Abstract
MicroRNAs (miRNAs) play critical roles in a variety of biological processes in diverse organisms, including mammals. In the mouse skeletal system, a global reduction of miRNAs in chondrocytes causes a lethal skeletal dysplasia. However, little is known about the physiological roles of individual miRNAs in chondrocytes. The miRNA-encoding gene, Mir140, is evolutionarily conserved among vertebrates and is abundantly and almost exclusively expressed in chondrocytes. In this paper, we show that loss of Mir140 in mice causes growth defects of endochondral bones, resulting in dwarfism and craniofacial deformities. Endochondral bone development is mildly advanced due to accelerated hypertrophic differentiation of chondrocytes in Mir140-null mice. Comparison of profiles of RNA associated with Argonaute 2 (Ago2) between wild-type and Mir140-null chondrocytes identified Dnpep as a Mir140 target. As expected, Dnpep expression was increased in Mir140-null chondrocytes. Dnpep overexpression showed a mild antagonistic effect on bone morphogenetic protein (BMP) signaling at a position downstream of Smad activation. Mir140-null chondrocytes showed lower-than-normal basal BMP signaling, which was reversed by Dnpep knockdown. These results demonstrate that Mir140 is essential for normal endochondral bone development and suggest that the reduced BMP signaling caused by Dnpep upregulation plays a causal role in the skeletal defects of Mir140-null mice.
INTRODUCTION
MicroRNAs (miRNAs) are endogenously produced, small RNAs that regulate gene expression mainly at the posttranscriptional level. Direct binding of miRNAs to their target RNAs usually suppresses gene expression and facilitates RNA degradation (1, 3, 39). It is suggested that miRNAs act not only as genetic buffers to suppress harmful leaky gene expression that may compromise the robustness of cellular phenotypes but also as drivers of phenotypical diversity (14). miRNAs have been shown to regulate important biological functions in diverse organisms, including mice (33). In mouse chondrocytes, global miRNA deficiency caused by conditional ablation of Dicer, a gene encoding an RNase III that catalyzes miRNA maturation, results in reduced proliferation and accelerated hypertrophic differentiation downstream of the parathyroid hormone-related peptide (PTHrP) signaling pathway, presumably at a level that inhibits premature hypertrophic differentiation (20). Because chondrocytes express several hundred detectable miRNAs, it is unclear which particular miRNAs are responsible for the skeletal defects of Dicer-deficient chondrocytes. Clarifying the physiological roles of individual miRNAs in chondrocytes is critical for us to further understand the miRNA-mediated gene regulatory system in the skeletal system and to ultimately harness this system for treatment of skeletal diseases.
MicroRNA-140 (miR-140) and -140*, encoded by the Mir140 gene, are expressed almost exclusively in chondrocytes (38, 40). Mir140 is evolutionarily conserved among vertebrates, but this gene is not present in invertebrates. These findings suggest that Mir140 confers certain advantages to vertebrate skeletal systems.
A previous study using zebrafish embryos demonstrated that miR-140 regulates palate chondrogenesis by inhibiting the expression of platelet-derived growth factor receptor, alpha polypeptide (PDGFRA) (9). Deregulation of PDGFRA signaling appears to influence the migration of neural crest-derived mesenchymal cells to the rostral region, which subsequently affects the formation of cartilaginous elements of the head. However, this study provides little insight into the role of Mir140 in chondrocytes, the major cell type expressing this miRNA gene.
In order to understand the physiological role of Mir140, we generated Mir140-null mice. Mice lacking Mir140 showed a shortening of endochondral bones, likely due to mildly accelerated chondrocyte differentiation into postmitotic hypertrophic chondrocytes. Furthermore, we have experimentally identified Dnpep as a Mir140 target whose upregulation may play a causal role in the skeletal defect of Mir140-null mice by dampening bone morphogenetic protein (BMP) signaling.
MATERIALS AND METHODS
Generation of Mir140-null mice.
The targeting vector was constructed to replace the 55-nucleotide sequence of the Mir140 gene by an Flp recombinant target (FRT)-flanked neomycin-resistant gene cassette (FRT-neo) (Fig. 1A to C). The 4.4-kb-long 5′ homologous arm was PCR amplified using the high-fidelity polymerase PrimeStar (Takara-Mirus) with the primers mir140-5-5, 5′-GAGTCTGGACCTGGGTGGTTTTAG-3′, and mir140-5-3-AgeI, 5′-CACCGGTAAAACCACTGGCAGGACACAGAG-3′. The correct sequence was confirmed. The 1.9-kb-long 3′ homologous arm was similarly amplified using the primers mi140-3-5Spe1Hind3, 5′-ACTAGTAAGCTTACTGGAGCACCCTCTGCATCGAAG-3′, and mi140-3-3Not1, 5′-GCGGCCGCCGGCAGTGAGTAACAGTCT-3′. FRT-neo, a kind gift from Susan Dymecki, was ligated between the homologous arms. A diphtheria toxin fragment A (DT-A)-negative selectable marker was placed outside the 3′ homologous arm. The linearized targeting vector was electroporated into V6.5 embryonic stem (ES) cells (10), selected in the presence of 250 μg/ml G418. Homologous recombination was determined by Southern blot analysis and long PCR using the Phusion polymerase (New England BioLabs). ES cells carrying FRT-neo were subjected to blastocyst injection to generate chimeric mice. F1 mice carrying the mutation were further crossed to flpe transgenic mice (35) to remove FRT-neo. Mice were then backcrossed to the C57BL/6 strain for three generations. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and were performed in accordance with its regulations and guidelines.
