Abstract
Heterocysts are specialized cells required for aerobic fixation of dinitrogen by certain filamentous cyanobacteria. Numerous genes involved in the differentiation and function of heterocysts in Anabaena sp. strain PCC 7120 have been identified by mutagenizing and screening for mutants that require fixed nitrogen for growth in the presence of oxygen. We have verified that 10 Anabaena sp. genes, all1338, all1591, alr1728, all3278, all3520, all3582, all3850, all4019, alr4311, and all4388, identified initially by transposon mutagenesis, are such genes by complementing or reconstructing the original mutation and by determining whether the mutant phenotype might be due to a polar effect of the transposon. Elucidation of the roles of these genes should enhance understanding of heterocyst biology.
INTRODUCTION
Anabaena sp. strain PCC 7120 (hereinafter referred to as Anabaena sp.) is a filamentous cyanobacterium in which in the absence of fixed nitrogen, approximately every 10th cell differentiates into a specialized cell called a heterocyst. Heterocysts are the site of nitrogen fixation by the oxygen-sensitive enzyme nitrogenase. Within heterocysts, nitrogenase is protected from oxygen by a double-layered envelope comprising a laminated layer of heterocyst envelope glycolipids (HGL) that impedes entry of oxygen into the cell and a homogeneous layer of heterocyst envelope polysaccharide (HEP) that protects HGL layers from physical damage, an increased rate of respiration, and inactivation of at least the oxygen-generating complex of photosystem II (39). An estimate that ca. 15 to 25% of the Anabaena sp. DNA sense strand is transcribed only in heterocysts (21) could not distinguish how many of the genes involved are actually essential for development or diazotrophy.
Numerous genes involved in the differentiation and function of heterocysts in Anabaena sp. have been identified by mutagenizing and screening for mutants that require fixed nitrogen for growth in the presence of oxygen. Having identified such mutants that nonetheless reduced acetylene under anoxic conditions, Ernst et al. (9) wrote that “the inability to grow on molecular nitrogen in the presence of 0.2 atm … of O2 does not preclude the possibility that nitrogenase may be expressed under other conditions, for example, in the absence of oxygen.” Therefore, they coined the term “Fox” mutants, mutants that are “incapable of fixation in the presence of oxygen,” to distinguish them from “Fix” mutants, unable to fix N2 under all conditions. Fan et al. (11) identified conR (all0187) as a gene that requires fixed nitrogen for growth in the presence of oxygen. Their observation was confirmed by Mella-Herrera et al. (24), who observed that for at least a short period after N2 fixation normally starts, a conR mutant shows aerobic nitrogenase activity. (They suggested that the inability of the cells to grow on N2 was caused by the inability of heterocysts to transfer fixed nitrogen to neighboring cells.) What have been called Fox mutants have been tested for growth, or the lack of growth, in the presence of oxygen and absence of fixed nitrogen, but they have seldom been tested for aerobic nitrogenase activity. Because a conR mutant could express some nitrogenase activity aerobically, we propose that Fox mutants be defined slightly more broadly as those “requiring fixed nitrogen for growth in the presence of oxygen” rather than the original definition (9), those “incapable of fixation in the presence of oxygen,” and will use the broader definition. By either definition, mutants are often easily distinguished because they soon yellow and cease growth under aerobic conditions in a medium lacking fixed nitrogen (9). Often, the mutation is evidently heterocyst related, e.g., hep and hgl genes that are required specifically for the biosynthesis of HEP and HGL, respectively, have heretofore been proven to be Fox genes. In other instances, a mutation may affect all cells of a filament, e.g., a mutation in a gene such as sepJ (fraG) (13, 26) that leads to extensive fragmentation of filaments. In that instance, heterocysts may soon lack nutrients with which to fix nitrogen or to remain internally anoxic. Similarly, a hglK mutant has a phenotype in vegetative cells as well as in heterocysts, but it is a Fox gene perhaps because of its effect on N2 fixation in heterocysts (2).
Not all genes that regulate differentiation are Fox genes. For example, hetR is a Fox gene, because in its absence, no heterocysts differentiate and so no nitrogen fixation takes place (3), whereas patA and patS are not Fox genes. In a patA mutant, heterocysts form only at the ends of long filaments, but those cells that do differentiate fix nitrogen, so that the filaments grow (20). When a patS mutant is deprived of fixed nitrogen, the initial response is that contiguous vegetative cells differentiate into heterocysts, but within a few days, spaced heterocysts again become the norm (44). Genes required for heterocyst-specific metabolism can be Fox genes, e.g., nifH, nifD, and nifK, that encode the subunits of nitrogenase, but fdxH, a ferredoxin that is heterocyst specific is—although important—not essential for aerobic N2 fixation and so is not a Fox gene (23). Mutations in one of the two cytochrome oxidase operons, coxABCII and coxABCIII, allow continued diazotrophy under oxic conditions, so that these are not Fox genes, but a combination of mutations in coxII and coxIII does not allow aerobic nitrogen fixation (36), so that the dual mutation has a Fox− phenotype. Similarly, mutation of two protein kinase genes, pkn30 and pkn44, but neither alone, results in a Fox− phenotype (31). In short, a gene need not be a Fox gene to be important for heterocyst formation or function, and a Fox gene—while it need not be expressed only in heterocysts—evidently has, in the presence of oxygen, some critical importance for heterocyst development or function but is not essential for growth on fixed nitrogen.
We have used transposon screening to identify Fox genes. However, a transposon mutation that confers a Fox− phenotype can also result from a combination of a spontaneous mutation unrelated to the presence of the transposon and antibiotic resistance conferred by the transposon or by a polar effect of the transposon on downstream genes. That a transposon-intercepted gene is a Fox gene can be verified by reconstruction of the mutation or by complementation, provided that the possibility of a polar effect is carefully considered.
