases in the regulation of raf gene expression, we measured the expression levels of each potential raffinose operon by qRT-PCR using rafK, aga, rafR, and rafG as target genes in D39 pneumococci lacking RafK (D39-ΔRafK) and RafE (D39-ΔRafE) and compared them to the levels in D39 pneumococci. The RafK-negative strain lacked any detectable transcript of rafK (Fig. 3 A) and had a significantly decreased expression of both aga (53-fold; P < 0.001) and rafG (81-fold; P < 0.001) (Fig. 3BC) but had an unaltered expression of the regulatory gene rafR (Fig. 3D). This decrease suggests that RafK exhibits an important role in the activation of raffinose gene transcription, by allowing the import of raffinose that is known to activate transcription (36) and/or through sequestering of transcriptional suppressor activity, analogous to the binding of the maltose regulator MalT to the maltose ATP-binding protein MalK in E. coli (5). RafK-negative pneumococci showed unaltered expression of dldh, suggesting that there was no cross-regulation between the systems (Fig. 3E).
Fig. 3.
Expression of genes involved in raffinose uptake. Levels of gene expression were quantitated by qRT-PCR using the standard curve method and normalized to levels of cyclophilin D (SPD_1367 in the D39 genome). Genes examined were as follows: (A) rafK: raffinose ABC transporter ATP-binding protein; (B) aga: alpha-galactosidase; (C) rafG: raffinose ABC transporter permease; (D) rafR: potential raffinose transport transcriptional activator; (E) dldh: dihydrolipoamide dehydrogenase. Strains compared: D39, wild type; DLDH, dldh mutant; P2A1, control mutant that lacks expression of lplA; RafK, rafK deletion with Janus insertion; RafE, rafE interruption resulting in polar effects on the raf operon. Bars represent starting quantity (SQ) and are the combined data from duplicates of two or more experiments. Error bars represent standard deviations. All statistical tests were Student t tests in comparison to data for D39. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ND, not determined. (F) Expression of RafK protein in wild-type and DLDH-negative bacteria (C0832). Cell wall, membrane, and cytosolic fractions from the bacteria were separated by gel electrophoresis, and RafK was detected with anti-RafK antibodies by Western blotting. Lanes: 1, D39 cell wall fraction; 2, C0832 cell wall fraction; 3, D39 membrane fraction; 4, C0832 membrane fraction; 5, D39 cytosolic fraction; 6, C0832 cytosolic fraction.
The rafE-negative strain showed, as expected, a polar effect on the expression of the downstream raf operon genes, as demonstrated by the 257-fold decrease in expression of rafG (Fig. 3C). However, RafE-negative cells also showed a major decrease in expression of the upstream gene, aga (69.1-fold; P < 0.001) (Fig. 3B), to a degree similar to that seen in the RafK-mutant pneumococci. These results suggest that raffinose per se provides the major activating signal for the expression of aga and rafEFG.
The presence of raffinose in the growth medium has been shown to be associated with an upregulation of alpha-galactosidase activity, whereas sucrose, but not glucose, has been shown to inhibit the expression of alpha-galactosidase activity (36). However, whether these carbon sources act through transcriptional regulation alone or whether inducer exclusion exists in this system has not been shown. To investigate this, we grew D39 pneumococci in THY medium, washed them in PBS, and exposed them to semidefined medium with no carbohydrate or in medium in the presence of 20 mM glucose, raffinose, sucrose, or lactose as a sole carbon source over a time (15 min) that did not alter the expression of the raf operon (measured by alpha-galactosidase activity of a bacterial lysate). Under these conditions, both sucrose and glucose significantly inhibited raffinose uptake, suggesting that inducer exclusion exists in the pneumococcus and works on the raffinose transporter (Fig. 4). The presence of lactose had no effect on transport of raffinose under these conditions (Fig. 4).
Fig. 4.