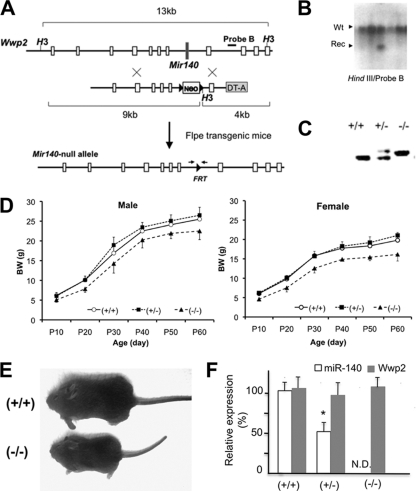
Fig. 1.
Generation of Mir140-null mice. (A) The Mir140 gene was replaced by an FRT-flanked, neomycin-resistant gene (Neo), which was subsequently removed by crossing mice with Flpe transgenic mice. (B) Southern blot analysis shows that an ES cell clone has both wild-type (Wt) and homologous recombinant (Rec) alleles. (C) PCR genotyping of mice using primers spanning FRT (see the small arrows in panel A). (D) Mir140-null mice show growth retardation in both male (left) and female (right) mice, whereas heterozygotes are indistinguishable from wild-type mice (n > 5). The body weight (BW) of Mir140-null mice was significantly lower than that of wild-type or heterozygous mice (n = 5; P < 0.05, t test) at all data points. (E) Picture of 3-week-old female mice. (F) miR-140 expression in primary rib chondrocytes is not detectable (N.D.) in Mir140-null mice, whereas miR-140 expression is reduced by approximately 50% in heterozygotes. The expression level of the host gene of Mir140, Wwp2, is not affected in Mir140-null mice.
Mouse genotyping.
The FRT-neo-free Mir140-null and wild-type alleles were detected by PCR using the primers Mi140-5S2, 5′-CTTGGTGGGCATCTGGTGTGGCTC-3′, and Mi140-3N, 5′-GAGCTAAACAATTGGGGAACAATC-3′, as 256-bp- and 233-bp-long PCR amplicons, respectively. Primer sequences to genotype Cre transgenic mice (19) and floxed Pdgfra mice (37) were described previously.
Skeletal preparation and histology.
Alizarin red and Alcian blue staining was performed using a modified McLeod method (25). Carcasses were fixed in 95% ethanol, stained with Alcian blue and Alizarin red, cleared in 1% KOH, and kept in 50% glycerol. For histological analysis, mice were dissected, fixed in 10% formalin, decalcified in 10% EDTA, paraffin processed, cut, and subjected to hematoxylin-eosin staining, in situ hybridization, and BrdU staining. In situ hybridization was carried out as previously described (28). The probe for Col10a1 was described previously (19).
BrdU labeling and detection.
For BrdU labeling, 50 μg BrdU/g of body weight was given to mice intraperitoneally 2 h before sacrifice. BrdU was detected using the BrdU staining kit (Invitrogen). The BrdU labeling index was calculated as the ratio of the number of BrdU-positive nuclei over the total number of nuclei in proliferating columnar chondrocytes of the growth plate.
Primary chondrocyte isolation and culture.
The frontal part of the rib cage was removed from 1.5-day-old mice, rinsed with phosphate-buffered saline (PBS), and incubated in a digestion medium containing Dulbecco modified Eagle medium (DMEM), 10% fetal calf serum (FCS), and 0.2% (approximately 600 U/ml) collagenase type II (Worthington) at room temperature for 10 min. Muscle, soft tissues, and mineralized rib and sternal bones were removed from the rib cage using fine tweezers and surgical scissors under a dissection microscope. This procedure was repeated until most of the noncartilaginous tissues were removed from the specimen. Rib cartilage was further digested in fresh digestion medium at 37°C for 4 h. Cells were released from the cartilage matrix by gentle pipetting, passed through nylon mesh strainers (BD Falcon), spun to remove collagenase, counted, plated at the concentration of 5 × 105 cells/ml in the DMEM medium containing 10% FCS, and cultured overnight.
Ago2-IP and microarray analysis.
Primary rib chondrocytes were cultured overnight. Total RNA was isolated from 5% of cells using SurePrep columns (Fisher) for input analysis. The rest of the cells were subjected to Ago2 immunoprecipitation (Ago2-IP) using the microRNA isolation kit Mouse Ago2 (Wako Chemicals) according to the manufacturer's instructions. For a negative control, immunoprecipitation was performed using nonimmune IgG beads prepared with the antibody immobilization bead kit (Wako Chemicals). RNA was amplified using the Ovation Pico WTA system (Nugen), labeled, and subjected to microarray analysis using HT-MG-430-PM array plates or mouse genome 430 2.0 gene chips (Affymetrix). Ago2-IP enrichment scores were calculated as normalized signal intensity ratios of total RNA and Ago2-IP RNA preparations. Genes whose Ago2-IP enrichment scores were greater than 2 were considered to be enriched in the Ago2-IP fraction.
qRT-PCR.