We present 10 genes whose mutants have a Fox− phenotype and that have not previously been shown conclusively to be Fox genes in Anabaena sp. Mutations in some of these genes result in heterocysts that have structural abnormalities; some others have metabolic defects. Bioinformatic analysis provides hints as to what some of the latter defects may be. Considering that approximately 75 Fox genes have been published (see Table S1 in the supplemental material), the 10 genes reported in this paper represent more than 10% of the currently known Fox genes. Additionally, if the estimate is correct that Anabaena sp. has approximately 100 to 140 Fox genes (38), the majority of Fox genes have been discovered, many of them by our transposon mutagenesis screen (10, 11, 13, 17, 19, 37, 40).
MATERIALS AND METHODS
Cyanobacteria were grown, with shaking, in flask cultures of AA/8 liquid medium, with or without nitrate (16a), or in medium AA, with or without nitrate, solidified with 1.2% home-purified (Difco) Bacto agar (16a) and supplemented with antibiotics as appropriate, at 30°C. Cultures were illuminated initially as described previously (10, 17) and more recently with Philips F32T8/TL741Universal/Hi-Vision lamps (ca. 30 μmol s−1 m−2) (LI-COR Bioscience light meter LI-250A). Mutagenesis with transposon Tn5-1063, screening of mutants, complementation, and insertional mutagenesis were performed as described previously (10, 17). Strains and plasmid constructions are listed in Table 1. Samples stained with an aqueous solution (17) of Alcian Blue (13a) were visualized with a Wild M20 microscope and photographed with a Nikon CoolPix 4300 digital camera.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics and/or derivationa |
|---|---|
| Fox− derivatives of Anabaena sp. strain PCC 7120 | |
| FQ202 | Bmr Nmr Smr; alr1728::Tn5-1063 |
| FQ204 | Bmr Nmr Smr; all1591::Tn5-1063 |
| FQ211 | Bmr Nmr Smr; all3520::Tn5-1063 |
| FQ228 | Bmr Nmr Smr; all3278::Tn5-1063 |
| FQ324 | Bmr Nmr Smr; alr1728::Tn5-1063 |
| FQ384 | Bmr Nmr Smr; all3850::Tn5-1063 |
| FQ406 | Bmr Nmr Smr; all4019::Tn5-1063 |
| FQ747 | Bmr Nmr Smr; all1591::Tn5-1063 |
| FQ807 | Bmr Nmr Smr; alr4311::Tn5-1063 |
| FQ1265 | Bmr Nmr Smr; all1338::Tn5-1063 |
| FQ1470 | Bmr Nmr Smr; all1338::Tn5-1063 |
| FQ1580 | Bmr Nmr Smr; all3582::Tn5-1063 |
| FQ1595 | Bmr Nmr Smr; all4388::Tn5-1063 |
| SR2816a | Cmr Emr; mutation in all3850 resulting from single recombination with pRL2816a; see below |
| SR2821 | Cmr Emr; mutation in alr1728 resulting from single recombination with pRL2821; see below |
| Escherichia coli strains | |
| DH5αMCR | Invitrogen Corp., received courtesy of J. C. Meeks |
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74recA1endA1araD139 Δ(ara leu)7697galUgalKrpsLnupG λ− |
| HB101 | F−thi-1hsdS20 (rB− mB−) supE44recA13ara-14leuB6proA2lacY1galK2rpsL20 (Smr) xyl-5mtl-1 |
| Plasmids | |
| anc0756 | Cmr Emr; Anabaena sp. chromosomal DNA from bp 4233705 to 4250432 in the BamHI site of pRL838 |
| anc1006 | Cmr Emr; Anabaena sp. chromosomal DNA from bp 3952462 to 3970608 in the BamHI site of pRL838 |
| anc1512 | Cmr Emr; Anabaena sp. chromosomal DNA from bp 2070875 to 2084189 in the BamHI site of pRL838 |
| anp00427 | Apr; Anabaena sp. chromosomal DNA from bp 5161083 to 5168600 in the BamHI site of pUC18 |
| anp00676 | Apr; Anabaena sp. chromosomal DNA from bp 4325494 to 4333569 in the BamHI site of pUC18 |
| anp00920 | Apr; Anabaena sp. chromosomal DNA from bp 4640078 to 4648267 in the BamHI site of pUC18 |
| anp02032 | Apr; Anabaena sp. chromosomal DNA from bp 5253901 to 5262374 in the BamHI site of pUC18 |
| anp02580 | Apr; Anabaena sp. chromosomal DNA from bp 4321373 to 4329600 in the BamHI site of pUC18 |
| anp03035 | Apr; Anabaena sp. chromosomal DNA from bp 4241824 to 4248722 in the BamHI site of pUC18 |
| anp03337 | Apr; Anabaena sp. chromosomal DNA from bp 1859242 to 1867412 in the BamHI site of pUC18 |
| anp04001 | Apr; Anabaena sp. chromosomal DNA from bp 4833192 to 4840653 in the BamHI site of pUC18 |
| anp04055 | Apr; Anabaena sp. chromosomal DNA from bp 1510438 to 1518550 in the BamHI site of pUC18 |
| anp04490 | Apr; Anabaena sp. chromosomal DNA from bp 1584150 to 1591582 in the BamHI site of pUC18 |
| pGEM-T Easy | Apr; cloning vector (Promega Corp.) |
| pK18 | Kmr; cloning vector (27) |
| pRL443 | Apr Tcr; conjugative plasmid (10) |
