Abstract
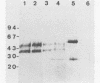
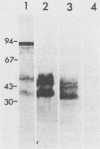
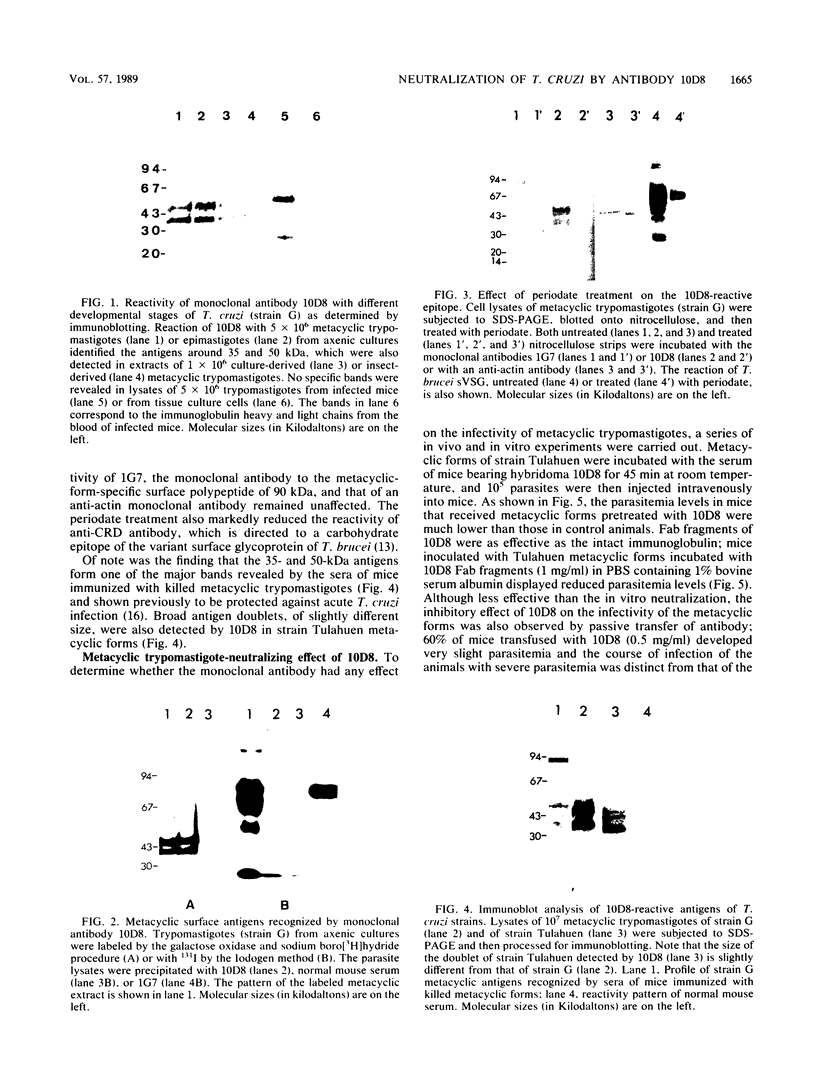
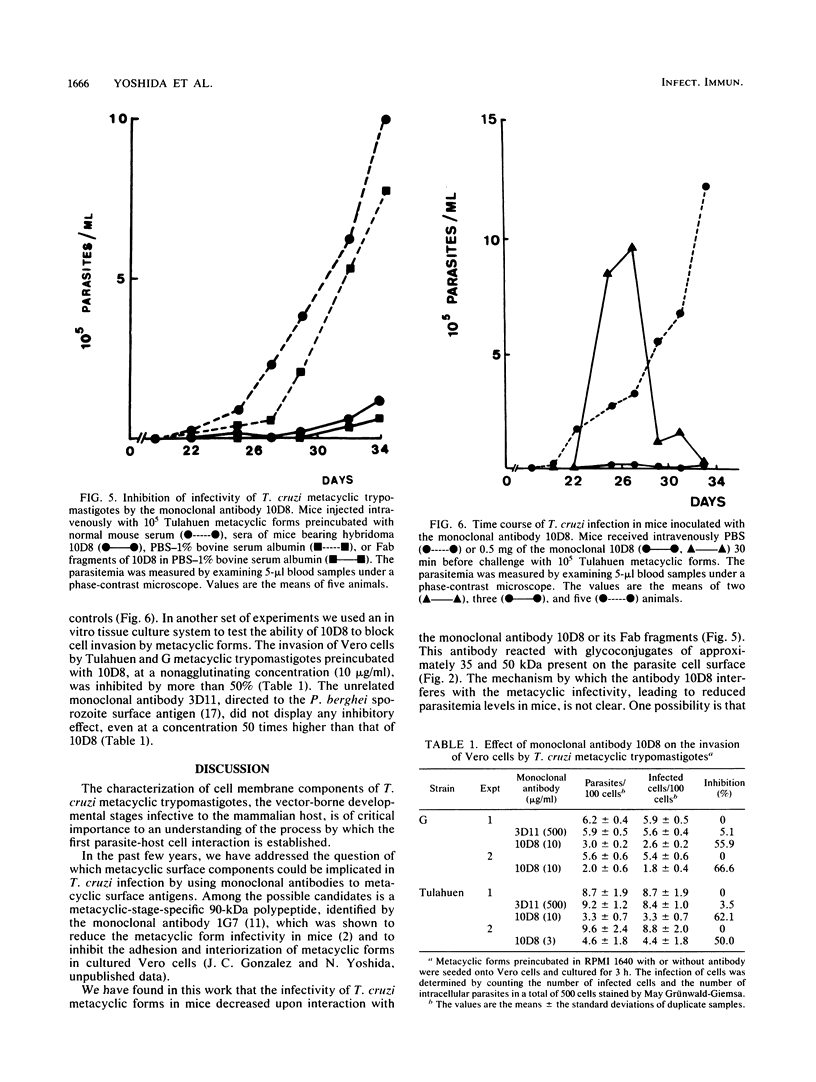
It was shown in this work that the infectivity of metacyclic forms of Trypanosoma cruzi was affected upon interaction with the monoclonal antibody (10D8), which reacts with a carbohydrate epitope of the 35- and 50-kilodalton (kDa) surface glycoconjugates. The invasion of Vero cells by metacyclic forms of strains Tulahuen and G was inhibited 50 to 67% in the presence of 10D8 (10 micrograms/ml), whereas a nonrelated monoclonal antibody to Plasmodium berghei had no such effect. In mice that were inoculated with metacyclic forms preincubated with 10D8 or that had passively received 10D8 before challenge with metacyclic forms, a considerable decrease in the parasitemia levels was observed. The 35- and 50-kDa antigens were detectable by the galactose oxidase and sodium boro[3H]hydride procedure but not by surface iodination or metabolic labeling with [35S]methionine, suggesting that they may be of glycolipid nature. The finding that the 35- and 50-kDa antigens are major bands recognized by sera of mice immunized with killed metacyclic forms and protected against acute infection, in addition to the results with 10D8, indicate that these glycoconjugates may play an important role in the metacyclic form-host cell association that initiates T. cruzi infection.
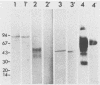
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alves M. J., Abuin G., Kuwajima V. Y., Colli W. Partial inhibition of trypomastigote entry into cultured mammalian cells by monoclonal antibodies against a surface glycoprotein of Trypanosoma cruzi. Mol Biochem Parasitol. 1986 Oct;21(1):75–82. doi: 10.1016/0166-6851(86)90081-2. [DOI] [PubMed] [Google Scholar]