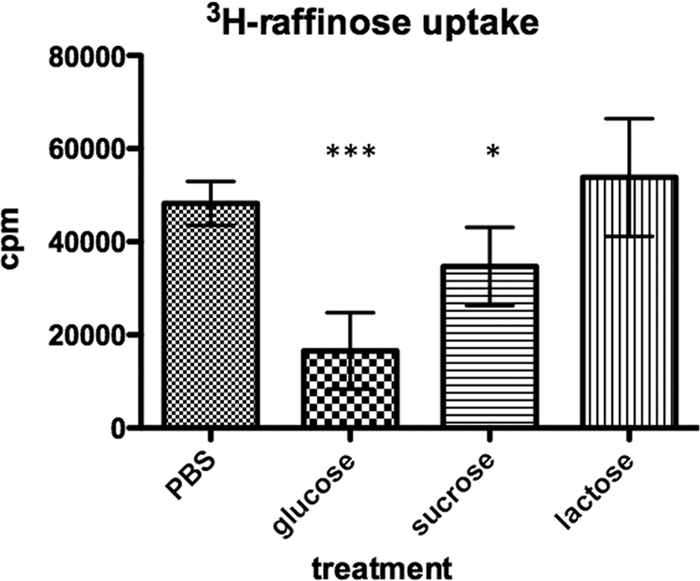
Raffinose transport is reduced in the presence of glucose or sucrose. Bacteria were grown in THY, washed, and resuspended in SH medium alone or in the presence of 20 mM glucose, sucrose, or lactose. After a 15-min incubation, raffinose transport was assessed. Data shown are combined data from two experiments. Error bars represent standard deviations. Statistical tests were Student t tests in comparison to results for PBS-alone treatment. *, P < 0.05; ***, P < 0.001.
Role of DLDH in regulation of raffinose transport.
Pneumococci lacking DLDH displayed a more than 80% reduction in raffinose uptake compared with that of wild-type bacteria and grew slowly with raffinose or stachyose as the sole carbon source (Fig. 2A and B). The DLDH mutant strain is also a functional mutant for the downstream gene encoding a lipoate protein ligase (LplA) due to polar effects of the dldh mutation. We therefore included LplA-negative bacteria (P2A1) in this study to verify that the effects were directly related to DLDH. LplA is responsible for attaching lipoic acid to the lipoyl domain of DLDH, and this function was not important for transport of raffinose (Fig. 2A) or bacterial virulence (41).
The decreased transport of raffinose in DLDH-negative bacteria prompted us to investigate how DLDH might be regulating the raffinose uptake system. First, DLDH could be affecting the expression of the genes involved in raffinose uptake. We therefore measured the expression of rafK, aga, rafR, and rafG by qRT-PCR in DLDH-negative bacteria and compared it to the expression measured in the wild-type strain. DLDH-negative bacteria showed a slight decrease in rafK expression (1.7-fold; P < 0.01) that although significantly different from expression in wild-type bacteria was not very substantial (Fig. 3A). The P2A1 strain showed a similar slight decrease (1.6-fold; P < 0.05) in the expression of rafK compared to the result for D39 (Fig. 3A). As P2A1 showed no decrease in raffinose transport and grew normally with raffinose or stachyose as the sole carbon source, we conclude that this small decrease in expression of rafK is not biologically significant. This was further verified on the protein level. A Western blot experiment using anti-RafK antibodies indicated that the levels of RafK were not different in DLDH-positive and -negative bacteria and that RafK was distributed primarily to the membrane in both strains (Fig. 3F).
In contrast, the expression was markedly reduced for aga (6.1-fold, P < 0.001) and rafG (6.6-fold; P < 0.001) in DLDH-negative pneumococci compared to that for wild-type bacteria (Fig. 3B and C). The level of aga expression correlated well with the decreased alpha-galactosidase activity we have observed earlier in DLDH-negative cells (41). Expression of the putative transcriptional activator, rafR, was not significantly altered in the DLDH-negative bacteria compared with that in the wild-type pneumococci (Fig. 3D). These data indicate that DLDH has a role in activating the expression of raffinose transporter genes, especially expression of alpha-galactosidase and the permeases.
DLDH binds to RafK.