| pRL838 | Cmr Emr; BAC vector (18) (GenBank accession no. AF403425) |
| pRL2801 | Apr; internal fragment of all3850 amplified by PCR using genomic DNA from Anabaena sp. as template and primers IDT147 (5′-TAAATTCTCAGGCAGCAGATGA-3′) and IDT148 (5′-TGTAACACCGATGATTCTGGAG-3′), cloned in pGEM-T Easy |
| pRL2807 | Apr; internal fragment of alr1728 amplified by PCR using genomic DNA from Anabaena sp. as template and primers IDT161 (5′-AATCTCGCTACTCGCTTACCAG −3′) and IDT162 (5′-GTTTAGCATGACGGGGTTTAAG-3′), cloned in pGEM-T Easy |
| pRL2816a | Apr Cmr Emr; PstI fragment containing C.CE3-oriT cassette from pRL2665b (17) transferred to the PstI site of pRL2801 |
| pRL2821 | Apr Cmr Emr; PstI fragment containing C.CE3-oriT cassette from pRL2665b (17) transferred to the PstI site of pRL2807 |
| pRL2831a | Smr Spr; PglnA-containing RSF1010 derivative (17, 40) |
| pRL2831b | Smr Spr; same as pRL2831a, but PglnA oppositely oriented (17) |
| pRL2833a | Cmr Emr; PglnA-containing pDU1 derivative (10) |
| pRL2833b | Cmr Emr; same as pRL2833a, but PglnA oppositely oriented (10) |
| pRL2879 | Smr Spr; all1590, downstream of all1591, was excised as an EaeI-RsaI fragment of anp03337 and transferred between PspOMI and StuI of pRL2831a |
| pRL2888 | Smr Spr; all4018-containing XmnI-NheI fragment of anp04001 transferred between XbaI and StuI of pRL2831a |
| pRL3006 | Smr Spr; all3520-containing HpaI-Acc65I fragment of anp03035 transferred between StuI and BsiWI of pRL2831a |
| pRL3007 | Smr Spr; all1338-containing HincII-EaeI fragment of anp04490 transferred between StuI and PspOMI of pRL2831a |
| pRL3008 | Smr Spr; all3582-containing HindIII-HincII fragment of anp00676 transferred between HindIII and Ecl136II of pRL2831b |
| pRL3009 | Smr Spr; all4388-containing NlaIII-HincII fragment of anp02032 transferred between SphI and Ecl136II of pRL2831b |
| pRL3026 | Smr Spr; alr4311-containing Eco47III-NlaIII fragment of anp00427 transferred between Ecl136II and SphI of pRL2831b |
| pRL3038 | Smr Spr; all4019-containing SphI-HindIII fragment of anp04001 transferred between the same sites in pRL2831b |
| pRL3050 | Smr Spr; all3278-containing NheI fragment of anc1006 transferred into BlnI of pRL2831a |
| pRL3106 | Kmr; genes downstream of all3582 were excised as an XcmI (blunted)-PstI fragment of anp02580 and cloned between SmaI and PstI of pK18 |
| pRL3118 | Cmr Emr; genes downstream of all3582 were excised as a SacI-SphI fragment of pRL3106 and transferred between the same sites in pRL2833b |
| pRL3132 | Kmr; all4387-containing SpeI-Cac8I fragment of anp02032 cloned between XbaI and SmaI of pK18 |
| pRL3134 | Cmr Emr; genes downstream of all3520 were excised as a SapI (blunt)-BsrGI fragment of anc0756 and transferred between StuI and BsiWI of pRL2833a |
| pRL3142 | Cmr Emr; all4387-containing PstI-Acc65I fragment of pRL3132 transferred between NsiI and BsiWI of pRL2833a |
| pRL3155 | Apr Cmr Emr; genomic DNA of SR2821 was digested with HindIII, religated, and transformed to E. coli. Plasmids recovered on plates containing Cm contain pRL2821 and its neighboring genomic sequences |
| pRL3170 | Cmr Emr; all1591-containing EaeI-PvuII fragment of anp03337 transferred between PspOMI and StuI of pRL2833a |
| pRL3171 | Cmr Emr; all3850-containing PstI-DraI fragment of anp00920 transferred between NsiI and StuI of pRL2833a. This fragment extends 41 bp 3′ and 396 bp 5′ from all3850 (88 bp 3′ from the next upstream orf, asl3851) |
| pRL3172 | Cmr Emr; all4018-containing NsiI-XhoI fragment of pRL2888 transferred between the same sites of pRL2833a |
| pRL3180 | Smr Spr; asl3849-containing ApaI-XmnI fragment, which overlaps the 3′ ends of neighboring ORFs asr3848 and all3850, of anp00920 transferred between ApaI and StuI of pRL2831a |
| pRL3810 | Kmr Nmr; the coliphage T7 terminator as a BamHI-BglII fragment of pET-3 (28) inserted into the BamHI site at the end of the polylinker of pRL3040a (40) |
| pRL3811 | Kmr Nmr; SacI-XhoI fragment of pRL3171 transferred between the SacI and XhoI sites of pRL3810 |
| pUC18 | Apr; cloning vector (43) |
Drug resistance is indicated by a superscript r. The drugs are abbreviated as follows: Ap, ampicillin; Bm, bleomycin; Cm, chloramphenicol; Em, erythromycin; Km, kanamycin; Nm, neomycin; Sm, streptomycin; Sp, spectinomycin; Tc, tetracycline. Anabaena sp., Anabaena sp. strain PCC 7120; BAC, bacterial artificial chromosome.
