Abstract
The internalization and degradation of vascular endothelial growth factor receptor 2 (VEGFR-2), a potent angiogenic receptor tyrosine kinase, is a central mechanism for the regulation of the coordinated action of VEGF in angiogenesis. Here, we show that VEGFR-2 is ubiquitinated in response to VEGF, and Lys 48-linked polyubiquitination controls its degradation via the 26S proteosome. The degradation and ubiquitination of VEGFR-2 is controlled by its PEST domain, and the phosphorylation of Ser1188/Ser1191 is required for the ubiquitination of VEGFR-2. F-box-containing β-Trcp1 ubiquitin E3 ligase is recruited to S1188/S1191 VEGFR-2 and mediates the ubiquitination and degradation of VEGFR-2. The PEST domain also controls the activation of p38 mitogen-activated protein kinase (MAPK) through phospho-Y1173. The activation of p38 stabilizes VEGFR-2, and its inactivation accelerates VEGFR-2 downregulation. The VEGFR-2-mediated activation of p38 is established through the protein kinase A (PKA)/MKK6 pathway. PKA is recruited to VEGFR-2 through AKAP1/AKAP149, and its phosphorylation requires Y1173 of VEGFR-2. The study has identified a unique mechanism in which VEGFR-2 stability and degradation is modulated. The PEST domain acts as a dual modulator of VEGFR-2; the phosphorylation of S1188/S1191 controls ubiquitination and degradation via β-Trcp1, where the phosphorylation of Y1173 through PKA/p38 MAPK controls the stability of VEGFR-2.
INTRODUCTION
The accurate and specific signaling by receptor tyrosine kinases (RTKs) requires that intracellular signals emanated by RTK qualitatively and quantitatively are monitored by tight regulatory mechanisms. The ubiquitin-mediated degradation of RTKs in eukaryotic cells is a common method that has evolved to fine-tune RTK signaling (20). Vascular endothelial growth factor receptor 2 (VEGFR-2) is the main endothelial cell RTK involved in angiogenesis, and upon binding to VEGF family ligands VEGFR-2 forms a dimer, resulting in its increased tyrosine kinase activity and the phosphorylation of multiple cytoplasmic tyrosine residues (29). Upon phosphorylation, these tyrosine sites provide docking sites for downstream effectors, such as phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ1 (PLCγ1), Src kinases, IQGAP1, and c-Cbl (6, 22, 29). c-Cbl interacts with phospho-Y1054 and phospho-Y1057, which are located in the kinase domain of VEGFR-2, mediates the ubiquitination of PLCγ1, the major substrate of VEGFR-2, and uniquely suppresses the tyrosine phosphorylation of PLCγ1 without degradation (13, 23, 37). Interestingly, c-Cbl activity is dispensable for VEGFR-2 ubiquitination and degradation (37, 38). Given the critical role of VEGFR-2 signaling during embryonic development and diverse pathological conditions, ranging from cancer to age-related macular degeneration (4, 8), the regulation of VEGFR-2 activation, particularly through posttranslational modification, represents an important rate-limiting mechanism for angiogenesis. VEGFR-2 has been shown to be ubiquitinated in endothelial cells in response to VEGF stimulation (38). However, the mechanisms that regulate the ubiquitination of VEGFR-2, as well as the exact mechanisms by which VEGFR-2 ubiquitination impacts its function, is not clear. Hence, a systematic analysis of the molecular pathways determining the cellular outcome of VEGFR-2 activation is required for the development of antiangiogenic therapies based on these signaling cascades.
Central to the proper regulation of the angiogenic activity of VEGFR-2 is the process by which VEGFR-2 triggers its own internalization and degradation, consequently terminating its angiogenic signaling. VEGFR-2 associates with caveolin-1 in cholesterol-rich endothelial cell membranes, which are thought to mediate its clathrin-independent endocytosis (2, 17). Upon stimulation with VEGF family proteins, VEGFR-2 subsequently is removed from the cell membrane, dissociates from caveolin-1 (2, 14), and undergoes incompletely understood processes through clathrin-dependent endocytosis (19), degradation (38), and recycling (9). The tyrosine kinase activity of VEGFR-2 and the activation of the protein kinase C (PKC) pathway are required for the efficient degradation of VEGFR-2 (38). The progressive deletion of the carboxyl terminal of VEGFR-2 has been shown to inhibit the ligand-dependent degradation of VEGFR-2 (24), suggesting that the carboxyl terminal, through posttranslational modification and recruitment of signaling proteins to VEGFR-2, significantly controls the stability and signaling of VEGFR-2. Moreover, the ligand-dependent degradation of VEGFR-2 occurs through a mechanism independent of regulated intramembrane proteolysis (RIP) processing, and the presence of the carboxyl-terminal region is required for the efficient PKC-mediated degradation of VEGFR-2 (38).
Ubiquitin-mediated proteasomal degradation is a common mechanism to control protein homeostasis, and a recent study indicates that VEGFR-2 is ubiquitinated, suggesting that the degradation of VEGFR-2 is linked to its degradation (38). The presence of the PEST motif (rich in proline [P], glutamic acid [E], serine [S], and threonine [T]) is considered a signature of short-lived proteins degraded by the ubiquitin pathway (32). It is thought that PEST sequences are unstructured regions in certain protein sequences, possibly serving as a phosphodegron for the recruitment of F-box-containing ubiquitin E3 ligases that lead to ubiquitination and degradation (5, 49). In this study, we demonstrate that VEGFR-2 contains a unique PEST domain-like sequence that regulates the ubiquitination and downregulation of VEGFR-2. Phosphoserine 1188/Ser1191 of the PEST domain is required for ubiquitination and degradation, where phosphotyrosine 1173, through the protein kinase A (PKA)/p38 mitogen-activated protein kinase (MAPK) pathway, attenuates the degradation of VEGFR-2. Hence, the PEST domain-based dual regulation of VEGFR-2 downregulation provides novel insights into the regulatory mechanism of the angiogenic signaling of VEGFR-2.
MATERIALS AND METHODS
Growth factors and antibodies.
Recombinant murine vascular endothelial growth factor A-164 (recVEGF-A164) was purchased from R&D Systems (Minneapolis, MN). Anti-phospho-KDR/Flk-1/VEGFR2 (Tyr1054), clone D1W, anti-phosphotyrosine 4G10 (IgG2b), anti-KDR/Flk-1/VEGFR2, and clone CH-11 were purchased from Millipore (Temecula, CA). Anti-FLAG M2 antibody was purchased from Stratagene (La Jolla, CA). Mouse monoclonal antibody to ubiquitylated proteins (clone FK2; IgG1) was purchased from BIOMOL International (Plymouth Meeting, PA). Rabbit polyclonal anti-phospholipase Cγ1 antibody (pY783) was purchased from Biosource (Camarillo, CA). Rabbit polyclonal anti-VEGFR-2 sera were raised against either a glutathione S-transferase (GST)–VEGFR-2 kinase insert domain fusion protein (1410) or a GST-VEGFR–2 carboxyl-terminal fusion protein (28). Phospho-Ser1188 antibody was made against phosphorylated Ser1188 peptide and purified through a protein A-agarose column. The following antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): preadsorbed goat anti-rabbit IgG (sc-2054), goat anti-mouse IgG (sc-2055), and donkey anti-goat IgG (sc-2020) secondary antibodies conjugated to horseradish peroxidase (HRP), anti-ERK1 (sc-94), anti-PLCγ1 (sc-81), anti-glycogen synthase kinase 3α/β (anti-GSK3α/β) (sc-7291), anti-PKAα cat (sc-28315), anti-MEK-6 (sc-1991), and anti-p38α/β (sc-7149). Anti-phospho-MAPK (pT202/pY204), anti-phospho-PKA C (Thr197), and anti-phospho-p38 MAPK (Thr180/Tyr182; 28B10) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-β-catenin antibody was kindly provided by I. Dominguez (Boston University Medical Campus).
Plasmids and siRNAs.
The following plasmids were purchased from Addgene: pcDNA3-EGFP-Cdc42(WT) and pcDNA3-EGFP-Cdc42 (3); pCDNA3-Flag MKK6(glu), pCDNA3-Flag MKK6, and pCDNA3-Flag MKK6(K82A) (30); pCalpha EV (PKA catalytic subunit Cα) (44); M7pdnPKA-GFP (45); and pCDNA3 Flag p38α (agf) pCDNA3 Flag p38 β2 and pCDNA3 Flag p38 β2 (7). pMT3 p38 (from the J. Kyriakis laboratory) and hemagglutinin (HA) GSK3β S9A pcDNA3 (40) also were used. A-kinase anchoring protein 1 (AKAP1), also called AKAP149/AKAP121 (clone 30549315), was purchased from Open Biosystems and was further cloned into pcDNA3.1/Myc-His(−)B via NotI and EcoRI sites. GSK3β-pCMV-SPORT6 (clone 2648507) was purchased from Open Biosystems. All of the F-box-containing E3 ligase cDNAs used in the manuscript were generously provided by J. W. Harper (Department of Pathology, Harvard Medical School, Boston, MA) (47). Flag-tagged β-Trcp1 and F-box-deleted Flag-tagged β-Trcp1 plasmids were kindly provided by Michele Pagano (Cancer Institute, NYU). Wild-type ubiquitin and lysine mutant constructs were generously provided by Ted Dawson (Johns Hopkins University, Institute for Cell Engineering, Baltimore, MD) and Cam Paterson (University of North Carolina). Short hairpin RNA (shRNA) β-Trcp1, β-Trcp2, and pSuper.puro plasmid were generously provided by A. J. Singh and R. Koshravi-Far (Beth Israel Deaconess Medical Center, Boston, MA). p38α small interfering RNA (siRNA) was purchased from Santa Cruz Inc.
Immunoprecipitation and Western blot analysis.