RafK carries a regulatory domain homologous to the C-terminal domain of MalK of the maltose transporter in E. coli and certain other ATP-binding proteins (Fig. 5; 5, 12, 39). In the maltose ABC transporter in E. coli, this regulatory domain is important both for regulation of maltose gene expression through sequestering the transcriptional activator MalT in the presence of maltose and for inducer exclusion and catabolite repression through binding the dephosphorylated EIIA component of the glucose PTS system produced in the presence of glucose in the growth medium (5, 39). As in MalK, the regulatory domain in RafK contains a lipoyl-like TOBE_2 fold similar to the one found in the E2 dihydrolipoamide transferase protein (transport-associated OB, type 2, PF08402, CATHDB homologous superfamily 2.40.50.100), the attachment point for lipoic acid, and the substrate for DLDH (41). As maltose transport in E. coli is also regulated by DLDH (35) and as the regulatory domains contain a lipoyl-like fold with high homology to the DLDH substrate, we were interested in investigating if DLDH interacts with RafK.
Fig. 5.
Alignment of RafK and MalK protein sequences showing homology. Annotated are typical ABC transporter motifs (Walker A, Lid, Signature, Walker B, D-loop, Switch), the start of the regulatory domain (RD Start), regulatory domain motif 1 (RDM1), and regulatory domain motif 2 (RDM2). Residues in bold are considered important conserved residues in the regulatory domain. Secondary structures of the regulatory domain of E. coli MalK are given under the sequences, with α representing α-helices with corresponding numbers and β representing β sheets with corresponding numbers.
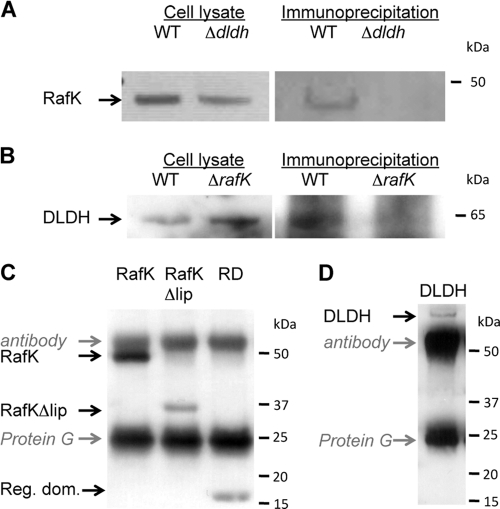
To test for a direct or indirect interaction between these two proteins, anti-DLDH polyclonal antibodies bound to magnetic beads were used to pull down DLDH and its binding partners from a whole-cell lysate (Fig. 6 A). Captured proteins were separated by SDS-PAGE, and a number of bands, one of which corresponded to RafK, were found in the eluate. This band was verified to be RafK in a Western blot using anti-RafK antibodies for detection (Fig. 6A, right panel). To verify the specificity of the reaction, immunoprecipitations were also done using DLDH-negative cells, which failed to capture RafK (Fig. 6A). To confirm these results, the immunoprecipitation was done in reverse as well, using anti-RafK antibodies to pull out RafK and its binding partners. This procedure pulled out a number of proteins, one of which was identified to be DLDH in a Western blotting reaction using anti-DLDH antibodies for detection (Fig. 6B, right panel). Again, the specificity of the capture was confirmed using RafK-negative bacteria that failed to capture DLDH (Fig. 6B). Combined, these results suggest that DLDH and RafK interact directly or indirectly in vivo.
Fig. 6.
DLDH binds to RafK. (A) Western blot of immunoprecipitation of RafK from bacterial lysate incubated with magnetic beads carrying anti-DLDH antibodies. (B) Western blot of immunoprecipitation of DLDH from bacterial lysate incubated with magnetic beads carrying anti-RafK antibodies. (C) Western blot of immunoprecipitation of recombinant RafK, RafKDlip, and the RafK regulatory domain (lipoyl domain) after incubation with recombinant DLDH and magnetic beads carrying anti-DLDH antibodies. (D) Western blot of immunoprecipitation of recombinant DLDH after incubation with recombinant RafK and magnetic beads carrying anti-RafK antibodies.