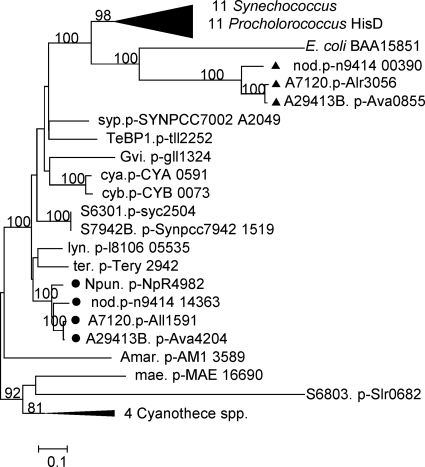
The evolutionary history of hisD was inferred using the neighbor-joining method (29). The optimal tree with the sum of branch length of 8.38 is shown. Bootstrap value (100 replicates) larger than 75% are shown above the branches (12). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Dayhoff matrix-based method (30) and are in the units of the number of amino acid substitutions per site. All positions containing gaps and missing data were eliminated from the data set (complete deletion option). The final data set totaled 381 positions. Phylogenetic analyses were conducted by using MEGA4 (34).
RESULTS AND DISCUSSION
We present below information concerning 10 genes that we have identified as Fox genes on the basis of corresponding mutants that require fixed nitrogen to grow aerobically. Table 2 summarizes the information.
Table 2.
Ten genes newly identified and/or validated as Fox genes and their known properties
| Gene | Fig. 1 panel(s) | Annotationa | Morphological phenotype | Microarray resultb | Comment(s)c |
|---|---|---|---|---|---|
| all1338 | a and b | Hypothetical protein | Sometimes a gap between protoplast and envelope | No change | Protein conserved among cyanobacteria, no defined domains |
| all1591 | c | Histidinol dehydrogenase | Heterocysts often open at end | Significant increase at 3 and 8 h | hisD; alr3056 also annotated hisD: do heterocysts make their own His? |
| alr1728 | d | Hypothetical protein with FHA domain | Some heterocysts are open at one end | Significant increase at 8 h | Regulatory, COG2339: prsW membrane proteinase, regulator of anti-sigma factor |
| all3278 | e | Hypothetical protein | Open polar region in at least one end of the heterocyst | No significant change, very low expression | Ykud domain, enzymes that may be involved in cell wall synthesis |
| all3520 | f | Unknown protein | Some heterocysts divide internally | Significant increase at 3 h | Only in heterocyst-forming cyanobacteria; signal peptide/transmembrane domain |
| all3582 | g | Hypothetical protein | Heterocysts vacuolate | Very low expression | Signal peptide/two transmembrane domains; entire protein highly conserved in cyanobacteria, ca. 70 aa similar to cell division proteins in other bacteria |
| all3850 | h | Unknown protein | Thick heterocyst envelope and sometimes polar bodies enlarged | Significant increase at 3 and 8 h | Peroxidase heme-binding signature at positions 339 to 349 appears only in cyanobacteria |
| all4019 | i | G6P dehydrogenase | No abnormal structural phenotype | Significant increase at 3, 8, and 24 h | One of two G6PDs |
| alr4311 | j | ATP-binding protein of ABC transporter | Some heterocysts are vacuolated | Very low expression | ABC transporter with no transmembrane domain. Likely an importer, functioning with Alr4309, Alr4310, or both |
| all4388 | k | Hypothetical protein | Cell envelope not stained by Alcian Blue | Significant increase at 3, 8, and 24 h | Polysaccharide transporter |
FHA domain, forkhead-associated domain; G6P, glucose-6-phosphate; ABC, ATP-binding cassette.
Microarray result for data in reference 7 analyzed by the method in reference 41. The strains were deprived of fixed nitrogen and studied after 3, 8, and 24 hours of nitrogen deprivation.
aa, amino acids; G6PD, glucose-6-phosphate dehydrogenase.
all1338.
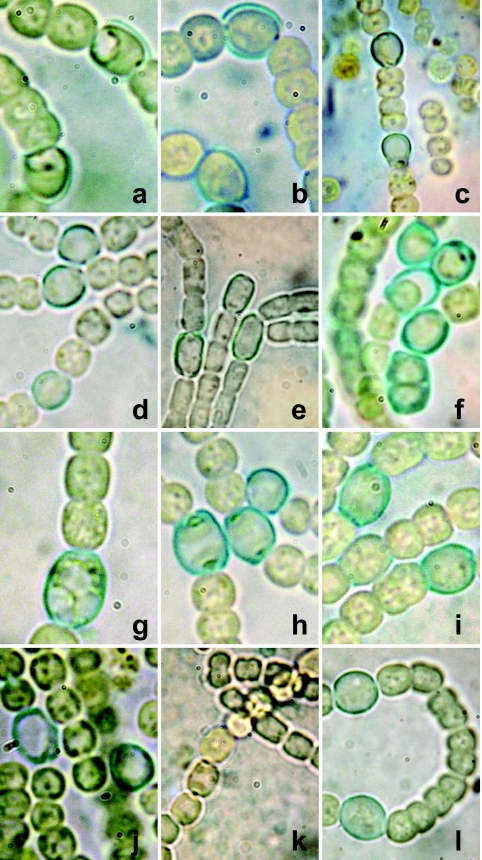
all1338 was intercepted by a transposon twice in our mutagenesis study and is the last gene of a possible operon. FQ1470, a strain with a mutation in this gene, was complemented by pRL3007, which bears all1338 as its only intact gene, implying that it is a Fox gene. The heterocyst envelopes of FQ1470 and of a second all1338 mutant, FQ1265, are abnormal (Fig. 1 a and b). In addition, internal vacuoles (Fig. 1a) are more frequently observed in heterocysts of FQ1265 than in FQ1470. All1338 is annotated as a hypothetical protein. It is unique to, and conserved among, all 42 genomically sequenced cyanobacteria from different taxonomic groups. It is rich in aspartic and glutamic acids and appears to be soluble. No function can now be assigned to Alr1338 on the basis of sequence similarity.