Cells were prepared for immunoprecipitation as described previously (24). Briefly, cells were grown in 10-cm culture dishes until 80 to 90% confluence, and after serum starvation cells were left resting or were stimulated with VEGF or as indicated in the figure legends. Cells were lysed, and normalized whole-cell lysates were subjected to immunoprecipitation by incubation with appropriate antibodies. Immunocomplexes were captured by incubation with either protein A-Sepharose or protein G-agarose beads, and immunoprecipitated protein was subjected to Western blot analysis. Occasionally, membranes were stripped by incubating them in a stripping buffer containing 6.25 mM Tris-HCl, pH 6.8, 2% SDS, and 100 mM β-mercaptoethanol at 50°C for 30 min, washed in Western rinse, and reprobed with the antibody of interest. On some occasions the blots were scanned and subsequently quantified using ImageJ (NIH).
Site-directed mutagenesis.
Site-directed mutagenesis was performed using a PCR-based site-directed mutagenesis strategy (22, 24). The identity of mutations was confirmed by sequencing the plasmids. cDNAs were cloned into pcDNA3.1His.Myc vector or into retroviral vector pLNCX2. In some cases, the PCR products were cloned into pGEX2T vector and used to make a GST fusion protein in Escherichia coli. For virus production, the retroviral vector was transfected into 293-GPG cells and viral supernatants were collected for 5 days, concentrated by centrifugation, and used as previously described (27).
GST pulldown assay.
In vitro GST fusion protein binding experiments were performed as described previously (27). Briefly, equal numbers of HEK293 cells expressing VEGFR-2 were grown to 90% confluence prior to serum starvation overnight. Unstimulated or ligand-stimulated cells were lysed in ice-cold lysis buffer supplemented with 2 mM Na3VO4 and a protease inhibitor cocktail. Equal amounts of the appropriate immobilized GST fusion proteins were incubated with normalized whole-cell lysates by rocking for 3 h at 4°C. The beads were washed in the presence of protease inhibitors and Na3VO4, and proteins were eluted and analyzed by Western blotting using appropriate antibody.
Cell culture.
Three cell lines were used in this study: PAE (porcine aortic endothelial cells) and HEK293 cells were grown in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) plus antibiotics, and HUVEC (primary human umbilical vascular endothelial cells) were grown in HUVEC growth medium. PAE cells were established to express VEGFR-2 (28). PAE cells expressing PEST(−)VEGFR-2, PEST(+)VEGFR-2, and serine mutant constructs were made as previously described using PCR-based site-directed mutagenesis (24, 38). All of the transient-transfection experiments were performed in HEK293 cells as indicated in the figure legends. For silencing the expression of p38 in HUVEC cells, we used p38α siRNA (h), which was a pool of four target-specific 19- to 25-nucleotide (nt) siRNAs designed to knock down the expression of p38α, and it was purchased from Santa Cruz, Inc. HUVEC were transfected according to the manufacturer's recommendations. The Matrigel assay was performed as described elsewhere (23).
Downregulation assay.
The downregulation of VEGFR-2 was performed as described previously (37, 38). Briefly, serum-starved cells were pretreated with cycloheximide (20 mM for 90 min), followed by stimulation with VEGF for various time points as indicated in the figure legends. Also, in certain experiments cells were further incubated with inhibitor or other pharmacological inhibitors as outlined in the figure legends. Cells were lysed, and whole-cell lysates were subjected to Western blot analysis using anti-VEGFR-2 antibody.
Immunofluorescence microscopy.
Serum-free PAE cells expressing VEGFR-2, PEST(−)VEGFR-2, and PEST(+)VEGFR-2 either were stimulated with VEGF for 10 min or were left unstimulated. Cells were fixed with 4% paraformaldehyde for 30 min, washed with PBS (three times), and permeabilized with 0.01% Triton X-100 for 1 min. Cells were incubated with 5% bovine serum albumin (BSA) for 45 min with rocking and then incubated with anti-VEGFR-2 antibody. After extensive washes and incubation with secondary antibody, they were mounted with antifade/4′,6′-diamidino-2-phenylindole (DAPI) (Vectastain). Confocal microscopy was performed as described elsewhere (23). Fluorophores were excited using the 405-nm line of a diode laser (DAPI) and the 488-nm line of a Kr/Ar laser (Alexa 488, Cy2).
RESULTS
K48-linked polyubiquitination controls VEGFR-2 degradation.
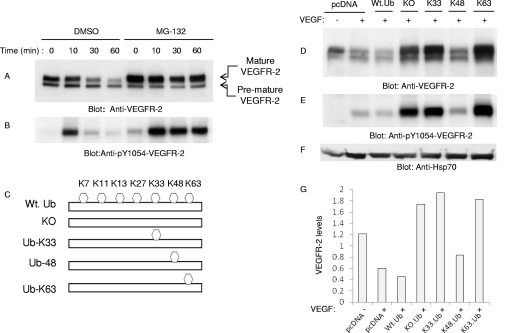
Ubiquitinated proteins often are targeted for 26S proteasome- and lysosome-dependent degradation. Our initial observation indicated that the treatment of cells with MG132, a potent inhibitor of 26S proteasome, significantly inhibits the degradation of VEGFR-2, suggesting that VEGFR-2 is specifically targeted for 26S proteasome-dependent degradation (Fig. 1 A). MG132 also selectively induced the accumulation of mature VEGFR-2 without altering the biogenesis of its premature form (Fig. 1A). Also, bortezomib, another 26S proteasome inhibitor, similarly inhibited the ligand-mediated degradation of VEGFR-2 (data not shown). MG132-dependent inhibition of degradation of VEGFR-2 also resulted in the accumulation of activated VEGFR-2 protein, as was demonstrated by phospho-Y1054-VEGFR-2-specific antibody (Fig. 1B). Tyrosine 1054 is located in the kinase domain of VEGFR-2, and its phosphorylation is required for the activation of VEGFR-2 (22).
Fig. 1.
VEGFR-2 undergoes Lys 48-dependent proteasomal degradation. Serum-starved PAE cells expressing VEGFR-2 were preincubated with dimethylsulfoxide (DMSO) or with MG132 for 30 min, and then cells were left unstimulated (0) or were stimulated with VEGF for 10 and 30 min. Whole-cell lysates were immunoblotted with anti-VEGFR-2 antibody (A) or with anti-phospho-Y1054-VEGFR-2 antibody (B). HEK293 cells expressing VEGFR-2 were transfected with an empty vector (pcDNA 3.1His.Myc) or with ubiquitin constructs, including wild-type ubiquitin (Wt. Ub), a Lys mutant ubiquitin in which all of the lysines were mutated (KO), and ubiquitin constructs containing only Lys 33 (Ub-K33), Lys 48 (Ub-K48), or Lys63 (Ub-K63) in HEK293 cells. Cells were left unstimulated (−) or were stimulated with VEGF for 10 min (+), and whole-cell lysates were immunoblotted with anti-VEGFR-2 antibody (D), anti-phospho-Y1054-VEGFR-2 antibody (E), and anti-Hsp70 antibody for protein loading (F). (G) The quantification of the downregulation of VEGFR-2 by ubiquitin constructs.
Ubiquitin molecules can be attached to a target protein either by means of monoubiquitination (i.e., the attachment of a single ubiquitin to one or multiple lysine residues), or ubiquitin can be attached to a target protein in the form of polyubiquitination, where multiple ubiquitin molecules are attached to a single lysine residue (31). There are seven different lysine residues in ubiquitin that can be employed for ubiquitin chain assembly. Lys48- and Lys29-linked polyubiquitination generally is associated with the degradation of target proteins by the 26S proteasome (31). VEGFR-2 undergoes both mono- and polyubiquitination, as detected by monoubiquitin- and polyubiquitin-specific antibodies (data not shown). To test the role of polyubiquitination and specific lysine residues involved in the ligand-mediated degradation of VEGFR-2, we coexpressed VEGFR-2 with wild-type ubiquitin or various lysine mutant ubiquitin constructs in HEK293 cells and analyzed the downregulation of VEGFR-2. In principle, if the degradation of VEGFR-2 requires polyubiquitin chain formation, the overexpression of forms of ubiquitin constructs harboring one or all lysine residues mutated is expected to terminate chain assembly formation and, hence, to inhibit the degradation of VEGFR-2 (i.e., acting in a dominant-negative fashion). The coexpression of a KO-ubiquitin construct with VEGFR-2, lacking all of the lysine residues, resulted in the accumulation of VEGFR-2 (Fig. 1D), suggesting that the polyubiquitination of VEGFR-2 targets VEGFR-2 for degradation. The coexpression of VEGFR-2 with ubiquitin K33 (K33-Ub) and K63 (K63-Ub), where all of the lysine residues except K33 and K63 were mutated to arginine, also prevented the degradation of VEGFR-2, similarly to the KO-ubiquitin construct. This suggests that K33- and K63-linked polyubiquitination are not involved in the degradation of VEGFR-2 (Fig. 1D). Unlike K33 and K63 ubiquitin constructs, the coexpression of VEGFR-2 with the K48 ubiquitin construct, where all of the lysine residues except K48 are mutated to arginine, did not prevent the downregulation of VEGFR-2, suggesting that the K48-linked polyubiquitination of VEGFR-2 mediates its degradation (Fig. 1D). The quantification of the downregulation of VEGFR-2 by ubiquitin constructs also is shown (Fig. 1G). Furthermore, the coexpression of the ubiquitin construct in which only K48 is mutated (K48R) and the rest of lysine residues were present also inhibited the degradation of VEGFR-2 (data not shown). Consistently with the role of the K48-mediated downregulation of VEGFR-2, ubiquitin constructs, including KO, K33, and K63 but not K48, all increased the active form of VEGFR-2, as measured by an anti-phospho-Y1054-VEGFR-2 antibody (Fig. 1E). The data indicate that VEGFR-2 degradation is mediated primarily by K48-linked polyubiquitination.
PEST domain controls VEGFR-2 degradation.