cDNA synthesis was performed using random hexamers and the DyNAmo cDNA synthesis kit (Finnzymes). Quantitative PCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems) and the EvaGreen quantitative reverse transcription-PCR (qRT-PCR) mix (Solis BioDyne). Primer sequences are as follows: GAPDH-L, 5′-CACAATTTCCATCCCAGACC-3′, and GAPDH-R, 5′-GTGGGTGCAGCGAACTTTAT-3′; Col2a1-L, 5′-AACACTTTCCAACCGCAGTC-3′, and Col2a1-R, 5′-TCTGCCCAGTTCAGGTCTCT-3′; Acan-L, 5′-GAAGAGCCTCGAATCACCTG-3′, and Acan-R, 5′-ATCCTGGGCACATTATGGAA-3′; Hmga2-L, 5′-ATCCAACTTTCTCCCCGTTC-3′, and Hmga2-R, 5′-AGGTATTGCCACAAGCAAGC-3′; Wwp2-L, 5′-ATTCTGGGCAAGGTGACAAC-3′, and Wwp2-R, 5′-GCTTTGGTCTGCTCTTCCAC-3′; Pdgfra-L, GATCCGGGCTAAGGAAGAAG-3′, and Pdgfra-R, 5′-CCAAAATGGATGCAGGAACT-3′; mouse Dnpep-L, 5′-CAGACCCTCACCCTTTTCAA-3′, and mouse Dnpep-R, 5′-TTGAAGGGGTGGAAGACAAC-3′; zebrafish Dnpep-L, 5′-CTGCGCTCATCCAGATGTTA-3′, and zebrafish Dnpep-R, 5′-GGTTGTCCAGACGAGGAGAG-3′; and zebrafish GAPDH-L, 5′-GATTGCCGTTCATCCATCTT-3′, and zebrafish GAPDH-R, 5′-GCCATCAGGTCACATACACG-3′.
Levels of expression of miR-140 and the normalization control, U6, were determined using the mirVana qRT-PCR miRNA detection kit (Ambion).
Cloning of mouse and rat Dnpep cDNA.
Mouse Dnpep cDNA was PCR amplified and cloned into the pCS2+ expression vector at the EcoRI and XhoI sites using primers R1-Dnpep5, 5′-CAgaattcGCCAGTGAAGTCCTCGGAAGC-3′ and Xh-Dnpep3, 5′-CTctcgagTGAAAATCTACTTTAATAACCAACA-3′ (lowercase letters indicate attached restriction enzyme sites). Rat Dnpep cDNA was purchased from Open Biosystems and further subcloned into the pCS2+ vector.
Zebrafish experiments.
Zebrafish maintenance and injection were performed as described previously (29). Injection of the miR-140 duplex was performed according to the protocol described previously (9).
Mir140 binding site prediction.
The binding sites of miR-140 and miR-140* were predicted using STarMir (http://sfold.wadsworth.org/cgi-bin/starmir.pl) (7).
Transfection and luciferase assay.
The predicted Mir140-binding sequence of Dnpep (designated BD), 5′-AACCAGGGCCTCTCGGTGCCACTGATGAGCGGCACCACTC-3′, was oligonucleotide synthesized, with the restriction enzyme sites SpeI and HindIII at its 5′ and 3′ ends, and subcloned into the pMIR-REPORT luciferase vector (Ambion). Mutant BD (5′-AACCAGGGCCTCTCGGTGggtgacATGAGCGGCtggtga3′; mutated nucleotides are shown in lowercase letters) was similarly synthesized and subcloned. One one hundredth microgram of the luciferase constructs, 0.02 μg of the Renilla luciferase control vector pRL-SV40, and 5 nmol of the miR-140 precursor or control (Ambion) per well of 96-well plates were cotransfected into ATDC5 cells using the Attractene transfection reagent (Qiagen). Luciferase and Renilla activities were measured 24 h after transfection using the dual-luciferase reporter assay system (Promega).
To assay the BMP response, the BMP reporter luciferase construct, carrying four copies of a BMP-2 responsive element in the Id1 gene (18), and pRL-SV40 were cotransfected into BMP-responsive C2C12 cells or primary rib chondrocytes using the Attractene transfection reagent with or without a Dnpep expression vector or a small interfering RNA (siRNA) duplex. The sequences of the Dnpep siRNA duplex are 5′-CCAAUGAGGCUGAAGCCAUUGCCAGGA-3′ and 5′-CUGGCAAUGGCUUCAGCCUCAUUGG-3′. Dnpep siRNA and control siRNA with a scrambled sequence were purchased from Integrated DNA Technologies. Transfected cells were cultured for 24 h with or without BMP-2 (Shenandoah Biotechnology, Inc.) in 10% FCS-containing DMEM.
Western blot analysis.
Anti-phospho-Smad1/5/8 antibody (Cell Signaling Technology), anti-PDGFRA antibody (Millipore), anti-Dnpep antibody (Protein Tech Group, Inc.), anti-Smad4 antibody (Genway Biotech, Inc.), and antiactin antibody (Santa Cruz Biotechnology) were purchased. Western blot analysis was performed according to the standard procedure.
Microarray data accession number.
The microarray data are available in the Gene Expression Omnibus (GEO) database under the accession number GSE27177.
RESULTS
Mir140-null mice show defects in endochondral bone growth.
In order to investigate the physiological role of the chondrocyte-specific miRNA gene, Mir140, mice lacking Mir140 were generated by gene targeting (Fig. 1A to C). Mir140-null mice survived postnatally and reached adulthood but showed impaired growth (Fig. 1D and E). The Mir140 gene is present in an intron of the Wwp2 gene, which encodes an E3 ubiquitin ligase. The deletion of the Mir140 gene did not alter the expression level of Wwp2 in primary chondrocytes, whereas miR-140 miRNA was not detectable in Mir140-null mice (Fig. 1F).
As suggested by the postnatal growth retardation of Mir140-null mice, long bones, which grow through endochondral bone formation, were significantly shorter in Mir140-null mice than in control mice (Fig. 2A and B). Another characteristic skeletal phenotype of Mir140-null mice was a defect in the longitudinal growth of the skull (Fig. 2C and D). Like long bones, longitudinal growth of the basal skull is mediated primarily by the growth plate cartilage. The shortening of these bones in the basal skull was detectable even in embryonic stages (Fig. 2E). A sagittal section of the basisphenoidal bone, flanked by bidirectional growth plates, at postnatal day 5.5 (P5.5) showed a reduction in longitudinal length (Fig. 2F).
Fig. 2.