To determine if DLDH and RafK interacted directly with each other, immunoprecipitations were done using recombinant DLDH and RafK (Fig. 6C and D). Using anti-DLDH polyclonal antibodies bound to magnetic beads, recombinant RafK was pulled down only in the presence of DLDH, indicating a direct interaction between these two proteins (Fig. 6C). To determine the importance of the RafK regulatory lipoyl domain in this interaction, we expressed the RafK protein without the regulatory domain as well as the regulatory domain by itself. Both recombinant proteins were found pulled down to various degrees in the presence of DLDH (Fig. 6C). Because the truncated proteins may have contained significant hydrophobic surfaces not normally exposed, we cannot conclude from these experiments if binding of DLDH to RafK takes place exclusively to the regulatory domain. To confirm the specificity of this interaction, we also performed reverse immunoprecipitation. Using anti-RafK polyclonal antibodies bound to magnetic beads, we were able to pull out recombinant DLDH only in the presence of RafK (Fig. 6D). These experiments demonstrate that RafK interacts with DLDH both in vivo and in vitro.
RafK-deficient pneumococcus are attenuated in virulence in mice.
Several ABC transporters have been identified as pneumococcal virulence factors, with some transporters being both necessary for uptake of nutrients and also involved in attachment to various ligands that could help the bacteria colonize and invade its host (4, 8, 13, 18, 22, 30). As DLDH-negative pneumococci have a striking avirulent phenotype in murine colonization and infection models, we were interested in better understanding the contribution of the raffinose transport system to this avirulent phenotype.
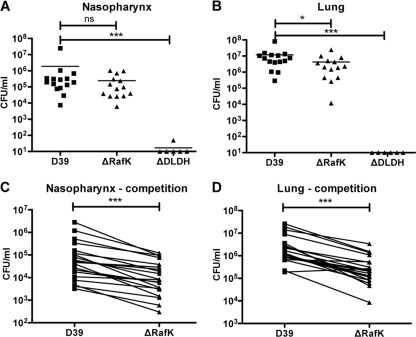
RafK-negative pneumococci were introduced intranasally, alone or together with wild-type bacteria, into the lungs of CBA/CaHN-Btkxid/J mice by aspiration and colonization of the nasopharyngeal tissue, and the lungs were examined 24 h postinoculation. When mice were inoculated individually with either wild-type or RafK-negative pneumococci, the bacterial load of RafK-negative bacteria was identical to that of wild-type pneumococci in the nasopharynx (Fig. 7 A) and was slightly, yet significantly, lower in the lungs (Fig. 7B). DLDH-negative bacteria, on the other hand, completely cleared from both the lungs and the nasopharynx of the mice over the time course of this experiment (Fig. 7A and B). However, when RafK-negative bacteria were inoculated together with the D39 parental strain in a competition experiment, the RafK-mutants were significantly outcompeted: almost 7-fold in the nasopharynx (median CI of 0.15) (Fig. 7C) and almost 8-fold in the lungs (median CI of 0.13) (Fig. 7D). This indicates that RafK provides an advantage for pneumococcal fitness in the host. However, as the RafK-negative pneumococci were almost as virulent as wild-type bacteria when given alone, the significant virulence attenuation of the DLDH mutant cannot be explained solely by the lack of uptake of raffinose or stachyose. These data suggest that DLDH affects other pathogenesis mechanisms in pneumococci.
Fig. 7.
Virulence attenuation of RafK-negative pneumococci. (A and B) CBA/CaHN-Btkxid/J mice were infected intranasally with ∼1 × 107 CFU of D39, RafK-deficient, or DLDH-deficient pneumococci, and the bacterial load was determined after 24 h from nasopharyngeal (A) and lung (B) tissues from the mice. RafK-deficient pneumococci showed a slight but significant lower bacterial load than D39 pneumococci in the lung (P < 0.05) but not in the nasopharynx (P = 0.12). DLDH-negative bacteria were completely cleared from both tissues (P < 0.001 compared to D39). (C and D). CBA/CaHN-Btkxid/J mice were infected intranasally with ∼1 × 107 CFU each of D39 and RafK-deficient pneumococci in competition, and the bacterial load was determined for each strain after 24 h from nasopharyngeal (C) and lung (D) tissues. A line for each individual mouse links the bacterial load of the strains. RafK-negative pneumococci were outcompeted by D39 wild-type bacteria in both lung and nasopharynx (both P < 0.001), with median competitive indices of 0.13 in the lung and 0.15 in the nasopharynx.