Fig. 1.
Light micrographs of mutant filaments deprived of nitrogen on agar plates for 3 to 7 days and stained with Alcian Blue before microscopic observation. (a) Strain FQ1265 (all1338); (b) FQ1470 (all1338); (c) SR2813a (all1591); (d) FQ202 (alr1728); (e) FQ228 (all3278); (f) FQ211 (all3520); (g) FQ1580 (all3582); (h) SR2816a (all3850); (i) FQ406 (all4019); (j) FQ807 (all4311); (k) FQ1595 (all4388); (l) wild-type Anabaena sp. Aberrant overall coloration resulting from differing intensities of illumination was partially corrected with Adobe Photoshop CS5.
all1591.
all1591 was intercepted by a transposon 12 times in our mutagenesis study, one resulting mutant being FQ747. Its downstream gene, all1590, is similarly oriented. FQ747 was complemented by pRL3170, which carries all1591, and was not complemented by pRL2879, which carries all1590. PCR analysis of the complemented mutant, performed as described in reference 17, showed that pRL3170 has, in part, integrated into the genome of FQ747 upstream of the transposon and provided no evidence of integration downstream of the transposon. We conclude that only all1591 and not all1590 could have contributed to the complementation, implying that all1591 is a Fox gene. An all1591 insertional mutant, SR2813a, is also unable to grow in the absence of combined nitrogen. FQ747 and SR2813a, when deprived of fixed nitrogen, have the same phenotype: the heterocyst envelope, as seen with a microscope, ranges in shape from a slightly to widely open horseshoe (Fig. 1c). all1591 is annotated as hisD. hisD codes for histidinol dehydrogenase, an enzyme that catalyzes the last step of histidine biosynthesis from 5-phosphoribosyl-α-pyrophosphate. Three of the four heterocyst-forming cyanobacteria with a fully sequenced genome have two copies of HisD, but Nostoc punctiforme has only a single copy, which is similar to All1591. Phylogenetic analysis of HisD from cyanobacteria, with the protein from Escherichia coli as a reference, shows that All1591 and its orthologs from other heterocyst-forming cyanobacteria cluster with HisD from Lyngbya sp. and Trichodesmium erythraeum, cyanobacteria that are also filamentous, whereas the other HisD copy, Alr3056 and its orthologs, cluster with the homologs from E. coli and unicellular cyanobacteria (Fig. 2). Interestingly, heterocyst-forming cyanobacteria but no other cyanobacteria also have two copies of hisC (corresponding to all4966 and alr2092 in Anabaena sp.), coding for histidinol phosphate aminotransferase which functions upstream of HisD in the metabolic pathway. Perhaps heterocysts employ specialized enzymes for histidine biosynthesis, although there is no evidence that either hisC gene is required for heterocyst function. Why a modification of histidine biosynthesis should affect heterocyst morphology (Fig. 1c) is unclear. Microarray data (data in reference 7 analyzed by the method in reference 41) showed that the expression of all1591 increased significantly after 3 and 8 h of nitrogen deprivation, whereas the expression of alr3056 did not. Similarly, the expression of hisC (alr2092) increased after 8 h of N deprivation, and that of all4966 did not change significantly.
Fig. 2.
Phylogenetic analysis of 46 HisD sequences from cyanobacteria. The small black circles represent orthologs of All1591 in heterocyst-forming cyanobacteria, and the small black triangles represent their paralogs in the same organisms. HisD from E. coli (GenBank accession no. BAA15851) was used as a reference. Strain abbreviations: A7120, Anabaena sp.; A29413B, Anabaena variabilis ATCC 29413; Amar, Acaryochloris marina MBIC11017; cya, Synechococcus sp. OS type a′; cyb, Synechococcus sp. OS type b′; Gvi, Gloeobacter violaceus PCC 7421; lyn, Lyngbya sp. strain PCC 8106; mae, Microcystis aeruginosa NIES843; nod, Nodularia spumigena; Npun, Nostoc punctiforme ATCC 29133; S6803, Synechocystis sp. strain PCC 6803; S7942B, Synechococcus elongatus PCC 7942; syp, Synechococcus sp. strain PCC 7002; TeBP1, Thermosynechococcus elongatus bp1; ter, Trichodesmium erythraeum IMS101. The bar indicates 0.1 substitution per site.
alr1728.
alr1728 was intercepted by a transposon four times in our mutagenesis study and so it is unlikely to be a false-positive result. Because the gene downstream from alr1728 is encoded on the opposite strand of DNA, if the phenotype-causing mutation was within alr1728, no polar effect of the transposon on the downstream gene would need to be considered. Strains FQ202 and FQ324 were complemented by plasmid anc1512 (Table 1), implying that if the mutation causing the phenotype is not in alr1728 (bp 2077564 to 2078901), it is within 7 kb of that gene. Rather than continue with complementation, we took the alternative approach of reconstructing the mutation, in this instance by homologous recombination with an internal fragment of the gene. We thereby obtained mutant strain SR2821 (Table 1). To determine whether the mutational construction was actually within alr1728, genomic DNA of the mutant was cut with HindIII, diluted, recircularized by ligation, and transferred to E. coli by electroporation. Because pRL2821 includes a replication origin and antibiotic resistance determinants that function in E. coli and has sites for HindIII only within its internal fragment of alr1728, we could recover (as pRL3155) a portion of pRL2821 fused to a portion of the flanking region of insertion. We thereby determined that the insertion was within alr1728. SR2821 proved to be Fox−, showing that alr1728 is a Fox gene. Moreover, SR2821 shared with transposon mutant FQ202 (Fig. 1d) the structural phenotype that some but not all of its heterocysts were visibly open at one end.