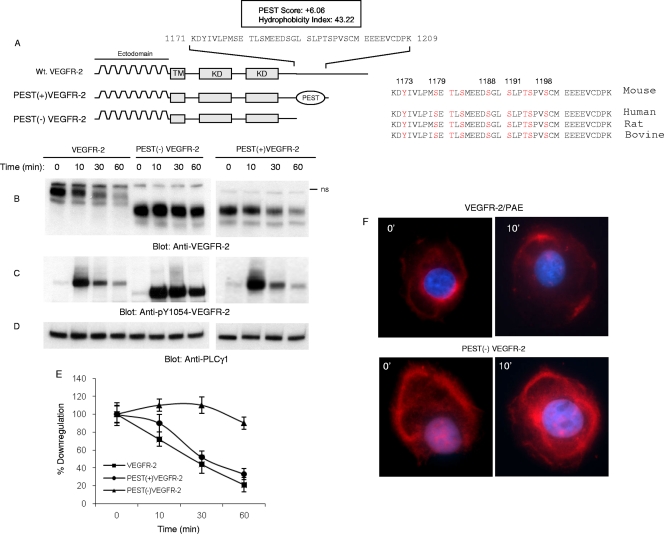
The presence of a carboxyl terminal of VEGFR-2 encompassing 55 amino acids distal to the kinase domain is required for the downregulation of VEGFR-2 (38). A closer look at the primary amino acid sequences of VEGFR-2 and the use of an online computational prediction program provided evidence for the existence of a putative PEST domain in the carboxyl terminus of VEGFR-2. The PEST domain is a polypeptide sequence rich in proline (P), glutamic acid (E), serine (S), and threonine (T) and commonly is flanked by positively charged residues such as K (lysine), R (arginine), or H (histidine) (32). The PEST domain is thought to act as a signal for the proteolytic degradation of certain proteins via the proteasome pathway involving ubiquitin E3 ligases (10, 33). The computational analysis of VEGFR-2 by the PESTFIND online prediction program (http://www.at.embnet.org/toolbox/pestfind/) revealed two possible PEST domains in mouse VEGFR-2 protein. The first PEST region was predicted at amino acids 1171 to 1209 with a score of +6.06. The second PEST domain was predicted at amino acids 1235 to 1248 with a score of +4.1 (Fig. 2 A). A score greater than +5 is considered a likely PEST domain. The PEST domain on VEGFR-2 encompassing residues 1171 to 1209 also displays a low hydrophobicity index of 43.2, suggesting that this region is surface exposed and accessible for a protein-protein interaction and serine and threonine phosphorylation. Indeed, from the 39 amino acids (residues 1171 to 1209), 8 amino acids (i.e., 21%) correspond to serine and threonine. The alignment of mouse VEGFR-2 with other species showed that this region is highly conserved among the mouse, human, rat, and bovine VEGFR-2 proteins (Fig. 2A), suggesting a regulatory role for this region in VEGFR-2 function. The second putative PEST domain (amino acids 1235 to 1248) with a lower PEST score (+4.1) was not considered for further studies. Our previous work also has shown that the presence of this region is not required for the downregulation of VEGFR-2 (38).
Fig. 2.
PEST domain is required for ligand-mediated degradation of VEGFR-2. The schematic of VEGFR-2 and the presence of the putative PEST domain are shown. The PEST domain was predicted using the online program PESTFIND as outlined in Materials and Methods. The schematic of truncated VEGFR-2 encompassing PEST domain PEST(+)VEGFR-2 and PEST domain-deleted VEGFR-2, PEST(−)VEGFR-2, also are shown. (A) The PEST domain is conserved among mouse, human, bovine, and rat VEGFR-2. Wild-type VEGFR-2, PEST(+)VEGFR-2, and PEST(−)VEGFR-2 were expressed in PAE cells by a retroviral system, and their downregulation in response to VEGF in a time-dependent manner was measured. (B) Whole-cell lysates were subjected to Western blot analysis using anti-VEGFR-2 antibody. ns, nonspecific. The same cell lysates were blotted with anti-pY1054-VEGFR-2 antibody (C) and anti-PLCγ1 antibody (D). (E) The quantification of the downregulation of VEGFR-2, PEST(+)VEGFR-2, and PEST(−)VEGFR-2 in response to VEGF. The graph is an average from two independent experiments. (F) Confocal microscopy of VEGFR-2, PEST(+)VEGFR-2, and PEST(−)VEGFR-2 stimulated with VEGF for 10 min or left unstimulated (0).
To test the possible function of the PEST domain (residues 1171 to 1209) in the degradation of VEGFR-2, we constructed two VEGFR-2 truncated constructs, PEST(+)VEGFR-2, which harbors a PEST domain, and PEST(−)VEGFR-2, in which the PEST domain is deleted from the carboxyl terminus (Fig. 2A). Subsequently, wild-type VEGFR-2 and C-terminal-truncated VEGFR-2 were expressed in PAE (porcine aortic endothelial) cells by a retroviral system and analyzed for their downregulation in response to stimulation with VEGF-A. The stimulation of wild-type VEGFR-2 and PEST (+)VEGFR-2 with VEGF-A induced the time-dependent downregulation of VEGFR-2, whereas PEST(−)VEGFR-2 persisted in VEGF-A-induced downregulation (Fig. 2B and E), suggesting that a PEST domain is required for the downregulation of VEGFR-2. Consistently with its sustained stabilization, PEST(−)VEGFR-2 showed robust tyrosine phosphorylation in response to stimulation with VEGF-A (Fig. 2C).
To determine the effect of the loss of the PEST domain in VEGFR-2 localization, we analyzed the cellular localization of VEGFR-2 using immunofluorescence confocal microscopy. As shown, PEST(−)VEGFR-2 is resistant to ligand-stimulated downregulation and is accumulated largely in various intracellular compartments (Fig. 2F). The cellular localization of PEST(+)VEGFR-2 was similar to that of wild-type VEGFR-2, and upon stimulation with VEGF for 10 min, they were largely gone from membrane areas (Fig. 2F). As recently reported, the majority of VEGFR-2 protein is not cell surface exposed but rather is present in the endosomal compartments (9). Taken together, the data suggest that the presence of the PEST domain is required for the degradation of VEGFR-2.
The presence of a PEST domain is required for the ubiquitination of VEGFR-2.
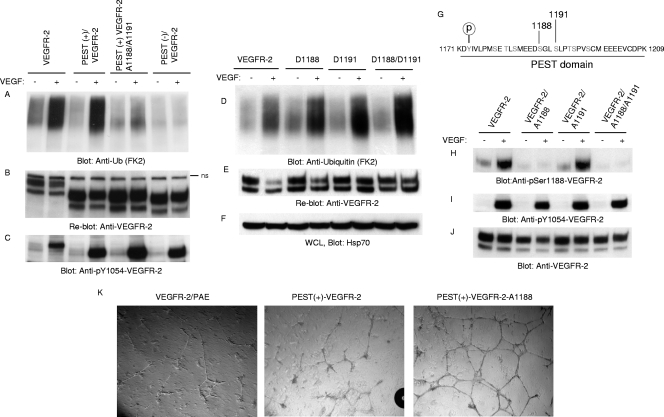
To test whether the PEST domain of VEGFR-2 is required for its ubiquitination, we analyzed the VEGF-induced ubiquitination of VEGFR-2. The results showed that the deletion of the PEST domain abolishes the ubiquitination of VEGFR-2, whereas preserving the PEST domain allows the full ubiquitination of VEGFR-2. Indeed, wild-type VEGFR-2 and PEST(+)VEGFR-2 (encompassing the PEST domain) were ubiquitinated equally (Fig. 3 A). Moreover, the mutation of two putative serine phosphorylation sites, namely serine 1188 and serine 1191, significantly reduced the ability of PEST(+)VEGFR-2 to undergo ubiquitination, suggesting that the phosphorylation of these sites is important for the ubiquitination of VEGFR-2 (Fig. 3A). The mutation of serine 1188 alone also significantly reduced the ubiquitination of VEGFR-2 in the context of PEST(+)VEGFR-2, where the mutation of serine 1191 alone just moderately reduced the ubiquitination of VEGFR-2 (data not shown), suggesting that Ser1188 plays a more important role than Ser1191. Moreover, the mutation of Ser1191 along with Thr1194, Ser1195, and Ser1198 had no significant effect on the ubiquitination of VEGFR-2 (data not shown). Moreover, the mutation of Ser1188 and Ser1191 in the context of full-length VEGFR-2 also markedly reduced the ubiquitination of VEGFR-2 (data not shown). Taken together, the data show that the ubiquitination of VEGFR-2 is regulated by the presence of the PEST domain, and Ser1188 and Ser1191 play a central role in the PEST domain-dependent ubiquitination of VEGFR-2.
Fig. 3.
Phosphorylation of serine 1188 is required for ubiquitination of VEGFR-2. (A) VEGFR-2, PEST(+)VEGFR-2, PEST(−)VEGFR-2, and PEST(+)VEGFR-2/A1188/A1191 (where Ser1188 and Ser1191 were mutated to Ala) were stimulated with VEGF for 10 min (+) or were left unstimulated (−), and cells were lysed, immunoprecipitated with anti-VEGFR-2 antibody, and immunoblotted with antiubiquitin (Anti-Ub; FK2) antibody. (B) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (C) Whole-cell lysates were blotted for phospho-Y1054-VEGFR-2. (D) PAE cells expressing VEGFR-2, D1188/VEGFR-2, D1191/VEGFR-2, and D1188/D1191/VEGFR-2 (Ser-to-Asp mutation) were stimulated as described for panel A and blotted with antiubiquitin antibody. (E) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (F) Whole-cell lysates (WCL) shown in panel D were blotted with anti-Hsp70 for internal protein levels. (G) Putative phosphorylation sites in the PEST domain are shown. PAE cells expressing wild-type VEGFR-2, VEGFR-2/A1188, VEGFR-2/A1188/A1191, and VEGFR-2/A1191 were stimulated with VEGF, and whole-cell lysates were blotted with anti-phospho-Ser1188-VEGFR-2 antibody (H), phospho-Y1054-VEGFR-2 antibody (F), and anti-VEGFR-2 antibody (I). (J) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (K) PAE cells expressing wild-type VEGFR-2, PEST(+)VEGFR-2, or PEST(+)VEGFR-2/A1188 (in which serine 1188 is mutated to alanine) were plated on the 24-well plates coated with Matrigel in the presence of VEGF, and pictures were taken under a light microscope equipped with a digital camera.