The longitudinal growth of endochondral bones is impaired in Mir140-null mice. Whole-mount Alizarin staining (A to D) shows the shortening of the indicated long bones (A), skull (C), and palate (D) of a 4-week-old male Mir140-null mouse. Brackets indicate palatal bones. (B) The lengths of the femur and tibia of 2-week-old Mir140-null mice are significantly smaller than those of wild-type littermates (n = 3; P < 0.05, t test). (E) Alizarin staining shows mild shortening of bones of the basal skull (right) of E17.5 Mir140-null embryos. BO, basioccipital bone; BS, basisphenoidal bone. (F) Hematoxylin and eosin-stained sections of the basisphenoidal bone (BS) of 5.5-day-old mice. The basisphenoidal bone (brackets) is shortened in Mir140-null mice. The bidirectional growth plates flanking this bone are slightly shortened. P, pituitary gland.
Mir140 deficiency results in mild acceleration of chondrocyte differentiation and bone development.
As in endochondral bones of the skull, mild skeletal abnormalities were observed in endochondral bones in other parts of the body at embryonic and neonatal stages. At birth, we found that talus bone development was consistently advanced in Mir140-null mice, as indicated by its large mineralized area (Fig. 3A and B). Similarly, the initial mineralization of the radius, ulna at embryonic day 14.5 (E14.5), and the hyoid horn at P1.5 was advanced in Mir140-null mice (Fig. 3C and see Fig. 5D). In situ hybridization to detect Col10a1, a specific marker of hypertrophic chondrocytes, indicated an increase in the size of the hypertrophic region of the talus at P0.5 and tibia at E14.5 in Mir140-null mice (Fig. 3D and E). The size of the hypertrophic region of the tibia was significantly larger in Mir140-null mice (0.473 ± 0.003 mm versus 0.503 ± 0.008 mm) (n = 3 each; P < 0.05, t test) than in normal mice. These findings suggest that initial hypertrophic differentiation and subsequent bone formation occur prematurely in the absence of Mir140.
Fig. 3.
Mild acceleration of bone development in Mir140-null mice. Whole-mount skeletal staining (A to C) shows an advanced mineralization of the talus (arrow) in newborn Mir140-null hind limbs (A). The sizes of Alizarin red-stained mineralized areas of the talus (B) and forearms (C) are greater in Mir140-null mice than in wild-type or heterozygous littermates. Arrows indicate mineralization of the ulna. (D, E) The size of the hypertrophic region marked by Col10a1 (brackets) is greater in the talus at P0.5 (D) and the tibia at E14.5 (E) than in control animals, suggesting accelerated hypertrophic differentiation of chondrocytes. Brackets and black lines indicate the hypertrophic region. H/E, hematoxylin and eosin stain. (F) Growth plates of the Mir140-null tibia at P5.5 exhibit no significant proliferation defects in proliferating columnar chondrocytes.
Fig. 5.

PDGFRA is not a physiologically important target of Mir140 in mouse chondrocytes. (A) PDGFRA expression is not upregulated in Mir140-null primary chondrocytes. Multiple bands detected by immunoblot analysis using anti-PDGFRA antibody in primary rib chondrocytes (left). The top two bands (arrowhead) migrate at the expected molecular masses (150 and 170 kDa). Pdgfra mRNA expression determined by qRT-PCR was not significantly altered in Mir140-null primary chondrocytes. N.S., not significant. (B) A chondrocyte-specific PDGFRA knockout (cKO) shows mild shortening of the longitudinal length of the skull and long bones. Skeletal preparation of the skull (left) and hind limbs (middle) at P10.5. Mineralization of the talus at P0.5 is slightly advanced in cKO mice (right). (C, D) Conditional ablation of Pdgfra does not rescue the skeletal defect of Mir140-null mice. (C) At P1.5, the shortening of the long bones of Mir140-null mice is not rescued by conditional Pdgfra ablation in chondrocytes. (D) The advanced mineralization of the hyoid horns (arrowheads) of 1.5-day-old Mir140-null mice was even augmented but not rescued in doubly mutant mice.
Premature differentiation of proliferating chondrocytes into postmitotic hypertrophic chondrocytes causes shortening of bones by reducing the number of proliferating chondrocytes, as exemplified by mutant mice lacking parathyroid hormone-related peptide (PTHrP) signaling, a pivotal regulator of chondrocyte differentiation (16, 22). It is therefore possible that mild acceleration of differentiation into postmitotic hypertrophic chondrocytes reduced the net mass of growth plate chondrocytes over time, leading to the growth defects observed in Mir140-null mice.
We did not find an alteration in the proliferation of columnar chondrocytes in Mir140-deficient tibiae at P5.5 (Fig. 3F), P21, P14, P3.5, or E17.5 (data not shown). These results suggest that impairment of endochondral bone growth in Mir140-null mice is caused primarily by altered chondrocyte differentiation.
Reciprocal change in the sizes of the resting and columnar zones in the Mir140-null growth plate.
In growth plates, chondrocytes in the resting zone proliferate slowly and then differentiate into vigorously proliferating columnar chondrocytes. Changes in cellular differentiation at this step influence the columnar length (21). Chondrocytes in the resting zone are small and do not form distinct columns, whereas columnar chondrocytes are flat and form orderly columns.
The growth plate morphology at early postnatal stages was relatively normal (data not shown). However, at later stages, when the secondary ossification center is already formed, we found that Mir140-null growth plates showed an increase in the size of the resting zone and a modest decrease in the size of the columnar zone (Fig. 4A to C). This finding suggests a possible inhibition of chondrocyte differentiation from the resting zone into proliferating columnar chondrocytes. We did not find significant shortening of the overall growth plate length in Mir140-null mice.