DISCUSSION
Fitness of a bacterium in the host environment is largely dependent upon its ability to obtain and utilize available energy sources and cofactors. Energy sources vary greatly with the different niches bacteria may survive in, and to conserve energy, bacteria have developed refined systems of regulating gene expression to most optimally sense and utilize only those substrates that are available around them. Understanding how bacteria transport and utilize substances from their environment may provide insight into the development of disease, especially disease caused by organisms such as the pneumococcus, which is commonly carried by a large percentage of the population yet rarely causes disease except in children and the elderly (33). It is also important, as the pneumococcus lacks many basic biosynthetic pathways and relies on numerous transport systems to acquire the majority of nutrients and cofactors needed for growth (46).
Previous work by our laboratory has demonstrated a role for DLDH in the uptake and utilization of raffinose, stachyose, and galactose by different ABC transport systems (41). DLDH was found to be required for ABC transport, and this defect was related to lost fitness of the bacteria in the host. In this study, we have further characterized the role of DLDH in raffinose transport. We identified the raffinose ATP-binding protein as open reading frame SPD_1409 in the D39 genome and showed that it was the only ATP-binding protein responsible for raffinose transport, as a knockout mutant was unable to grow in the presence of either raffinose or stachyose and was unable to import radiolabeled raffinose. We also confirmed that the raffinose operon constituted the other part of the transport machinery, as a strain deficient in rafEFG failed to grow in the presence of raffinose as a sole carbon source and failed to import radiolabeled raffinose. As the Raf system is homologous with the MSM system in S. mutans but does not transport or metabolize as many sugars, we named SPD_1409 rafK to conform to the terminology presented by Rosenow (36).
The regulation of raffinose transport has been previously addressed to some degree. Rosenow et al. have shown that α-galactosidase activity is induced by raffinose and downregulated by carbon catabolite repression (CCR) in the presence of sucrose (36). CCR is a mechanism whereby genes of less-needed catabolic pathway genes are downregulated and transport of nonfavored catabolites is decreased in the presence of a favored carbon source (36). In Gram-positive organisms, the LacI/GalR family transcriptional repressor catabolite control protein A (CcpA) and its homologs are thought to be the main players in transcriptional control (21) and have been shown to be important during colonization and lung infection in mice (14, 19). The system also uses other factors, such as HPr, that can act as corepressors or coactivators to the CcpA protein, which can then bind to CREs at upstream regions or in coding regions of catabolic genes to either activate or repress them, respectively (47). The aga gene has a CRE site in its promoter (36), but CcpA does not regulate the gene (36, 49). Although the specific molecules involved in regulation are not known, this study indicated that the importance of raffinose was as a strong activator of the transcription of the aga and rafEFG genes, as mutations that blocked import of raffinose (ΔRafK and ΔRafE) caused equal and major inhibition of gene transcription (50- to 80-fold). We were also able to show that the raffinose ABC transporter, similar to the maltose transporter in E. coli, is regulated by inducer exclusion, as raffinose transport was inhibited both in the presence of glucose and in the presence of sucrose. To our knowledge, this is the first report of inducer exclusion control of ABC transport in pneumococci.
DLDH has been shown to have numerous roles in various prokaryote and eukaryote cells besides that of the E3 component in 2-oxo acid dehydrogenase complexes (2, 20, 23, 24, 41, 48). As an enzyme involved in metabolism, it may well be a major player in the control of metabolic “sensing,” and part of this role could be as a coregulatory molecule in catabolite repression. To investigate how DLDH regulates raffinose transport, we first studied its role in transcriptional regulation. In the absence of DLDH, rafK expression was reduced 2-fold, which was not biologically relevant, as an LplA-negative strain that showed no effect on raffinose import also showed a similar transcriptional decrease and as it did not appear to affect the amount or distribution of expressed protein significantly based on Western blot analysis of bacterial extracts. There was no effect on the transcription of the regulatory genes rafR and rafS, but the expression of aga and the rafEFG genes was reduced about 7-fold in DLDH-negative cells, which would indicate that DLDH plays a coregulatory role mainly for the aga and rafEFG genes. It is therefore possible that DLDH interferes directly with the transcriptional regulators responsible for regulating aga and rafEFG transcription. Alternatively, DLDH may play a role in transcriptional repression by binding directly to the regulatory domain of RafK. In E. coli, expression of the maltose uptake system is regulated by MalT, an activator of transcription that is sequestered on MalK when MalK is not actively transporting it substrates (5, 39). By binding to RafK, DLDH may affect the affinity of other regulatory proteins, such as the putative transcriptional regulators RafS and RafR, which directly regulate expression of the raf genes (36). It is also possible that DLDH directly affects transporter activity by interfering with inducer exclusion. This might happen through a mechanism similar to that of E. coli MalK, where nonphosphorylated EIIAGlc binds to the regulatory domain of MalK, thereby inhibiting its ability to hydrolyze ATP and preventing further transport (5, 39). Studies of these regulatory pathways are currently being pursued.