To further corroborate the data obtained from mutations of serine to alanine, we also created a phosphomimetic mutation in which Ser1188 and Ser1191 were mutated to aspartic acid alone or together, expressed in PAE cells, and analyzed for their effect on the ubiquitination of VEGFR-2. Phosphomimetic mutations of Ser1188 and Ser1191, in contrast to mutation to alanine, not only did not inhibit the ubiquitination of VEGFR-2 but also significantly increased the ubiquitination of VEGFR-2 (Fig. 3D). Taken together, the data strongly suggest that the phosphorylation of Ser1188 and Ser1191 is important for the ubiquitination of VEGFR-2.
Serine 1188 of VEGFR-2 is phosphorylated in vivo, and its mutation increases angiogenesis.
The PEST domain is highly enriched in serine and threonine residues. There are eight possible serine and threonine phosphorylation sites in this region of VEGFR-2 (Fig. 3G). The mutation of serines 1188 and 1191 severely impaired the ubiquitination of VEGFR-2 (Fig. 3A), suggesting that these sites serve as a phosphodegron for the recruitment of F-box-containing ubiquitin E3 ligases, leading to the ubiquitination of VEGFR-2. To examine whether these sites are phosphorylated in vivo, we raised rabbit polyclonal antibodies against a peptide corresponding to the phosphoserine 1188 and phosphoserine 1191 antibodies. After initial enzyme-linked immunosorbent assay (ELISA) analysis, it was found that the sera containing anti-phosphoserine 1191 antibody contained no significant antibody, whereas sera containing anti-phospho-serine 1188 antibody were found to be worth purifying for further analysis. The initial analysis showed that this antibody recognizes ligand-stimulated VEGFR-2 in Western blotting, and that recognition was inhibited by the preincubation of the antibody with a synthesized phosphoserine 1188-containing peptide (data not shown).
To test the status of the phosphorylation of serine 1188 on VEGFR-2, PAE cells expressing wild-type VEGFR-2, and serine 1188 and serine 1191 mutant VEGFR-2s, in which these residues, individually or together, were mutated to alanine (A), were stimulated with VEGF and analyzed for the phosphorylation of serine 1188. The results showed that serine 1188 is phosphorylated on VEGFR-2 upon stimulation with VEGF, and the mutation of serine 1188 to alanine abolished the ability of the phosphoserine 1188 antibody to recognize VEGFR-2, whereas the mutation of serine 1191 did not impair the phosphorylation of VEGFR-2 on serine 1188 (Fig. 3H). Also, the mutation of neither of these sites affected the tyrosine phosphorylation of VEGFR-2, as detected by anti-phospho-pY1054 antibody (Fig. 3I). Taken together, the data suggest that Ser1188 is phosphorylated in vivo, and its phosphorylation significantly contributes to the ubiquitination of VEGFR-2. To determine whether the phosphorylation of Ser1188 requires the kinase activation of VEGFR-2, we examined the phosphorylation of Ser1188 in the background of kinase-dead R866/VEGFR-2, where ATP binding site K866 was mutated to R (28). The data showed that Ser1188 phosphorylation is inhibited in response to ligand stimulation (data not shown), suggesting that the activation of VEGFR-2 is required for the subsequent phosphorylation of Ser1188.
To establish the biological significance of Ser1188 phosphorylation in VEGFR-2-driven cellular responses, we decided to analyze the angiogenic phenotype of PAE cells expressing wild-type VEGFR-2, PEST(+)VEGFR-2, or PEST(+)VEGFR-2/A1188 (where serine 1188 is mutated to alanine). As shown, both wild-type VEGFR-2 and PEST(+)VEGFR-2 were able to promote in vitro angiogenesis in a similar manner. However, Ser1188 mutant VEGFR-2 stimulated a robust angiogenic response compared to that of wild-type VEGFR-2 and PEST(+)VEGFR-2 (Fig. 3K). These findings demonstrate that the phosphorylation of Ser1188 is biologically important for fine-tuning the angiogenic function of VEGFR-2, and the prevention of it from phosphorylation elevates the angiogenic capacity of VEGFR-2.
β-Trcp1 mediates ubiquitination of VEGFR-2.
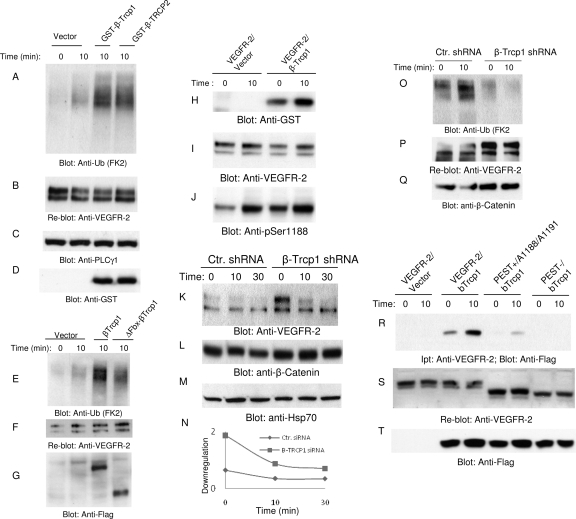
The presence of a PEST domain and the phosphorylation of Ser1188 within the PEST domain is required for the ubiquitination of VEGFR-2, suggesting that Ser1188 serves as a phosphodegron motif by recruiting certain F-box-containing ubiquitin E3 ligases to VEGFR-2, leading to the ubiquitination of VEGFR-2. F-box-containing ubiquitin E3 ligases such as β-Trcp are known for their ability to recognize phosphodegron (DpSGXXpS, where X represents any amino acid and p represents phosphorylation) sequences (36, 49). Interestingly, the sequence of Ser1188 (DS1188GL S1191) of VEGFR-2 is highly homologous to the known F-box recognition phosphodegron, the DpSGXXpS motif. Hence, we examined the putative role of β-Trcp in the ubiquitination of VEGFR-2. The coexpression of β-Trcp1 or β-Trcp2 with VEGFR-2 in HEK293 cells significantly increased the ubiquitination of VEGFR-2 (Fig. 4 A). The coexpression of F-box-deleted β-Trcp1 with VEGFR-2 did not increase the ubiquitination of VEGFR-2 (Fig. 4E). The β-Trcp1-mediated ubiquitination of VEGFR-2 appeared to be specific, since the coexpression of other F-box-containing E3 ligases, including Fbw7γ, Fbw7β, Fbw7α, Fbl3α, Fbl4, Fbl17, Fbl18, Fbx8, Fbx12, Fbx16, and Fbx37, had no effect on the ubiquitination of VEGFR-2, suggesting that β-Trcp is a main ubiquitin E3 ligase involved in the ubiquitination of VEGFR-2 (data not shown).
Fig. 4.
β-Trcp1 associates with VEGFR-2 and targets it for degradation. HEK293 cells expressing VEGFR-2 were transfected with an empty vector, GST-β-Trcp1, or with GST-β-Trcp2. (A) Cells were unstimulated (0) or stimulated with VEGF for 10 min, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with antiubiquitin (Anti-Ub; FK2) antibody. (B) The same membrane was reblotted with anti-VEGFR-2. Whole-cell lysates from panel A were blotted with anti-PLCγ1 (C) and anti-GST antibody (D). (E) HEK293 cells coexpressing VEGFR-2 with empty vector, with β-Trcp1, or with F-box-deleted β-Trcp1(ΔFbx-β-Trcp1) were left unstimulated (0) or stimulated for 10 min (8) with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with antiubiquitin antibody. (F) The same membrane was reblotted for VEGFR-2. (G) Whole-cell lysates (WCL) from the same lysates also were blotted for β-Trcp1 using anti-Flag antibody. (H) HEK293 cells coexpressing VEGFR-2 with empty vector or with β-Trcp1 were left unstimulated (0) or were stimulated for 10 min (10) with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with anti-GST antibody. (I) The same membrane was reblotted for VEGFR-2 levels. (J) Whole-cell lysates from the same cell lysate were blotted for phospho-Ser1188 antibody. Primary endothelial cells, HUVEC cells expressing pSuper.puro or shRNA.β-Trcp1.pSuper.puro, were stimulated with VEGF for the indicated periods of time, and whole-cell lysates were blotted with anti-VEGFR-2 antibody (K), β-catenin (L), and Hsp70 (M). Ctr., control. (N) Quantification of downregulation of VEGFR-2 is shown. (O) HUVEC expressing pSuper.puro vector or shRNA.β-Trcp1.pSuper.puro were left unstimulated (0) or were stimulated with VEGF for 10 min, and cells were lysed and immunoprecipitated with anti-VEGFR-2 and blotted with antiubiquitin antibody. The same membrane was reblotted for VEGFR-2 (P). Whole-cell lysates from the same cell lysates were blotted for β-catenin (L) and Hsp70 (Q). (S) HEK293 cells coexpressing VEGFR-2 with empty vector, VEGFR-2 with β-Trcp1, PEST(+)Ser1188/Ser1191 mutant VEGFR-2 with β-Trcp1, or PEST(−)VEGFR-2 with β-Trcp1 were unstimulated or stimulated with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with anti-Flag antibody. (T) The same membrane was reblotted for VEGFR-2. Ipt, immunoprecipitation. (U) Whole-cell lysates from the same cell lysates were blotted for β-Trcp1 using anti-Flag antibody.