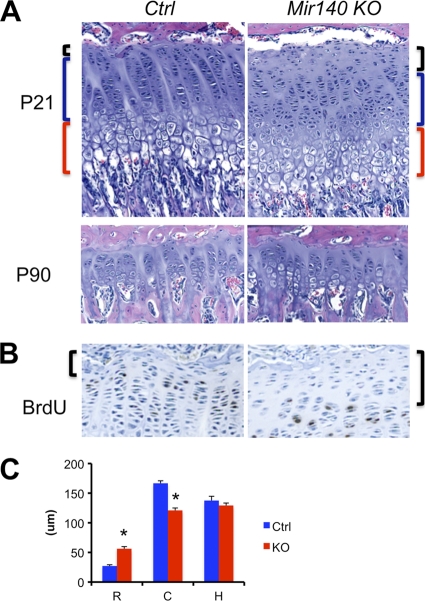
Fig. 4.
(A) Reciprocal change in the sizes of the resting and columnar zones of the Mir140-null growth plate. Hematoxylin and eosin-stained growth plates of the proximal tibia at P21 show an increase in the size of the resting zone (black brackets) and a modest decrease in the size of the columnar zone (blue brackets) in Mir140-null mice (KO) compared with those of control mice (Ctrl). This tendency is present but less appreciable in older mice (P90). (B) The resting zone (brackets), containing chondrocytes with low BrdU incorporation rates, is expanded in the Mir140-null growth plate. (C) The length of each layer was measured in 21-day-old control (wild-type and heterozygous mice) and Mir140-null littermates. The columnar zone (bars labeled C) was defined as the region containing columns formed by more than 5 chondrocytes per column. The resting (bars R) and hypertrophic (bars H) zones are defined as the cartilage area adjacent to the columnar zone (*, P < 0.05, n = 3, t test).
Pdgfra is not a physiologically important target of Mir140 in mouse skeletal development.
It has been shown that miR-140 targets Pdgfra to control palatal skeletogenesis in zebrafish (9). Since Pdgfra is also predicted to be a miR-140 target in mice, we tested whether PDGFRA expression was altered in mouse chondrocytes lacking Mir140. We did not find upregulation of PDGFRA expression in Mir140-null primary rib chondrocytes (Fig. 5A), in contrast to results for zebrafish with the miR-140 knockdown. In order to examine the possible causal role of the loss of Mir140-dependent Pdgfra suppression in the skeletal defect of Mir140-null mice, we tested whether genetic deletion of Pdgfra in chondrocytes could rescue the skeletal defect of Mir140-null mice. The Pdgfra gene was ablated in chondrocytes using the Cre-loxP binary system; Cre mice expressing Cre in chondrocytes under the control of a type II collagen promoter (32) were crossed to floxed Pdgfra (Pdgfrafl/fl) mice (37). Heterozygous conditional Pdgfra-null (Cre Pdgfrafl/+) and compound heterozygous (Cre Pdgfrafl/+ Mir140+/−) mice were indistinguishable from wild-type littermates. These mice were further crossed with Mir140-null mice to generate compound homozygous mutants (Cre Pdgfrafl/fl Mir140−/−). In the genetic background used in this study, chondrocyte-specific Pdgfra-deficient mice (conditional knockout [cKO]) survived postnatally and reached adulthood. However, Pdgfra cKO mice showed a mild reduction in growth. The body weight of Pdgfra cKO mice at P28 was about 10% less than that of control littermates (11.5 ± 0.4 g versus 13.2 ± 0.3 g), but the mice showed grossly normal appearance. Despite the mild reduction in size, mineralization of the talus at P0.5 was slightly greater in cKO mice and similar to that of Mir140-null mice (Fig. 5B). Mir140-null Pdgfra cKO doubly mutant mice were slightly smaller and had slightly shorter long bones than Mir140-null littermates, demonstrating that Pdgfra ablation was unable to rescue the defect in the long bone growth of Mir140-null mice (Fig. 5C). At P1.5, the hyoid horn of Mir140-null mice showed advanced mineralization similar to that of the talus, ulna, and radius at earlier stages. Again, simultaneous ablation of Pdgfra did not rescue the advanced hyoid mineralization (Fig. 5D). These results indicate that Pdgfra is not a physiologically important target of Mir140 in mouse chondrocytes.
Mir140 targets Dnpep.