Finally, as DLDH-negative pneumococci are drastically attenuated for virulence in mouse models, we investigated the contribution of the Raf transport system to pneumococcal virulence in a mouse model. As shown previously (41) DLDH-negative bacteria completely cleared the mouse nasopharynx and lungs within 24 h. RafK-deficient bacteria showed only a slight reduction in bacterial load after single-strain infections compared to the wild-type strain but showed a significant attenuation when infected together with the wild-type strain, suggesting that raffinose utilization is important for pneumococci on the mucosal surfaces in the lung and nasopharynx. The specific availability of raffinose in mucosal secretions has not been determined. Raffinose and stachyose are common galactooligosaccharides, found in large amounts in plants and plant products used as staple human foods, such as beans and soy (11, 26). They are not fermented by our bodies but are thought to be substrates for the intestinal microflora (50). However, raffinose is known to be absorbed by the intestinal epithelium to a significant degree (29) and even if most of it is excreted in urine it is likely that some of the carbohydrate reaches mucosal surfaces, where it would act as an alternative energy source, available to bacteria colonizing the nasopharynx.
Although RafK-deficient pneumococci were less virulent in mice, they were not attenuated to the degree that was observed in DLDH-negative bacteria, suggesting that attenuation in the DLDH-negative strain was due to additional events beyond just the repression of the Raf system. Interestingly, three other ATP-binding proteins in the pneumococcal genome (the iron transport protein PitB, the polyamine transport protein PotA, and the ORF SPD_1608 with unknown function [8, 40]) possess a regulatory domain similar to those of RafK and MalK, and initial investigation of the transcriptional levels of these transport genes in DLDH-negative bacteria, as well as functional transport assays, has shown decreased expression, suggesting that they are regulated similarly to the raffinose transport system.
To summarize, in this study, we have characterized the raffinose transporter ATP-binding protein RafK. We have demonstrated that RafK is the sole ATP binding protein that works with the raf operon and have shown it to be the sole transporter of the α-galactosides raffinose and stachyose. We have also shown that DLDH regulation of this transport system on a transcriptional level may be associated with its ability to bind directly to RafK and potentially interfere with CCR. As the regulatory domain found on RafK is present only on ATP-binding proteins in the archaeal and bacterial kingdoms, a better understanding of DLDH's binding to this domain may lead to important breakthroughs that could be utilized for future drug development.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Avery O. T., MacLeod C. M., McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a dexoxyribonuceic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babady N. E., Pang Y. P., Elpeleg O., Isaya G. 2007. Cryptic proteolytic activity of dihydrolipoamide dehydrogenase. Proc. Natl. Acad. Sci. U. S. A. 104:6158–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balachandran P., Brooks-Walter A., Virolainen-Julkunen A., Hollingshead S. K., Briles D. E. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basavanna S., et al. 2009. Screening of Streptococcus pneumoniae ABC transporter mutants demonstrates that LivJHMGF, a branched-chain amino acid ABC transporter, is necessary for disease pathogenesis. Infect. Immun. 77:3412–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bohm A., Diez J., Diederichs K., Welte W., Boos W. 2002. Structural model of MalK, the ABC subunit of the maltose transporter of Escherichia coli: implications for mal gene regulation, inducer exclusion, and subunit assembly. J. Biol. Chem. 277:3708–3717 [DOI] [PubMed] [Google Scholar]
- 6. Briles D. E., Novak L., Hotomi M., van Ginkel F. W., King J. 2005. Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect. Immun. 73:6945–6951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown J. S., Gilliland S. M., Holden D. W. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572–585 [DOI] [PubMed] [Google Scholar]