Since β-Trcp1 stimulates the ubiquitination of VEGFR-2, we examined its association with VEGFR-2 by in vitro pulldown assay. We show that the full-length recombinant GST-tagged β-Trcp1 binds to VEGFR-2, and its binding to VEGFR-2 increases after stimulation with VEGF (data not shown), suggesting that the binding of β-Trcp to VEGFR-2 is regulated by ligand-mediated events. β-Trcp also binds to VEGFR-2 in vivo, as was demonstrated by its coimmunoprecipitation with VEGFR-2 (Fig. 4H). It should be noted that the association of β-Trcp with VEGFR-2 prior to VEGF stimulation likely is due to the basal phosphorylation of Ser118 of VEGFR-2 (Fig. 4J). To further analyze the biological importance of β-Trcp in VEGFR-2 downregulation, we decided to silence the expression of β-Trcp in primary endothelial cells, HUVEC (human umbilical endothelial cells), by shRNA and analyze the downregulation of VEGFR-2. Silencing the expression of β-Trcp1 significantly inhibited VEGFR-2 downregulation (Fig. 4F and I), demonstrating that the β-Trcp1-mediated ubiquitination of VEGFR-2 targets VEGFR-2 for degradation. As a control to monitor the efficiency of the silencing of β-Trcp1, we also analyzed the expression of β-catenin (Fig. 4G). Moreover, silencing the expression of β-Trcp in HUVEC blocked the ubiquitination of VEGFR-2 (Fig. 4O). Finally, we tested whether the binding of β-Trcp to VEGFR-2 requires the PEST domain, in particular, whether Ser1188 and Ser1191 are important for the recruitment of β-Trcp to VEGFR-2. For this purpose, we coexpressed β-Trcp1 with A1188/A1191/VEGFR-2 and PEST(−)VEGFR-2 and analyzed the binding of β-Trcp1 with VEGFR-2. As shown, β-Trcp1 was coprecipitated with wild-type VEGFR-2 but not with PEST(−)VEGFR-2, and Ser1188 and Ser1191 also are critically required for the recruitment of β-Trcp1 to VEGFR-2 (Fig. 4S). Taken together, the data demonstrate that β-Trcp mediates the ubiquitination and downregulation of VEGFR-2 in a PEST domain-dependent manner, and Ser188 and Ser1191 are required for the recruitment of β-Trcp to VEGFR-2.
Phosphorylation of tyrosine 1173 negatively regulates downregulation of VEGFR-2 and controls phosphorylation of p38 MAPK.
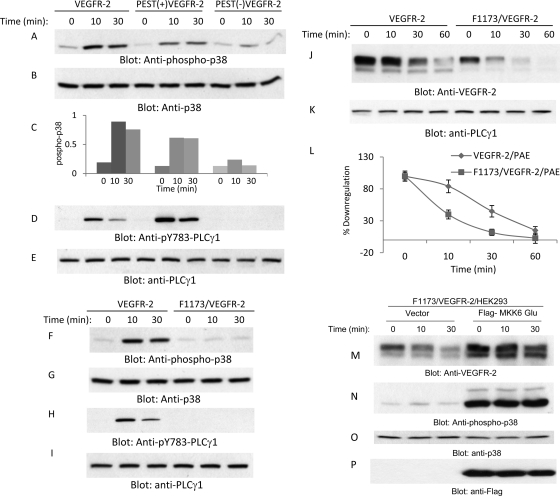
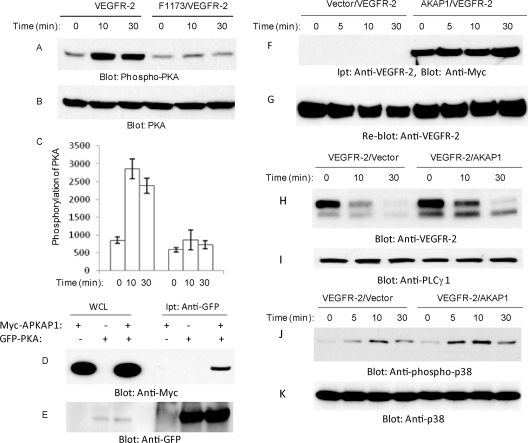
Tyrosine 1173 is located in the PEST domain, and its phosphorylation is considered to play a key role in VEGFR-2-mediated angiogenesis and the activation of several important signaling proteins, including PLCγ1 and PI3 kinase (6, 21, 41). Since the deletion of the PEST domain also removes tyrosine 1173, we decided to analyze the effect of the deletion of the PEST domain on the phosphorylation of several key signaling proteins that are activated by VEGFR-2. Unexpectedly, we noted that the deletion of PEST prevents VEGFR-2 from activating p38 MAPK, where both wild-type VEGFR-2 and PEST(+)VEGFR-2 were able to activate p38 MAPK at comparable levels (Fig. 5 A). The quantification of the activation of p38 also is shown (Fig. 5C). As expected, PEST(−)VEGFR-2 also failed to activate PLCγ1, as the Y1173 site no longer was operational in the PEST domain-deleted VEGFR-2 (Fig. 5C). We then examined whether the phosphorylation of Y1173 contributes to the activation of p38 MAPK and whether the p38 MAPK activity is involved in the PEST domain-dependent downregulation of VEGFR-2. To this end, we used previously established F1173/VEGFR-2, where Y1173 was mutated to F (21, 37), and analyzed it for its ability to activate p38 MAPK. Our analysis showed that the mutation of Y1173 severely impairs the VEGFR-2-mediated activation of p38 MAPK (Fig. 5F), suggesting that the inability of F1173/VEGFR-2 and PEST(−)VEGFR-2 to activate p38MAPK contributes to the downregulation of VEGFR-2.
Fig. 5.
PEST domain controls the phosphorylation of p38 MAPK. Serum-starved PAE cells expressing VEGFR-2, PEST(+)VEGFR-2, and PEST(−)VEGFR-2 were stimulated with VEGF for the indicated periods of time, cells were lysed, and whole-cell lysates were subjected to Western blot analysis and blotted for phospho-p38 (A), total p38 (B), phospho-PLCγ1 (D), and total PLCγ1 (E). (C) Quantification of activation of p38. Serum-starved PAE cells expressing VEGFR-2 and F1173/VEGFR-2 were stimulated with VEGF for the indicated periods of time, cells were lysed, and whole-cell lysates were subjected to Western blot analysis and immunoblotted for phospho-p38 (F), total p38 (G), phospho-PLCγ1 (H), and total PLCγ1 (I). Serum-starved PAE cells expressing VEGFR-2 and F1173/VEGFR-2 were preincubated with cycloheximide for 90 min, and then cells were stimulated with VEGF for the indicated periods of time. Cells were lysed, and whole-cell lysates were immunoblotted for VEGFR-2 (J) and total PLCγ1 (K). (L) Quantification of VEGFR-2 protein levels from blot I is shown. The graph shows averages from two independent experiments. HEK293 cells coexpressing F1173/VEGFR-2 with an empty vector or constitutive active MKK6 (MKK6-Glu) were stimulated with VEGF for the indicated periods of time, and whole-cell lysates were immunoblotted for VEGFR-2 (M), phospho-p38 MAPK (pT180/pY182) (N), p38 MAPK (O), and MKK6 using anti-Flag antibody (P).
To determine whether the phosphorylation of Y1173 contributes to the downregulation of VEGFR-2, we analyzed the ligand-dependent downregulation of Y1173 mutant VEGFR-2 (F1173/VEGFR-2). The result, unlike what we were anticipating, showed that the mutation of Y1173 resulted in the acceleration of the ligand-mediated downregulation of VEGFR-2 (Fig. 5J and L). To further examine the contribution of Y1173 to the downregulation of VEGFR-2, we generated phosphomimetic Y1173 by replacing Y with E (E1173/VEGFR-2) and analyzed its VEGF-dependent downregulation. As shown, the ligand-stimulated downregulation of E1173/VEGFR-2 was identical to that of the downregulation of wild-type VEGFR-2 (data not shown). The mutation of Y1173 also did not influence the phosphorylation of Ser1188 (data not shown), suggesting that the phosphorylation of Ser118 is not regulated through Y1173 of VEGFR-2. Taken together, the data indicate that Y1173 contributes to the stability of VEGFR-2 and raise the possibility that the activation of the p38 MAPK pathway is inhibiting the degradation of VEGFR-2, and the inability to activate this pathway elevates its degradation.
To address whether the failure to activate p38 MAPK is responsible for the accelerated degradation of F1173/VEGFR-2, we coexpressed F1173/VEGFR-2 with constitutively active MKK6, a known upstream activator of p38 (30). The data showed that the coexpression of MKK6-Glu prevents the accelerated degradation of F1173/VEGFR-2 (Fig. 5 M) and also stimulates p38 phosphorylation (Fig. 5N). Taken together, the data further support the idea that the inability to activate p38 is responsible for the increased downregulation of F1173/VEGFR-2. These results also suggest that the PEST domain of VEGFR-2 relays two opposing signaling events, one signaling pathway involving serine 1188 and serine 1191, which promote ubiquitination and degradation, and the second signaling pathway, emanating from Y1173, inhibits the degradation of VEGFR-2.
Activation of p38 pathway stabilizes VEGFR-2 protein.
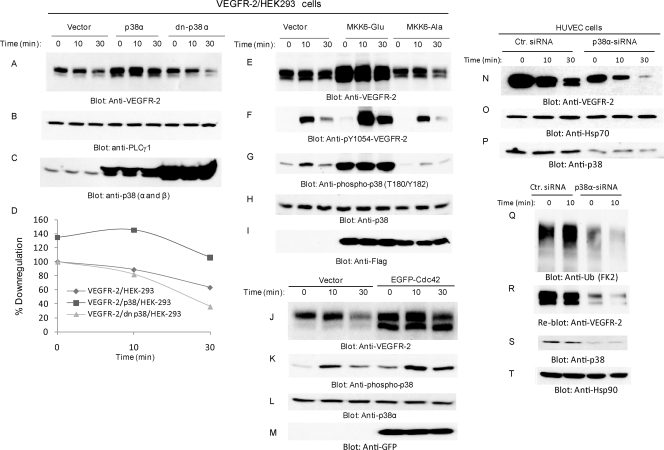
To examine the direct involvement of p38 MAPK in the ligand-mediated degradation of VEGFR-2, we coexpressed wild-type p38 or a dominant-negative form of p38 (dn-p38) with VEGFR-2 and analyzed their potential to influence the downregulation of VEGFR-2 in response to VEGF. As shown, the coexpression of wild-type p38 with VEGFR-2 considerably prevented the degradation of VEGFR-2, where the overexpression of the dominant-negative p38 accelerated the VEGF-induced degradation of VEGFR-2 (Fig. 6 A and D). The inhibitory effect of the overexpression of wild-type p38 MAPK was more profound than the positive effect of dn-p38 MAPK, suggesting that the mutation of Thr180 and Tyr182 is not sufficient to fully block the activity of p38. Moreover, the treatment of cells with the p38 inhibitor SB 203580 (10 μM) also inhibited the downregulation of VEGFR-2 (data not shown), further supporting a possible role of p38 in the negative regulation of VEGFR-2 degradation.