Alr1728 is annotated as a hypothetical protein. The C-terminal 2/3 of the protein belongs to COG2339 (35), which includes membrane proteinases that regulate anti-sigma factors. Alr1728 may belong to a group of regulated intramembrane proteolytic (RIP) proteins (8) that posttranslationally activate membrane-bound transcription factors (MTF) (16). Ruanbao Zhou (S. Dakota State University) (personal communication) is currently testing whether Alr1728 may interact with Alr4305, a putative membrane-bound transcription factor. The presence of such a Fox gene in Anabaena sp. suggests that one or more RIP-MTF interactions may be important in heterocyst differentiation. (Due to an annotational error, Fox gene All0187 [11] might, but should not, be interpreted as an MTF [24].) The N-terminal 1/3 of Alr1728 contains a forkhead-associated domain that may bind phosphoserine, phosphothreonine, or sometimes phosphotyrosine residues.
all3278.
all3278 was intercepted by a transposon 7 times in our mutagenesis study; its downstream gene is encoded on the opposite strand of DNA. Strain FQ228, mutated in this open reading frame (ORF), was complemented by pRL3050, implying that it is a Fox gene. When deprived of fixed nitrogen, heterocysts of FQ228 appear to have an open polar region in at least one end (Fig. 1e). All3278 is annotated as a hypothetical protein. Its product resembles Ykud domain enzymes (pfam03734), and its orthologs in N. punctiforme and Anabaena variabilis are annotated as ErfK/YbiS/YcfS/YnhG proteins. ErfK-like proteins are proteins that are involved in cell wall synthesis; hence, this gene product may be required for the cell wall reorganization that is thought to take place during heterocyst differentiation (19, 42, 45).
all3520.
all3520 was intercepted 3 times in our experiments. It encodes an unknown protein that is unique to heterocyst-forming cyanobacteria and contains a possible transmembrane helix or signal peptide domain. The second in a sequence of 7 ORFs that have the same orientation, it begins only 20 bp 3′ from all3521, and so is presumably cotranscribed with that gene. all3519, 224 bp 3′ from all3520, may have its own promoter. pRL3006, whose only intact ORF is all3520, complemented FQ211, whereas pRL3134, which bears the downstream genes, failed to complement FQ211. Therefore, all3520 appears to be a Fox gene. Remarkably, some heterocysts of nitrogen-deprived FQ211 appeared to divide internally (Fig. 1f, lower right). In microarray experiments, the gene was upregulated at 3 h of nitrogen deprivation (data in reference 7 analyzed by the method in reference 41). No functional domains could be identified using INTERPRO or Pfam searches, but a low similarity to bacterial surface proteins is seen in an iterative PSI-BLAST search (1). The relationship between the unusual mutant phenotype of all3520 and the protein it encodes is unclear.
all3582.
all3582, intercepted 5 times in our experiments, encodes a hypothetical protein that is highly conserved in many cyanobacteria. It contains a signal peptide or a transmembrane domain at its N terminus. The C terminus of the protein has similarity to the glyoxalase superfamily of proteins (cl00411) that contains some signal transduction and cell division proteins. The gene is the first in a sequence of 5 similarly oriented genes. FQ1580, mutated in this gene, was complemented by pRL3008, a replicating plasmid whose only intact ORF is all3582, and was not complemented by pRL3118, a plasmid that bears the four downstream genes, implying that all3582 is a Fox gene. Heterocysts of FQ1580 appear vacuolated and sometimes granular (Fig. 1g).
all3850.
Intercepted by a transposon 5 times in our experiments, all3850 encodes an unknown protein that is conserved in cyanobacteria but is otherwise unique. Plasmids pRL3171 and pRL3811 bear all3850, and pRL3180 bears the downstream gene, asl3849. Because all3850 mutant FQ384 was not complemented by pRL3171, pRL3180, or both plasmids, the mutation was reconstructed by insertional mutation with pRL2816a. In the resulting Fox− mutant, SR2816a, the heterocyst envelope appears unusually thick, and the polar bodies are sometimes quite enlarged (Fig. 1h). SR2816a was also not complemented by all3850 clone pRL3811, pRL3180, or both plasmids. It is unclear why neither all3850 mutation was complemented. All3850 has a peroxidase heme-binding signature at positions 339 to 349 (ProSite PS00435) that includes a conserved His residue that is involved in binding the ligand. After nitrogen deprivation, expression of all3850 is upregulated at 3 and 8 h, but not at 24 h, compared to a culture maintained with nitrate (data in reference 7 analyzed by the method in reference 41).
all4019.
Intercepted 11 times in our transposon mutagenesis experiments, all4019 is one of two genes in Anabaena sp. that encodes a glucose-6-phosphate dehydrogenase (G6PD). Plasmid pRL3038 bears all4019 as its only gene, and compatible plasmid pRL3172 bears all4018, the next gene downstream from all4019, as its only intact gene. Strain FQ406, mutated in all4019, was complemented by pRL3038 with or without pRL3172, whereas pRL3172 alone failed to complement FQ406. We conclude that if all4018 is a Fox gene, it is not the sole Fox gene affected by the FQ406 mutation, implying that all4019 is a Fox gene. all4018 is orthologous to opcA, a gene that in Nostoc punctiforme is cotranscribed along with zwf. In N. punctiforme, both genes are required for a Fox+ phenotype, and opcA is required for the activity of G6PD, of which it is an allosteric effector (14, 33). It is likely, then, that in Anabaena sp. both genes, all4019 and all4018, are required for heterocyst function. Although the transcript level of all4019 was significantly higher 3, 8, and 24 h after nitrogen deprivation compared to a nitrate-grown culture (data in reference 7 analyzed by the method in reference 41), the mutant shows no unusual morphological phenotype (Fig. 1i). Interestingly, not only is zwf, an ortholog of all4019, required for heterocyst differentiation in N. punctiforme (32, 33), but akinete-like cells can also differentiate more abundantly in zwf mutants than in wild-type N. punctiforme (4).