- Araguth M. F., Rodrigues M. M., Yoshida N. Trypanosoma cruzi metacyclic trypomastigotes: neutralization by the stage-specific monoclonal antibody 1G7 and immunogenicity of 90 kD surface antigen. Parasite Immunol. 1988 Nov;10(6):707–712. doi: 10.1111/j.1365-3024.1988.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Boschetti M. A., Piras M. M., Henríquez D., Piras R. The interaction of a Trypanosoma cruzi surface protein with Vero cells and its relationship with parasite adhesion. Mol Biochem Parasitol. 1987 Jun;24(2):175–184. doi: 10.1016/0166-6851(87)90104-6. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J. Mapping structural proteins of cultured cells by monoclonal antibodies. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):769–783. doi: 10.1101/sqb.1982.046.01.073. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- PIZZI T., RUBIO M., PRAGER R., SILVA R. Acción de la cortisona en la infección experimental por Trypanosoma cruzi; communicación preliminar. Bol Inf Parasit Chil. 1952 Apr-Jun;7(2):22–24. [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Teixeira M. M., Yoshida N. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol Biochem Parasitol. 1986 Mar;18(3):271–282. doi: 10.1016/0166-6851(86)90085-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. J., Cardoso de Almeida M. L., Gurnett A. M., Raper J., Ward J. Biosynthesis, attachment and release of variant surface glycoproteins of the African trypanosome. Curr Top Microbiol Immunol. 1985;117:23–55. doi: 10.1007/978-3-642-70538-0_2. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Nussenzweig R. S., Potocnjak P., Nussenzweig V., Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Yoshida N. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect Immun. 1983 May;40(2):836–839. doi: 10.1128/iai.40.2.836-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N. Trypanosoma cruzi: recognition of trypomastigote surface antigens by lytic antisera from mice resistant to acute infection. Exp Parasitol. 1986 Apr;61(2):184–191. doi: 10.1016/0014-4894(86)90151-7. [DOI] [PubMed] [Google Scholar]
- Zingales B., Colli W. Trypanosoma cruzi: interaction with host cells. Curr Top Microbiol Immunol. 1985;117:129–152. doi: 10.1007/978-3-642-70538-0_7. [DOI] [PubMed] [Google Scholar]