Fig. 6.
MKK6-dependent activation of p38 inhibits downregulation of VEGFR-2. HEK 293 cells coexpressing VEGFR-2 with an empty vector, VEGFR-2 with wild-type p38 MAPK, or VEGFR-2 with dominant-negative p38 (dn-P38) MAPK were preincubated with cycloheximide for 90 min and then stimulated with VEGF for the indicated periods of time. Whole-cell lysates were blotted for VEGFR-2 (A), PLCγ1 as a control for protein loading (B), and p38 (C). (D) The quantification of the downregulation of VEGFR-2 in response to ligand stimulation. Whole-cell lysates from HEK293 cells coexpressing VEGFR-2 either with an empty vector, constitutive active MKK6 (MKK6-Glu), or with dominant-negative MKK6 (MKK6-Ala) were immunoblotted for VEGFR-2 (E), phospho-VEGFR-2 (pY1054-VEGFR-2) (F), phospho-p38 MAPK (pT180/pY182) (G), p38 MAPK (H), and MKK6 using anti-Flag antibody (I). HEK293 cells expressing VEGFR-2 were transfected either with empty vector or with enhanced green fluorescent protein (EGFP)-tagged Cdc42. Serum-starved cells were stimulated with VEGF for the indicated times, and cells were lysed. Whole-cell lysates were blotted for VEGFR-2 (J), phospo-p38 (K), total p38 (L), and anti-GFP for Cdc42 expression (M). Cells also were treated with cycloheximide (20 mM for 90 min prior to stimulation with VEGF. HUVEC were transfected with control (Ctr.) siRNA or p38α siRNA after 24 h, and cells were starved overnight and stimulated with VEGF for the indicated periods of time. Cells were lysed, and whole-cell lysates were blotted with anti-VEGFR-2 antibody (N), anti-Hsp70 antibody (O), and anti-p38 antibody (P). (Q) Ubiquitination of VEGFR-2 in HUVEC in which p38α was silenced. (S) The same membrane was reblotted for VEGFR-2 levels. Whole-cell lysates were blotted for p38 (R) and Hsp90 (T) for protein loading.
MKK6/SAPK4 acts as an upstream activator of p38 (7, 30). To further link p38 activation to the inhibition of VEGFR-2 downregulation, we overexpressed either constitutively active MKK6 (MKK6-Glu, where Ser 207 and Thr 211 are replaced with Glu) or a dominant-negative form of MKK6 (MKK6-Ala, where Lys 82 is replaced with Ala) with VEGFR-2 and examined their effect on the downregulation of VEGFR-2. The data showed that the overexpression of constitutively active MKK6 significantly inhibits the downregulation of VEGFR-2 (Fig. 6E) and also stimulated the phosphorylation of p38 MAPK (Fig. 6G). The coexpression of the dominant-negative form of MKK6 (MKK6-Ala) with VEGFR-2 only modestly increased the degradation of VEGFR-2 (Fig. 6E). MKK6-Ala also reduced the VEGFR-2-dependent phosphorylation of p38 MAPK (Fig. 6G). The accumulation of VEGFR-2 protein due to the overexpression of MKK6 also resulted in the enhanced activation of VEGFR-2 (Fig. 6F). Moreover, Cdc42, a known activator of p38 (50), also stimulated p38 phosphorylation and inhibited the downregulation of VEGFR-2 (Fig. 6J and K). Taken together, the data demonstrate that the activation of the MKK6/Cdc42/p38 MAPK pathway inhibits the VEGF-mediated degradation of VEGFR-2. To further establish a role for p38 in VEGFR-2 protein stabilization, we analyzed the effect of GSK3. GSK3 is known to inhibit p38 activation. The treatment of cells with lithium chloride, a commonly used inhibitor of GSK3, and GSK3 inhibitor IX both increased the phosphorylation of p38 and inhibited the downregulation of VEGFR-2 (data not shown). The coexpression of VEGFR-2 with a kinase-inactive form GSK3 also prevented the downregulation of VEGFR-2 (data not shown).
To further establish the biological importance of p38 in the stability of VEGFR-2 protein, we silenced the expression of p38 in human primary endothelial HUVEC by siRNA. Transfection of p38α siRNA reduced the expression of p38 by more than 60% (Fig. 6P). Reducing the expression of p38 in HUVEC also accelerated the downregulation of VEGFR-2 (Fig. 6N). The baseline VEGFR-2 protein level also was reduced, suggesting that the constitutive and ligand-mediated downregulation of VEGFR-2 both are regulated by p38 (Fig. 6N). Moreover, silencing the expression of p38 in HUVEC cells also prevented the ubiquitination of VEGFR-2 (Fig. 6Q); however, the reduced ubiquitination of VEGFR-2 likely was due to the reduced expression of VEGFR-2 rather than its direct effect on the ubiquitination of VEGFR-2 (Fig. 6S). Taken together, the data strongly demonstrate that p38α plays a pivotal role in controlling VEGFR-2 protein stability.
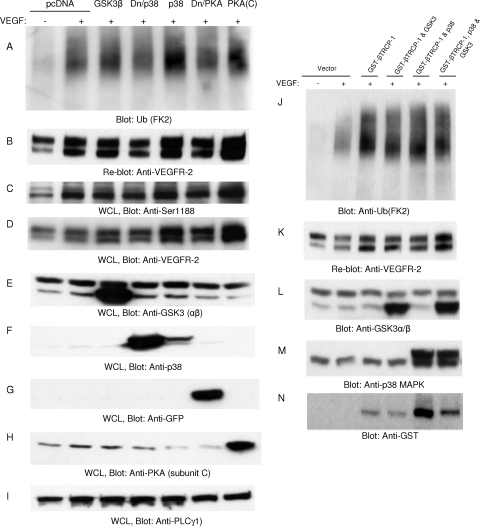
To examine the possible function of p38, PKA, and GSK3 in the ubiquitination of VEGFR-2, we coexpressed wild-type p38, dominant-negative p38, an active subunit of PKA (C subunit), and GSK3 with VEGFR-2 and analyzed the ubiquitination of VEGFR-2. As shown, none of these kinases was able to inhibit the ubiquitination of VEGFR-2 (Fig. 7 A). The coexpression of p38 and PKA with VEGFR-2 appeared to increase the ubiquitination of VEGFR-2; however, this was due mainly to the stabilization of VEGFR-2 protein by these kinases rather than an increase in their ubiquitination of VEGFR-2 (Fig. 7A and B). Moreover, the phosphorylation of Ser1188 also was not affected by these kinases (Fig. 7C). Again, the apparent increase in the phosphorylation of Ser1188 was due to the stabilization of VEGFR-2 protein (Fig. 7D). To further examine a possible role for p38 in the ubiquitination of VEGFR-2, we analyzed whether p38 can inhibit β-Trcp. The coexpression of β-Trcp1 and p38 with VEGFR-2 or β-Trcp1 and GSK3 with VEGFR-2 had no apparent effect on the ubiquitination of VEGFR-2 (Fig. 7J), suggesting that the p38-mediated stabilization of VEGFR-2 is not associated with the regulation of Ser1188 phosphorylation or the function of β-Trcp1 to ubiquitinate VEGFR-2.
Fig. 7.
p38 is not involved in the ubiquitination of VEGFR-2. HEK293 cells expressing VEGFR-2 were transfected with empty vector, GSK3β, dominant-negative p38 (Dn/p38), wild-type p38, dominant-negative PKA catalytic subunit C (Dn/PKA), and wild-type PKA catalytic subunit C [PKA(C)]. Cells were stimulated with VEGF for 10 min, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with antiubiquitin (Ub; FK2) antibody (A). (B) The same membrane was reblotted for VEGFR-2 levels. Whole-cell lysates (WCL) from panel A were blotted with anti-phospho-Ser1188 antibody (C), anti-VEGFR-2 antibody (D), anti-GSK3 antibody (E), anti-p38 antibody (F), anti-GFP antibody to detect dominant-negative PKA (G), anti-PKA antibody (H), and anti-PLCγ1 antibody (I). HEK293 cells expressing VEGFR-2 were transfected with GST-tagged β-Trcp1 alone or GST-tagged β-Trcp1 with GSK3β, β-Trcp1 with p38, or β-Trcp1 with p38 and GSK3β. (J) Cells were stimulated with VEGF for 10 min, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with anti-ubiquitin (FK2) antibody. (K) The same membrane was reblotted for VEGFR-2. (L to N) Whole-cell lysates from panel J were blotted for GSK3, p38, and β-Trcp1.
AKAP1/PKA pathway controls downregulation of VEGFR-2 via p38 MAPK activation.
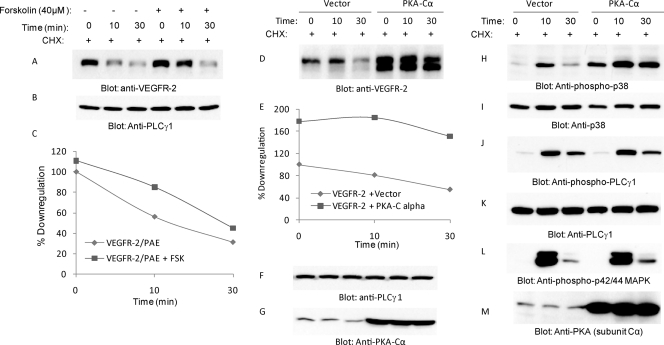
Our data, consistently with previous publications, indicate that multiple upstream pathways, including PKA and MKK6, activate p38 (18, 34, 50). To determine the role of PKA in VEGFR-2-dependent p38 MAPK activation and the downregulation of VEGFR-2, we treated cells with a low concentration of forskolin (40 μM), a well-characterized agent known to activate PKA. The treatment of cells with forskolin also moderately inhibited the VEGF-dependent downregulation of VEGFR-2 (Fig. 8 A and C). Increasing the concentration of forskolin (100 μM) further inhibited the downregulation of VEGFR-2 (data not shown). To directly test the role of PKA in the inhibition of the downregulation of VEGFR-2, we coexpressed the catalytically active C subunit of PKA with VEGFR-2. The expression of the catalytically active C subunit of PKA with VEGFR-2 significantly inhibited the downregulation of VEGFR-2. Both the baseline and VEGF-dependent downregulation of VEGFR-2 were significantly inhibited by PKA (Fig. 8D and E). The overexpression of PKA also elevated p38 MAPK phosphorylation (Fig. 8H), where it had no effect on the VEGF-dependent phosphorylation of PLCγ1 and MAPK (p42/44) (Fig. 8J and 8L), suggesting that cyclic AMP (cAMP)-dependent PKA activation modulates the downregulation of VEGFR-2 through the activation of p38 MAPK.