To understand the molecular mechanism by which Mir140 regulates endochondral bone development, we sought to identify target transcripts of Mir140 miRNAs. We took advantage of the finding that RNA immunoprecipitation using anti-Argonaute (Ago) antibodies can identify miRNA-regulated genes (4, 8, 12, 13, 17, 42). We reasoned that (i) Ago-associated RNAs in wild-type chondrocytes should contain Mir140 targets, (ii) RNAs that are regulated predominantly by Mir140 should be associated with Ago to a lesser extent in Mir140-null than in wild-type chondrocytes, and (iii) genes regulated mainly by Mir140 should be upregulated in Mir140-null chondrocytes, since miRNA-RNA interaction usually leads to RNA degradation (11). We performed immunoprecipitation using an antibody against Argonaute 2, the most abundant Ago in mice (Ago2-IP). In wild-type chondrocytes, the well-known let-7 miRNA target transcript Hmga2 (23, 24), along with Dnpep, was immunoprecipitated with this antibody but not nonimmune IgG (Fig. 6B). We profiled Ago2-associated transcripts by microarray analysis and screened them according to the screening strategy depicted (Fig. 6A). We examined genes with relatively high signal intensities by quantitative reverse transcription-PCR (qRT-PCR) and confirmed that the gene encoding an aspartyl aminopeptidase, Dnpep, was enriched in the Ago2-IP fraction of wild-type but not Mir140-null chondrocytes (Fig. 6C). On the other hand, Hmga2 was similarly enriched in the Ago2-IP fractions of both wild-type and Mir140-null chondrocytes. As expected, Dnpep expression was upregulated in Mir140-null chondrocytes (Fig. 6D and E). To further confirm the interaction between miR-140 and Dnpep, we coinjected a miR-140 duplex and Dnpep mRNA into zebrafish embryos. Injection of miR-140 causes defects in palatal skeletogenesis in zebrafish (9). Coinjection of mouse Dnpep mRNA significantly reduced the occurrence of the miR-140-induced palatal defect (Fig. 7A). Interestingly, coinjection of rat Dnpep mRNA also had the same effect. Zebrafish Dnpep lacks strongly predicted miR-140 binding sites, and expression of endogenous zebrafish Dnpep was unchanged 1 and 3 days after miR-140 injection (data not shown), suggesting that miR-140 does not regulate zebrafish Dnpep; therefore, this rescue likely occurred because coinjected Dnpep sequesters miR-140. This result suggests a direct interaction between miR-140 and Dnpep transcripts. Bioinformatics analysis using STarMir (7) identified multiple potential binding sites of miR-140 in the full mouse and rat Dnpep sequence. However, only a single site located in the coding sequence was commonly predicted in mouse and rat Dnpep. This site is located in a 45-nucleotide-long region (designated BD) that contained two potential miR-140 binding sites in mouse Dnpep (Fig. 7B). To test whether the BD sequence could mediate the miR-140–Dnpep interaction, a luciferase reporter plasmid carrying the BD sequence was constructed. Cotransfection with a miR-140 miRNA precursor reduced the luciferase activity of the reporter construct with the BD but not a mutated BD sequence, suggesting that miR-140 suppresses Dnpep expression by binding to BD (Fig. 7C). Furthermore, transfection of the miR-140 precursor into chondrogenic ATDC5 cells reduced Dnpep expression (Fig. 7D).
Fig. 6.
miR-140 targets Dnpep in mouse chondrocytes. (A) Experimental strategy. RNA was prepared from the Ago2-IP fraction and total RNA from wild-type and Mir140-null primary chondrocytes and subjected to expression profiling. RNA enriched in the Ago2-IP fraction was first identified in wild-type (a) and Mir140-null (c) chondrocytes; genes that were enriched in the Ago2-IP fraction in wild-type but not in Mir140-null chondrocytes were selected. Among them, those upregulated in Mir140-null chondrocytes (b) were subjected to further investigation. (B) RNA immunoprecipitation followed by RT-PCR (RIP-RT-PCR) shows the specific association between Hmga2 and Dnpep transcripts and Ago2 in wild-type cells. (C) Dnpep is associated with Argonaute 2 (Ago2) in wild-type but not in Mir140-null chondrocytes. Ago2 association was determined by comparing transcript levels of Ago2 immunoprecipitants with those of total RNA (Input in panel B). Transcript levels were expressed relative to that of GAPDH of each fraction. Ago2-IP enrichment ratios were calculated by dividing normalized transcript levels in the Ago2-IP RNA by those in total RNA. The relative enrichment of Dnpep in the Ago2-IP fraction is significantly less in Mir140-null chondrocytes, whereas Hmga2, a quintessential target of let-7 miRNAs, is enriched in the Ago2-IP fractions of both Mir140-null and wild-type chondrocytes (*, P < 0.05, n = 4, t test). (D, E) Dnpep is upregulated in Mir140-null primary rib chondrocytes both at the RNA level (D) and at the protein level (E).
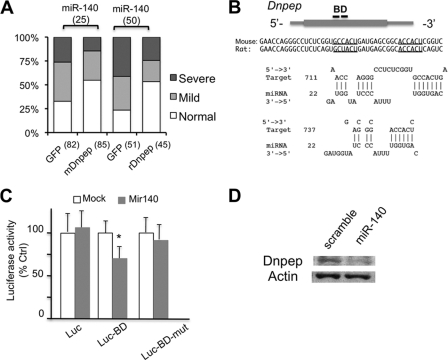
Fig. 7.
Direct interaction between miR-140 and Dnpep suppresses Dnpep expression. (A) Coinjection of mouse or rat Dnpep rescues miR-140-induced palatal defects in zebrafish. Zebrafish embryos were injected with 3 nl of 25 μM miR-140 or 50 μg of miR-140 duplex solution with 1 ng of the indicated synthesized mRNA. The total numbers of analyzed fish are indicated in parentheses below the graph. The severity of the skeletal phenotypes was judged according to the criteria described previously (9). Fractions of fish with normal development were significantly greater in mouse or rat Dnpep-coinjected animals than in control animals (P < 0.05, χ2 test). (B) A major miR-140 binding site (BD) and its sequence predicted by STarMir are shown. The coding and untranslated regions are indicated by a gray box and lines, respectively. Underlines indicate seed sequence binding sites. (C) A luciferase reporter construct carrying the 45-nucleotide-long BD sequence, but not a mutated BD, shows a reduction in luciferase activity upon miR-140 cotransfection. The luciferase activity was normalized to that of cotransfected Renilla luciferase. (*, P < 0.05, n = 4, t test). (D) miR-140 transfection into ATDC5 cells reduces Dnpep expression.
Dnpep antagonizes BMP signaling downstream of Smad activation.