- 8. Brown J. S., Gilliland S. M., Ruiz-Albert J., Holden D. W. 2002. Characterization of pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect. Immun. 70:4389–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danson M. J. 1988. Dihydrolipoamide dehydrogenase: a ‘new’ function for an old enzyme? Biochem. Soc. Trans. 16:87–89 [DOI] [PubMed] [Google Scholar]
- 10. Danson M. J., Conroy K., McQuattie A., Stevenson K. J. 1987. Dihydrolipoamide dehydrogenase from Trypanosoma brucei. Characterization and cellular location. Biochem. J. 243:661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz-Batalla L., Widholm J. M., Fahey G. C. J., Castano-Tostado E., Paredes-Lopez O. 2006. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 54:2045–2052 [DOI] [PubMed] [Google Scholar]
- 12. Diederichs K., et al. 2000. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis. EMBO J. 19:5951–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dintilhac A., Claverys J. P. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148:119–131 [DOI] [PubMed] [Google Scholar]
- 14. Giammarinaro P., Paton J. C. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454–5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorke B., Stulke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613–624 [DOI] [PubMed] [Google Scholar]
- 16. Guiral S., Mitchell T. J., Martin B., Claverys J. P. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. U. S. A. 102:8710–8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hakansson A. P., Smith A. W. 2007. Enzymatic characterization of dihydrolipoamide dehydrogenase from Streptococcus pneumoniae harboring its own substrate. J. Biol. Chem. 282:29521–29530 [DOI] [PubMed] [Google Scholar]
- 18. Hava D. L., Camilli A. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389–1406 [PMC free article] [PubMed] [Google Scholar]
- 19. Iyer R., Baliga N. S., Camilli A. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187:8340–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang Y. J., Chung K. S., Park C., Yoo H. S. 1997. Fission yeast dihydrolipoamide dehydrogenase gene is involved in G1/S cell cycle progression. Biochim. Biophys. Acta 1358:229–239 [DOI] [PubMed] [Google Scholar]
- 21. Kaufman G. E., Yother J. 2007. CcpA-dependent and -independent control of beta-galactosidase expression in Streptococcus pneumoniae occurs via regulation of an upstream phosphotransferase system-encoding operon. J. Bacteriol. 189:5183–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerr A. R., et al. 2004. The Ami-AliA/AliB permease of Streptococcus pneumoniae is involved in nasopharyngeal colonization but not in invasive disease. Infect. Immun. 72:3902–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim S. Y., Kim J. 2010. Roles of dihydrolipoamide dehydrogenase Lpd1 in Candida albicans filamentation. Fungal Genet. Biol. 47:782–788 [DOI] [PubMed] [Google Scholar]
- 24. Kim Y., Ingram L. O., Shanmugam K. T. 2008. Dihydrolipoamide dehydrogenase mutation alters the NADH sensitivity of pyruvate dehydrogenase complex of Escherichia coli K-12. J. Bacteriol. 190:3851–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuhnau S., Reyes M., Sievertsen A., Shuman H. A., Boos W. 1991. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J. Bacteriol. 173:2180–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar V., et al. 2010. Sucrose and raffinose family oligosaccharides (RFOs) in soybean seeds as influenced by genotype and growing location. J. Agric. Food Chem. 58:5081–5085 [DOI] [PubMed] [Google Scholar]
- 27. Lanie J. A., et al. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linton K. J., Higgins C. F. 1998. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 28:5–13 [DOI] [PubMed] [Google Scholar]
- 29. Lobley R. W., Burrows P. C., Warwick R., Dawson D. J., Holmes R. 1990. Simultaneous assessment of intestinal permeability and lactose tolerance with orally administered raffinose, lactose and L-arabinose. Clin. Sci. (Lond.) 79:175–183 [DOI] [PubMed] [Google Scholar]