alr4311.
alr4311, intercepted twice in our transposon mutagenesis experiments, encodes a putative ATP-binding cassette (ABC) protein. The gene is the last in a presumptive operon, downstream from three ORFs that encode hypothetical proteins. It is also immediately downstream from nrrA (all4312), a gene that on the opposite strand of DNA encodes for a nitrogen-responsive response regulator that is important in heterocyst differentiation (6, 7, 25). Strain FQ807, mutated in alr4311, was complemented by pRL3026, a plasmid that carries alr4311 as its only intact ORF, implying that alr4311 is a Fox gene. Heterocysts of FQ807 appear normal or (as in Fig. 1j) vacuolated. Although Alr4311 itself contains no predicted transmembrane domains, analysis of the predicted products, Alr4309 and Alr4310, of its two upstream genes show that they contain 4 and 3 transmembrane helices, respectively, suggesting that these three proteins may jointly compose a transporter (15). Because exporters normally contain the transmembrane domain and the ABC on a single polypeptide and ABC proteins that are not involved in transport normally contain two fused ABC domains, it is likely that Alr4311 with Alr4309 and/or Alr4310 function as an importer (5). Even if this were true, what they may transport is obscure.
all4388.
Intercepted by a transposon as described earlier (α21 [9, 22]) and intercepted 5 times in our more recent transposon mutagenesis experiments, all4388 encodes a putative polysaccharide transporter that was described as a Fox gene without the corresponding mutant having been complemented or reconstructed (22). As described in reference 22 for mutant α21 (Fig. 1l), the envelopes of heterocysts of FQ1595, which appear to retain a glycolipid layer, lack a polysaccharide layer according to light microscopy and staining with Alcian Blue. FQ1595 was complemented by pRL3009, whose only intact Anabaena sp. ORF is all4388. It was not complemented by pRL3142, which bears the downstream gene, all4387. These results support the proposition (22) that all4388 is a Fox gene. Expression of all4388 was upregulated 3, 8, and 24 h after nitrogen deprivation, whereas many of the genes required for deposition of HEP (17, 37) are first significantly upregulated at 8 h (data in reference 7 analyzed by the method in reference 41).
In summary, we have documented that 10 genes are required for heterocyst differentiation or function in Anabaena sp. These genes may be classified into three functional groups. (i) Presumptive regulatory genes include alr1728, whose sequence is similar to the sequences of known intramembrane proteolytic proteins, and all3520, which normally prevents internal division of heterocysts. (ii) Genes that are involved in the formation of structural elements of heterocysts include all3278, which may be involved in cell wall reconstruction; all4388, which is required for HEP deposition; and all1338, which may affect deposition of the heterocyst envelope. (iii) Genes that are involved in specialized heterocyst functions include all1591, presumably required for histidine biosynthesis in heterocysts; all3850, whose presumptive peroxidase heme-binding signature may be involved in sensing of reactive oxygen species or of O2 or in other redox reactions required for heterocyst function; all4019, encoding glucose-6-phosphate dehydrogenase, thought to be required in heterocysts for the oxidative pentose phosphate cycle and the transfer of electrons from sugar to respiration or to assimilation of N2 (33); and alr4311, which may encode an importer of an unidentified ligand. The role of all3582 remains obscure.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy grant DOE FG02-91ER20021.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black K., Buikema W. J., Haselkorn R. 1995. The hglK gene is required for localization of heterocyst-specific glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 177:6440–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buikema W. J., Haselkorn R. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321–330 [DOI] [PubMed] [Google Scholar]
- 4. Campbell E. L., Summers M. L., Christman H., Martin M. E., Meeks J. C. 2007. Global gene expression patterns of Nostoc punctiforme in steady-state dinitrogen-grown heterocyst-containing cultures and at single time points during the differentiation of akinetes and hormogonia. J. Bacteriol. 189:5247–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davidson A. L., Dassa E., Orelle C., Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72:317–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehira S., Ohmori M. 2006. NrrA directly regulates expression of hetR during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 188:8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehira S., Ohmori M. 2006. NrrA, a novel nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 59:1692–1703 [DOI] [PubMed] [Google Scholar]
- 8. Ellermeier C. D., Losick R. 2006. Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis. Genes Dev. 20:1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ernst A., et al. 1992. Synthesis of nitrogenase in mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst development or metabolism. J. Bacteriol. 174:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan Q., et al. 2005. Clustered genes required for synthesis and deposition of envelope glycolipids in Anabaena sp. strain PCC 7120. Mol. Microbiol. 58:227–243 [DOI] [PubMed] [Google Scholar]
- 11. Fan Q., et al. 2006. Signal transduction genes required for heterocyst maturation in Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6688–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felsenstein J. 1992. Estimating effective population size from samples of sequences: a bootstrap Monte Carlo integration method. Genet. Res. 60:209–220 [DOI] [PubMed] [Google Scholar]
- 13. Flores E., et al. 2007. Septum-localized protein required for filament integrity and diazotrophy in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:3884–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a. Gantar M., Elhai J., Jia J., Ow M. 1995 Abstr. 5th Cyanobacterial Mol. Biol. Workshop, p. 25. [Google Scholar]