Dnpep has been suggested to be involved in intracellular peptide metabolism, but its role in vivo is not known. In order to understand the possible role of Dnpep in vivo, first, we overexpressed Dnpep in zebrafish embryos. The majority of the injected embryos showed grossly normal development. However, we found that 4.2 to 6.3% of Dnpep-injected embryos showed dorsalization, whereas dorsalization was never observed in green fluorescent protein (GFP)-injected embryos (Fig. 8A and B). Dorso-ventral patterning in zebrafish embryos is controlled by BMP and Wnt signaling (36). Since dorsalized, Dnpep-injected embryos maintained anterior head structures, whose formation is antagonized by Wnt signaling, we speculated that dorsalization caused by Dnpep overexpression was due to reduced BMP signaling, rather than increased Wnt signaling. To test this possibility, we coinjected Bmp2b and Dnpep into zebrafish embryos to test whether Dnpep could antagonize the ventralization effect caused by Bmp2b overexpression (Fig. 8C and D). Dnpep coinjection caused a mild but significant inhibitory effect in the ventralization induced by Bmp2b. This result further supports the antagonistic effect of Dnpep in BMP signaling. In order to test whether this effect is conserved in mammalian cells, we overexpressed Dnpep along with a BMP reporter construct carrying the BMP-responsive element of the Id1 gene (18). Dnpep overexpression in BMP-responsive C2C12 cells showed significantly reduced responses to BMP-2 (Fig. 9A). Since Dnpep was upregulated in Mir140-null chondrocytes, we tested the BMP activity of Mir140-null primary rib chondrocytes. Primary rib chondrocytes were transfected with the BMP reporter construct and treated with BMP-2. We found that the reporter activity was consistently lower in Mir140-null chondrocytes than in the control (Fig. 9B) at any BMP concentration. However, upon stimulation with BMP-2, the reporter activity increased in a dose-dependent manner in both control and Mir140-null chondrocytes. Mir140-null chondrocytes showed a normal response in the phosphorylation of Smad1/5/8 upon BMP treatment, an immediate consequence of BMP receptor activation, and a normal level of the Smad1/5/8 binding partner, Smad4 (Fig. 9C and D). These results suggest that BMP-dependent gene transcription is compromised in Mir140-null chondrocytes at a position downstream of Smad activation. Next, we tested whether overexpression of Dnpep reduces the BMP reporter activity in primary chondrocytes. Primary chondrocytes were cotransfected with reporter constructs and a Dnpep expression vector and cultured in the presence of 50 ng/ml of BMP-2. Dnpep overexpression reduced the basal BMP reporter activity both in control and in Mir140-null chondrocytes (Fig. 9E). Conversely, Dnpep knockdown using siRNA increased BMP reporter activity in Mir140-null chondrocytes (Fig. 9F). These results show that Dnpep upregulation negatively regulates BMP signaling in Mir140-null chondrocytes.
Fig. 8.
Negative regulation of BMP activity by Dnpep. (A, B) Dorsalization in zebrafish overexpressing Dnpep. (A) Representative picture of dorsalized (white arrows) and normal (black arrow) embryos. Shortening of the tail and preservation of anterior structures of the head are observed at 30 h postfertilization. (B) About 4 to 6% of Dnpep-injected zebrafish show dorsalization (P < 0.05, χ2 test). The number of injected embryos per experimental group is indicated in parentheses above the graph. (C, D) Dnpep coinjection reduces the occurrence of ventralization caused by Bmp2b (P < 0.05, χ2 test). Numbers of injected animals are indicated in parentheses. Pictures were taken at 30 to 35 h postfertilization.
Fig. 9.
Dnpep dampens BMP signaling in mammalian cells. (A) BMP-responsive C2C12 cells were cotransfected with a BMP-responsive luciferase reporter (Id1-Luc), a Renilla luciferase (pRL-SV40) control, and an empty (control) or a Dnpep expression vector. Cells were treated with BMP-2 at the indicated concentrations (ng/ml) for 24 h. Relative luciferase activity normalized by that of Renilla luciferase was significantly reduced in Dnpep-overexpressing cells. (*, P < 0.05, n = 5, t test). RLU, relative light units. (B) Reduced BMP reporter activity in Mir140-null chondrocytes. Primary rib chondrocytes were transfected with Id1-Luc and treated with BMP-2 at the indicated concentrations for 24 h. Mir140-null chondrocytes show significantly lower normalized luciferase activities than those of the control (*, P < 0.05, n = 8, t test). (C, D) Smad phosphorylation (C) or the Smad4 level (D) is not affected in Mir140 deficiency. (E) Dnpep overexpression significantly reduced BMP reporter activity both in control and in Mir140-null (KO) chondrocytes (*, P < 0.05, n = 8, t test). Cells were cultured in the presence of 50 ng/ml of BMP-2 for 24 h. (F) An siRNA-mediated knockdown of Dnpep increased BMP reporter activity. siRNA duplexes were cotransfected to primary rib chondrocytes with Id1-Luc and pRL-SV40. Cells were cultured in the presence of 50 ng/ml of BMP-2 for 24 h. The Dnpep siRNA significantly increased the BMP reporter activity in Mir140-null chondrocytes (*, P < 0.05, n = 6, t test).
DISCUSSION
We have shown that loss of the chondrocyte-specific miRNA gene, Mir140, in mice causes defects in endochondral bone development. Since there were no overt changes in the proliferation of Mir140-null growth plate chondrocytes, the growth defects observed in Mir140-null mice are likely caused by the acceleration of chondrocyte differentiation into postmitotic hypertrophic chondrocytes. Although the acceleration of hypertrophic differentiation is relatively mild, causing few detectable histological changes, it is possible that, over time, these defects negatively influence the production of the net chondrocyte mass, leading to a visible reduction in the skeletal size of Mir140-null mice. A recent study describing separately generated Mir140-null mice reports a reduction in the proliferation and shortening of the growth plates (27). In this study, we did not find significant reductions in chondrocyte proliferation or growth plate length at multiple stages of development. The reason for these disparities is not clear. We backcrossed mice to the C57BL/6 strain for three generations, and it is possible that the difference in the genetic background may influence the phenotype. We found a relative increase in the size of the resting zone and a mild decrease in the size of the columnar zone in 3-week-old Mir140-null mice. This finding suggests a possible inhibition of resting chondrocyte differentiation into columnar chondrocytes. Stimulation of chondrocyte differentiation at this step increases the length of the columnar zone (21). Therefore, it is likely that inhibition of this step would cause a reduction in proliferating columnar chondrocytes. As with acceleration of hypertrophic differentiation, inhibition of this step would also decrease the number of proliferating chondrocytes, resulting in the shortening of endochondral bones.