Fig. 8.
PKA pathway inhibits downregulation of VEGFR-2 and activates p38. PAE cells expressing wild-type VEGFR-2 were pretreated with forskolin (40 μM) and then stimulated with VEGF for the indicated time periods. Cells were treated with cycloheximide for 90 min to inhibit protein synthesis. Whole-cell lysates were blotted with anti-VEGFR-2 antibody (A) and with anti-PLCγ1 antibody for protein loading (B). (C) The quantification of VEGFR-2 downregulation in the presence or absence of forskolin is shown. Serum-starved HEK293 cells coexpressing VEGFR-2 either an empty vector or the catalytic active subunit (Cα) of PKA were pretreated with cycloheximide (CHX) for 90 min, and then cells were stimulated with VEGF for the indicated time periods. Whole-cell lysates were immunoblotted with anti-VEGFR-2 antibody (D), anti-PLCγ1 antibody (F), and anti-PKA antibody (G). (E) The quantification of the downregulation of VEGFR-2. The same cell lysates also were blotted for phospho-p38 (H), total p38 (I), phospho-PLCγ1 (J), total PLCγ1 (K), phospho-MAPK42/44 (L), and total PKA (M).
To address the mechanism by which PKA is activated by VEGFR-2, we first examined whether Y1173 of VEGFR-2 contributes to the phosphorylation of PKA. To this end, we analyzed the ability of F1173/VEGFR-2 to stimulate the phosphorylation of PKA. The results showed that the stimulation of wild-type VEGFR-2 increased the phosphorylation of PKA, where F1173/VEGFR-2 failed to activate PKA (Fig. 9 A), suggesting that the activation of PKA by VEGFR-2 is mediated through phospho-Y1173. The intracellular targeting and association of PKA with other proteins is controlled through association with AKAPs (A-kinase anchoring proteins) (42, 48). To determine the nature of the association of PKA with VEGFR-2, we tested the hypothesis that PKA, through AKAP1 (also called AKAP149 and AKAP121), associates with VEGFR-2. To test this hypothesis, initially we examined the ability of PKA to interact with AKAP1. The coexpression of GFP-tagged PKA with Myc-tagged AKAP1 in HEK293 cells showed that PKA interacts with AKAP1 (Fig. 9D). We examined the association of AKAP1 with VEGFR-2. The data showed that AKAP1 constitutively binds to VEGFR-2, and the stimulation of cells with VEGF has no effect on its association with VEGFR-2 (Fig. 9F). The data further suggest that AKAP1 serves as an adaptor to link PKA to VEGFR-2. To determine whether the overexpression of AKAP1 influences the VEGF-induced downregulation of VEGFR-2, we measured kinetics of the downregulation of VEGFR-2 in response to ligand stimulation. As shown, the downregulation of VEGFR-2 was reduced in response to VEGF in cells overexpressing AKAP1 (Fig. 9H), suggesting that AKAP1 interaction is important for the VEGFR-2-dependent activation of PKA and for its subsequent role in VEGFR-2 stability. Since PKA stimulates the phosphorylation of p38, we tested the effect of the overexpression of AKAP1 in the phosphorylation of p38. The overexpression of AKAP1 also increased the phosphorylation of p38 (Fig. 9J). Overall, the data indicate that PKA, through AKAP1, interacts with VEGFR-2, and upon activation by VEGFR-2 it stimulates the phosphorylation of p38, leading to the stabilization of VEGFR-2.
Fig. 9.
Tyrosine 1173 of VEGFR-2 is required for PKA phosphorylation but not for its association with VEGFR-2. PAE cells expressing VEGFR-2 or F1173/VEGFR-2 were stimulated with VEGF for the indicated periods of time. Cells were lysed, and whole-cell lysates (WCL) were blotted for phospho-PKA (A) and total PKA (B). (C) Quantification of the phosphorylation of PKA is shown. It represents averages from two experiments. HEK293 cells were transfected with GFP-tagged PKA catalytic subunit C alone or with Myc-tagged AKAP1. (D) Cells were lysed, immunoprecipitated (Ipt) with anti-GFP antibody, and blotted with anti-Myc antibody. (E) Whole-cell lysates also were blotted for anti-GFP. Serum-starved HEK293 cells coexpressing VEGFR-2 with empty vector or with c-myc-tagged AKAP1 were stimulated with VEGF for the indicated periods of time (F). Cells were lysed, and VEGFR-2 was immunoprecipitated with anti-VEGFR-2 antibody and immunoblotted with anti-c-myc antibody. (G) The same membrane was stripped and reblotted for VEGFR-2. Whole-cell lysates from the same cell groups were immunoblotted for VEGFR-2 (H), total PLCγ1 (I), phospho-p38 (J), and total p38 (K).
DISCUSSION
Our study illustrates that the PEST motif of VEGFR-2 functions as a double-edged sword to regulate the degradation of VEGFR-2; the phosphorylation of Ser1188 regulates the ubiquitination of VEGFR-2, which promotes VEGFR-2 degradation by serving as a recruitment site for the F-box-containing ubiquitin E3 ligase β-Trcp1. On the other hand, the phosphorylation of Y1173 controls the activation of the PKA/p38 MAPK pathway, which, upon activation, acts to inhibit the ligand-mediated degradation of VEGFR-2. The presence of the PEST domain is linked to the degradation of certain polyubiquitinated proteins (26) and calpain-mediated proteolysis (43). The serine phosphorylation of PEST domains is known to act as a phosphodegron by recruiting F-box-containing ubiquitin E3 ligases (5, 49). The data presented here demonstrate that in addition to the known function of the PEST motif, which is thought to target proteins for ubiquitination and degradation (10, 33), the PEST motif of VEGFR-2 has the potential to protect VEGFR-2 from ligand-mediated degradation. The deletion of the PEST motif makes VEGFR-2 refractory to ligand-stimulated downregulation, and the mutation of Ser1188 significantly reduces the ubiquitination of VEGFR-2. In general, RTKs undergo tyrosine phosphorylation-dependent ubiquitination and degradation involving c-Cbl ubiquitin E3 ligase family proteins (20). VEGFR-2 ubiquitination and degradation is not mediated by c-Cbl (38). Unlike other RTKs, the data presented here demonstrate that VEGFR-2 ubiquitination and degradation are regulated by the PEST motif through Ser1188 phosphorylation. The phosphorylation of Ser1188 along with Ser1191 creates a phosphodegron motif that allows β-Trcp to recognize and target VEGFR-2 for ubiquitin-dependent degradation. The amino acids surrounding Ser1188 (DS1188GL S1191) of VEGFR-2 are highly homologues to the known β-Trcp recognition phosphodegron, the DpSGXXpS motif (5, 49). A recent study by Bruns (3) failed to demonstrate the β-Trcp-dependent ubiquitination of VEGFR by the overexpression of β-Trcp2 in endothelial cells. This was due most likely to the inefficient overexpression of β-Trcp2 in endothelial cells or high levels of endogenous β-Trcp in these cells, which might mask the effect of the ectopic expression of β-Trcp2.
While the phosphorylation of Ser1188 of the PEST motif targets VEGFR-2 for ubiquitination and degradation through β-Trcp, the phosphorylation of Y1173 of PEST motif counters VEGFR-2 degradation. The mechanism by which Y1173 of the PEST domain elicits its inhibitory role over the downregulation of VEGFR-2 is established by the PKA/MKK6/p38 MAPK pathway. The previously unknown ability of the PEST motif to protect protein from degradation suggests a larger role for the PEST motif in the regulation of protein stability than previously appreciated. Our molecular and pharmacological approaches illustrate that blocking p38 MAPK activation by VEGFR-2 accelerates the ligand-mediated downregulation of VEGFR-2, and increasing the activation of p38 MAPK prevents VEGFR-2 downregulation.
The data presented in the manuscript strongly implicate p38 MAPK in the stabilization of VEGFR-2. Overexpression, pharmacological inhibition, and siRNA strategies all point to a key role of p38 in the stabilization of VEGFR-2 protein. In addition, the upstream activators of p38 MAPK, including PKA, MKK6, and Cdc42, all blocked the ligand-mediated downregulation of VEGFR-2. Silencing the expression of p38α MAPK in primary endothelial cells significantly stabilized VEGFR-2, suggesting that this pathway is biologically important for VEGF angiogenic signaling. Interestingly, the loss of p38α MAPK in mice has been reported to be embryonic lethal mainly due to an apparent defect in angiogenesis and vascular remodeling (25). Unlike the loss of p38α MAPK, the losses of p38β and p38γ are not embryonic lethal and have no obvious abnormal phenotypes (16), suggesting that p38α MAPK plays a distinct role in angiogenic signaling. The activation of p38 MAPK also is linked to VEGF-mediated endothelial cell migration (35) and vascular permeability (15). The underlying mechanism for the p38 MAPK-mediated inhibition of the downregulation of VEGFR-2 is established by the activation of PKA. The phosphorylation of PKA is mediated by VEGFR-2 through Y1173. The mutation of Y1173 blocks the phosphorylation of PKA and p38. Although the phosphorylation of PKA by VEGFR-2 requires Y1173, the binding of PKA to VEGFR-2 is mediated through AKAP1/AKAP149. Interestingly, ubiquitination and Ser1188 phosphorylation are not affected by p38, suggesting that the p38-mediated stabilization of VEGFR-2 is not at the level of the phosphorylation of Ser1188. Another interesting and novel observation of the current study is the identification of AKAP1 as a binding partner of VEGFR-2. AKAP1/AKAP149 is known to bind protein kinases, such as PKC isoforms, as well as serine/threonine phosphatases (42). AKAPs are thought to anchor the kinases in an inactive state to their substrates (46). This theme is echoed by our observation that the interaction of AKAP1 with VEGFR-2 occurs prior to the activation of VEGFR-2 with ligand. AKAP1, by interacting both with PKA and VEGFR-2, positively stabilizes VEGFR-2 protein.