- 30. Marra A., Lawson S., Asundi J. S., Brigham D., Hromockyj A. E. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148:1483–1491 [DOI] [PubMed] [Google Scholar]
- 31. McDaniel L. S., Scott G., Kearney J. F., Briles D. E. 1984. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160:386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison T. B., Weis J. J., Wittwer C. T. 1998. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24:954–958, 960, 962 [PubMed] [Google Scholar]
- 33. O'Brien K. L., Santosham M. 2004. Potential impact of conjugate pneumococcal vaccines on pediatric pneumococcal diseases. Am. J. Epidemiol. 159:634–644 [DOI] [PubMed] [Google Scholar]
- 34. Perham R. N., Packman L. C., Radford S. E. 1987. 2-Oxo acid dehydrogenase multi-enzyme complexes: in the beginning and halfway there. Biochem. Soc Symp. 54:67–81 [PubMed] [Google Scholar]
- 35. Richarme G. 1985. Possible involvement of lipoic acid in binding protein-dependent transport systems in Escherichia coli. J. Bacteriol. 162:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenow C., Maniar M., Trias J. 1999. Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 9:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell R. R., Aduse-Opoku J., Sutcliffe I. C., Tao L., Ferretti J. J. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631–4637 [PubMed] [Google Scholar]
- 38. Saier M. H. J., et al. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217–230 [DOI] [PubMed] [Google Scholar]
- 39. Samanta S., et al. 2003. Disulfide cross-linking reveals a site of stable interaction between C-terminal regulatory domains of the two MalK subunits in the maltose transport complex. J. Biol. Chem. 278:35265–35271 [DOI] [PubMed] [Google Scholar]
- 40. Shah P., Nanduri B., Swiatlo E., Ma Y., Pendarvis K. 2011. Polyamine biosynthesis and transport mechanisms are crucial for fitness and pathogenesis of Streptococcus pneumoniae. Microbiology 157:504–515 [DOI] [PubMed] [Google Scholar]
- 41. Smith A. W., Roche H., Trombe M. C., Briles D. E., Hakansson A. 2002. Characterization of the dihydrolipoamide dehydrogenase from Streptococcus pneumoniae and its role in pneumococcal infection. Mol. Microbiol. 44:431–448 [DOI] [PubMed] [Google Scholar]
- 42. Spellerberg B., et al. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803–813 [DOI] [PubMed] [Google Scholar]
- 43. Stulke J., Hillen W. 1998. Coupling physiology and gene regulation in bacteria: the phosphotransferase sugar uptake system delivers the signals. Naturwissenschaften 85:583–592 [DOI] [PubMed] [Google Scholar]
- 44. Sung C. K., Li H., Claverys J. P., Morrison D. A. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tao L., Sutcliffe I. C., Russell R. R., Ferretti J. J. 1993. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans. J. Dent. Res. 72:1386–1390 [DOI] [PubMed] [Google Scholar]
- 46. Tettelin H., et al. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506 [DOI] [PubMed] [Google Scholar]
- 47. Thibault L., Vadeboncoeur C. 1985. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect. Immun. 50:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian J., et al. 2005. Mycobacterium tuberculosis appears to lack alpha-ketoglutarate dehydrogenase and encodes pyruvate dehydrogenase in widely separated genes. Mol. Microbiol. 57:859–868 [DOI] [PubMed] [Google Scholar]
- 49. van Opijnen T., Bodi K. L., Camilli A. 2009. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vinjamoori D. V., Byrum J. R., Hayes T., Das P. K. 2004. Challenges and opportunities in the analysis of raffinose oligosaccharides, pentosans, phytate, and glucosinolates. J. Anim. Sci. 82:319–328 [DOI] [PubMed] [Google Scholar]
- 51. Warner J. B., Lolkema J. S. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams C. H. 1992. Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric reductase—a family of flavoenzyme transhydrogenases, p. 121–211 In Müller F. (ed.), Chemistry and biochemistry of flavoenzymes, CRC, Boca Raton, FL [Google Scholar]
- 53. Yother J., Briles D. E. 1992. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J. Bacteriol. 174:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yother J., Handsome G. L., Briles D. E. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.