- 14. Hagen K. D., Meeks J. C. 2001. The unique cyanobacterial protein OpcA is an allosteric effector of glucose-6-phosphate dehydrogenase in Nostoc punctiforme ATCC 29133. J. Biol. Chem. 276:11477–11486 [DOI] [PubMed] [Google Scholar]
- 15. Higgins C. F., et al. 1986. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323:448–450 [DOI] [PubMed] [Google Scholar]
- 16. Hoppe T., Rape M., Jentsch S. 2001. Membrane-bound transcription factors: regulated release by RIP or RUP. Curr. Opin. Cell Biol. 13:344–348 [DOI] [PubMed] [Google Scholar]
- 16a. Hu N. T., Thiel T., Giddings T. H., Wolk C. P. 1981. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology 114:236–246 [DOI] [PubMed] [Google Scholar]
- 17. Huang G., et al. 2005. Clustered genes required for the synthesis of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 187:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneko T., et al. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205–213 [DOI] [PubMed] [Google Scholar]
- 19. Leganés F., et al. 2005. Wide variation in the cyanobacterial complement of presumptive penicillin-binding proteins. Arch. Microbiol. 184:234–248 [DOI] [PubMed] [Google Scholar]
- 20. Liang J., Scappino L., Haselkorn R. 1992. The patA gene product, which contains a region similar to CheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. U. S. A. 89:5655–5659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynn M. E., Bantle J. A., Ownby J. D. 1986. Estimation of gene expression in heterocysts of Anabaena variabilis by using DNA-RNA hybridization. J. Bacteriol. 167:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maldener I., Hannus S., Kammerer M. 2003. Description of five mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst differentiation and identification of the transposon-tagged genes. FEMS Microbiol. Lett. 224:205–213 [DOI] [PubMed] [Google Scholar]
- 23. Masepohl B., Schölisch K., Görlitz K., Kutzki C., Böhme H. 1997. The heterocyst-specific fdxH gene product of the cyanobacterium Anabaena sp. PCC 7120 is important but not essential for nitrogen fixation. Mol. Gen. Genet. 253:770–776 [DOI] [PubMed] [Google Scholar]
- 24. Mella-Herrera R. A., Neunuebel M. R., Golden J. W. 2011. Anabaena sp. strain PCC 7120 conR contains a LytR-CpsA-Psr domain, is developmentally regulated, and is essential for diazotrophic growth and heterocyst morphogenesis. Microbiology 157:617–626 [DOI] [PubMed] [Google Scholar]
- 25. Muro-Pastor A. M., Olmedo-Verd E., Flores E. 2006. All4312, an NtcA-regulated two-component response regulator in Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 256:171–177 [DOI] [PubMed] [Google Scholar]
- 26. Nayar A. S., Yamaura H., Rajagopalan R., Risser D. D., Callahan S. M. 2007. FraG is necessary for filament integrity and heterocyst maturation in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 153:601–607 [DOI] [PubMed] [Google Scholar]
- 27. Pridmore R. D. 1987. New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309–312 [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg A., et al. 1987. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene 56:125–135 [DOI] [PubMed] [Google Scholar]
- 29. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 30. Schwarz R., Dayhoff M. 1979. Matrices for detecting distant relationships, p. 353–358 In Dayhoff M. (ed.), Atlas of protein sequences. National Biomedical Research Foundation, New York, NY [Google Scholar]
- 31. Shi L., et al. 2007. Two genes encoding protein kinases of the HstK family are involved in synthesis of the minor heterocyst-specific glycolipid in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:5075–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Summers M. L., Meeks J. C. 1996. Transcriptional regulation of zwf, encoding glucose-6-phosphate dehydrogenase, from the cyanobacterium Nostoc punctiforme strain ATCC 29133. Mol. Microbiol. 22:473–480 [DOI] [PubMed] [Google Scholar]
- 33. Summers M. L., Wallis J. G., Campbell E. L., Meeks J. C. 1995. Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J. Bacteriol. 177:6184–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 35. Tatusov R. L., et al. 2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 29:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valladares A., Herrero A., Pils D., Schmetterer G., Flores E. 2003. Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol. Microbiol. 47:1239–1249 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y., et al. 2007. Predicted glycosyl transferase genes located outside the HEP island are required for formation of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 189:5372–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolk C. P. 2000. Heterocyst formation in Anabaena, p. 83–104 In Brun Y. V., Shimkets L. J. (ed.), Prokaryotic development. ASM Press, Washington, DC [Google Scholar]
- 39. Wolk C. P., Ernst A., Elhai J. 1994. Heterocyst metabolism and development, p. 769–823 In Bryant D. A. (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 40. Wolk C. P., et al. 2007. Paired cloning vectors for complementation of mutations in the cyanobacterium Anabaena sp. strain PCC 7120. Arch. Microbiol. 188:551–563 [DOI] [PubMed] [Google Scholar]
- 41. Xu X., Elhai J., Wolk C. P. 2008. Transcriptional and developmental responses by Anabaena to deprivation of fixed nitrogen, p. 383–422 In Herrero A., Flores E. (ed.), Genomics and molecular biology of cyanobacteria. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 42. Xu X., Khudyakov I., Wolk C. P. 1997. Lipopolysaccharide dependence of cyanophage sensitivity and aerobic nitrogen fixation in Anabaena sp. strain PCC 7120. J. Bacteriol. 179:2884–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanisch-Perron C., Vieira J., Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 44. Yoon H.-S., Golden J. W. 2001. PatS and products of nitrogen fixation control heterocyst pattern. J. Bacteriol. 183:2605–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu J., et al. 2001. HcwA, an autolysin, is required for heterocyst maturation in Anabaena sp. strain PCC 7120. J. Bacteriol. 183:6841–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.