Activated RTKs generally depart from the cell surface and are transported to an endosomal compartment, where they are recycled or targeted for degradation. Ubiquitination is thought to play a key role both in RTK endocytosis and degradation (20, 39). The monoubiquitination of cell surface receptors is linked to endocytosis, where polyubiquitination is suggested to target proteins for proteasome-mediated degradation (11, 12, 21). Our data demonstrate that the degradation of VEGFR-2 is mediated primarily by Lys48-linked polyubiquitination, although other lysine sites may contribute to other events involved in VEGFR-2 protein trafficking. The data presented here suggest that upon ligand binding, the PEST domain of VEGFR-2 is phosphorylated on tyrosine and serine sites, including Y1173 and S1188. The phosphorylation of VEGFR-2 proceeds via Lys-48-dependent polyubiquitination involving the ubiquitin E3 ligase β-Trcp, which promotes the degradation of VEGFR-2 by the 26S proteosome. The phosphorylation of serine 1188 plays a key role in the ubiquitination of VEGFR-2 (Fig. 10). On the other hand, the phosphorylation of Y11173 via AKAP1/PKA leads to the activation of p38, which attenuates the downregulation of VEGFR-2 (Fig. 10).
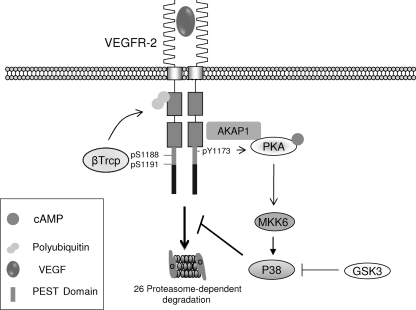
Fig. 10.
Proposed model for PEST-mediated downregulation of VEGFR-2. Upon ligand binding, the PEST domain of VEGFR-2 is phosphorylated on tyrosine and serine sites, including Y1173, S1188, and Ser1191. Ligand binding promotes β-Trcp1 association with VEGFR-2, which mediates the ubiquitination of VEGFR-2 through the Lys-48-dependent ubiquitin chain, leading to degradation by the 26S proteasome system. The activation of PKA by VEGFR-2 requires the AKAP1-mediated association of PKA with VEGFR-2, and its phosphorylation is mediated through Y1173 of VEGFR-2. The activation of PKA leads to the phosphorylation of p38. Activated p38 attenuates the downregulation of VEGFR-2.
A precise physiological balance between endogenous pro- and antiangiogenic regulators, a so-called angiogenic switch, controls endothelial cell functions such that endothelial cell growth normally is restrained. However, in pathological conditions such as tumor growth, a shift occurs in the balance of regulators favoring endothelial growth (1, 8). Central to the proper regulation of the angiogenic activity of VEGFR-2 is the process by which VEGFR-2 triggers its own internalization and degradation (i.e., downregulation), consequently terminating its angiogenic signaling. The PEST domain-dependent regulation of VEGFR-2 stability through the ubiquitin proteasome system and p38 MAPK pathway likely plays a profound role in fine-tuning VEGFR-2 function in vivo. The further elucidation of the mechanism of the p38-mediated inhibition of the downregulation of VEGFR-2 and the β-Trcp-mediated ubiquitination of VEGFR-2 is essential for a better understanding of the regulatory mechanism of angiogenic signaling events.
ACKNOWLEDGMENTS
This work was supported in part through grants from the National Institutes of Health (NIH/NEI) to N.R. This work also was supported by a grant from the Department of Pathology, Boston University, and Massachusetts Lions Foundation grant to the Department of Ophthalmology.
We thank E. Hartsough from Nader Rahimi's laboratory for reading and commenting on the manuscript.
We declare no conflicts of interest.
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Adams R. H., Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8:464–478 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharya R. 2005. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 19:1692–1694 [DOI] [PubMed] [Google Scholar]
- 3. Bruns A. F. 2010. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic 11:161–174 [DOI] [PubMed] [Google Scholar]
- 4. Cleaver O., Melton D. A. 2003. Endothelial signaling during development. Nat. Med. 9:661–668 [DOI] [PubMed] [Google Scholar]
- 5. Crusio K. M., King B., Reavie L. B., Aifantis I. 2010. The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene 29:4865–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dayanir V., Meyer R. D., Lashkari K., Rahimi N. 2002. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J. Biol. Chem. 277:27081–27087 [DOI] [PubMed] [Google Scholar]
- 7. Enslen H., Raingeaud J., Davis R. J. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741–1748 [DOI] [PubMed] [Google Scholar]
- 8. Folkman J. 2006. Angiogenesis. Annu. Rev. Med. 57:1–18 [DOI] [PubMed] [Google Scholar]
- 9. Gampel A. 2006. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartment. Blood 108:2624–2631 [DOI] [PubMed] [Google Scholar]
- 10. García-Alai M. M. 2006. Molecular basis for phosphorylation-dependent, PEST-mediated protein turnover. Structure 14:309–319 [DOI] [PubMed] [Google Scholar]
- 11. Haglund K., Di Fiore P. P., Dikic I. 2003. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28:598–603 [DOI] [PubMed] [Google Scholar]
- 12. Hicke L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2:195–201 [DOI] [PubMed] [Google Scholar]
- 13. Husain D., et al. 2010. Role of c-Cbl dependent regulation of phospholipase Cg1 activation in experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikeda S., et al. 2005. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 96:467–475 [DOI] [PubMed] [Google Scholar]
- 15. Issbrücker K. 2003. p38 MAP kinase-a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 17:262–264 [DOI] [PubMed] [Google Scholar]
- 16. Kuida K., Boucher D. M. 2004. Functions of MAP kinases: insights from gene-targeting studies. J. Biochem. 135:653–656 [DOI] [PubMed] [Google Scholar]
- 17. Labrecque L., Royal I., Surprenant D. S., Patterson C., Gingras D., Béliveau R. 2003. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol. Biol. Cell 14:334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamalice L., Houle F., Jourdan G., Huot J. 2004. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 23:434–445 [DOI] [PubMed] [Google Scholar]
- 19. Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Z., Hunter T. 2009. Degradation of activated protein kinases by ubiquitination. Annu. Rev. Biochem. 78:435–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer R. D., Latz C., Rahim N. 2003. Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J. Biol. Chem. 278:16347–16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer R. D., Sacks D. B., Rahimi N. 2008. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One 3:e3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer R. D., Husian D., Rahimi N. 2011. c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCγ1. Oncogene [Epub ahead of print.] doi: 10.1038/onc.2010.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer R. D., et al. 2004. Substitution of C-terminus of VEGFR-2 with VEGFR-1 promotes VEGFR-1 activation and endothelial cell proliferation. Oncogene 23:5523–5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudgett J. S. 2000. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 97:10454–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prakash S., Tian L., Ratliff K. S., Lehotzky R. E., Matouschek A. 2004. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat. Struct. Mol. Biol. 11:830–837 [DOI] [PubMed] [Google Scholar]
- 27. Rahimi N., Golde T. E., Meyer R. D. 2009. Identification of ligand-induced proteolytic cleavage and ectodomain shedding of VEGFR-1/FLT1 in leukemic cancer cells. Cancer Res. 69:2607–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahimi N., Dayanir V., Lashkari K. 2000. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J. Biol. Chem. 275:16986–16992 [DOI] [PubMed] [Google Scholar]
- 29. Rahimi N. 2006. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front. Biosci. 11:818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raingeaud J., Whitmarsh A. J., Barrettd T., Dérijar B., Davis R. J. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravid T., Hochstrasser M. 2008. Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 9:679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rechsteiner M., Rogers S. W. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267–271 [PubMed] [Google Scholar]
- 33. Rechsteiner M. 1990. PEST sequences are signals for rapid intracellular proteolysis. Semin. Cell Biol. 1:433–440 [PubMed] [Google Scholar]
- 34. Robidoux J. 2005. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38alpha MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol. Cell. Biol. 25:5466–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rousseau S., Houle F., Landry J., Huot J. 1997. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15:2169–2177 [DOI] [PubMed] [Google Scholar]
- 36. Sillibourne J. E., Bornens M. 2010. Polo-like kinase 4: the odd one out of the family. Cell Div. 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh A. J., et al. 2007. A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 104:5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh A. J., Meyer R. D., Band H., Rahim N. 2005. The carboxyl terminus of VEGFR-2 is required for PKC-mediated down-regulation. Mol. Biol. Cell. 16:2106–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorkin A., von Zastrow M. 2009. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stambolic V., Woodgett J. R. 1994. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 303:701–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takahashi T., Yamaguchi S., Chida K., Shibuya M. A. 2001. Single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taskén K., Aandahl E. M. 2004. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84:137–167 [DOI] [PubMed] [Google Scholar]
- 43. Tompa P., et al. 2004. On the sequential determinants of calpain cleavage. J. Biol. Chem. 279:20775–20785 [DOI] [PubMed] [Google Scholar]
- 44. Uhler M. D., McKnight G. S. 1987. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J. Biol. Chem. 262:15202–15207 [PubMed] [Google Scholar]
- 45. Ungar A. R., Moon R. T. 1996. Inhibition of protein kinase A phenocopies ectopic expression of hedgehog in the CNS of wild-type and cyclops mutant embryos. Dev. Biol. 178:186–191 [DOI] [PubMed] [Google Scholar]
- 46. Westphal R. S. 1999. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285:93–96 [DOI] [PubMed] [Google Scholar]
- 47. Winston J. T., Koepp D. M., Zhu C., Elledge S. J., Harper J. W. 1999. A family of mammalian F-box proteins. Curr. Biol. 9:1180–1182 [DOI] [PubMed] [Google Scholar]
- 48. Wong W., Scott J. D. 2004. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5:959–970 [DOI] [PubMed] [Google Scholar]
- 49. Yada M., et al. 2004. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23:2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang S. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934–23936 [DOI] [PubMed] [Google Scholar]