The skeletal abnormalities of Mir140-null mice differ from those reported in zebrafish with a miR-140 knockdown (9). Mice lacking Mir140 show a shortening of the palate rather than the elongation observed in zebrafish with miR-140 knocked down. The zebrafish study demonstrated that miR-140 suppressed the expression of PDGFRA, which regulates the migration of neural crest-derived mesenchymal cells to the rostral region. Although Pdgfra is also predicted to be a target of miR-140 in mice, we did not find significant alterations in PDGFRA expression in Mir140-null chondrocytes. In addition, genetic ablation of Pdgfra did not lessen the skeletal defect of Mir140-null mice. These data demonstrate that, unlike in zebrafish palatal development, Pdgfra is not a physiologically important target in mouse chondrocytes. This finding underscores the possibility that a miRNA may have substantially different sets of target transcripts and thus may play different physiological roles, dependent on animal species and cell types.
Identification of miRNA targets has been a challenge. Bioinformatics approaches have offered only limited solutions. Since bioinformatics approaches rely solely on base pairing of miRNAs and target RNAs, they do not take into account particular cellular contexts, such as expression levels of target RNAs and miRNAs. Therefore, to identify physiologically meaningful targets of miRNAs, it is necessary to experimentally examine miRNA-RNA interactions under specific conditions. A few methods have been developed to experimentally identify miRNA targets. Based on the fact that miRNAs bind to target RNAs through base pairing, miRNA-target RNA complexes were successfully isolated by pulldown assays using biotin- or digoxigenin-tagged miRNAs (15, 31). However, these methods may not reveal physiologically relevant interactions because tagged RNAs need to be exogenously added. In this study, we performed RNA immunoprecipitation using anti-Ago2 antibody (Ago2-IP) to isolate RNAs regulated by endogenous miRNAs in chondrocytes. Since Mir140 is one of the most abundant miRNAs in chondrocytes, we reasoned that Mir140 target RNAs should be enriched in the Ago2-IP fraction along with targets of other miRNAs expressed in chondrocytes and, therefore, that comparing profiles of Ago2-associated RNA between wild-type and Mir140-null chondrocytes would reveal target transcripts regulated mainly by Mir140. We found only 44 genes (data not shown), including Dnpep, that satisfied the screening criteria. Since many RNAs can be regulated by multiple miRNAs, it is likely that this strategy can identify only RNAs whose expression is regulated exclusively by Mir140. This screening did not find previously reported targets of miR-140, such as Pdgfra (9), Adamts5 (27), Hdac4 (38), or CxCl12 (30). It is possible that these genes may also be regulated by other miRNAs, and therefore, loss of miR-140 alone does not significantly reduce the interaction with Ago2.
Coinjection of both mouse and rat Dnpep transcripts suppressed the miR-140-induced palatal defect in zebrafish, but the only commonly predicted binding sequence was found in the coding sequence. It has been increasingly appreciated that miRNAs bind sequences beyond the 3′ untranslated region of genes (6). Since this short sequence was able to reduce reporter activity in a miR-140-dependent manner, it is likely that Dnpep expression is regulated by miR-140, at least in part, through this sequence.
Dnpep encodes an aspartyl aminopeptidase that catalyzes the sequential removal of amino acids from the unblocked N termini of peptides and proteins. Although Dnpep is expressed broadly, its physiological role in mammalian cells is poorly understood. It is speculated that aspartyl aminopeptidases are involved in protein and peptide metabolism and possibly in modulation of biologically active peptides such as angiotensin I (2). Dnpep overexpression in this study suggested that Dnpep had a weak antagonistic effect on BMP signaling. We found that the BMP reporter activity in Mir140-null chondrocytes was significantly reduced and that manipulation of Dnpep in primary chondrocytes confirmed that Dnpep negatively regulated the BMP reporter activity. In vivo reporter analysis has demonstrated a robust BMP-dependent transcriptional activity in growth plate chondrocytes (5). BMP signaling regulates cartilage development at multiple steps; it is essential for early chondrocyte differentiation (34, 41) and for maintaining the mass of proliferating chondrocytes by antagonizing fibroblast growth factor (FGF) signaling (26). Therefore, it is possible that the reduced BMP action in chondrocytes contributes to the skeletal defects of Mir140-null mice.
The mechanism by which Dnpep antagonizes BMP signaling is not clear at the moment. Smad phosphorylation and the Smad4 level in Mir140-null chondrocytes were unaffected. Although the BMP reporter activity in Mir140-null chondrocytes is constantly reduced compared with that in the control, Mir140-null chondrocytes respond reasonably well to exogenous BMP-2. These data suggest that Dnpep inhibits BMP action at a position downstream of Smad activation. For example, Dnpep might degrade cofactors of Smads in the regulation of gene transcription.
ACKNOWLEDGMENTS
We thank the MGH Transgenic Core Facility for blastocyst injection. We thank the BIDMC Genomics and Proteomics Center for microarray analysis.
This work was supported by the National Institutes of Health (grants AR054500 and AR056645 to T.K.) and the American Society for Bone and Mineral Research (grant CEA0811 to T.K.).
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Ambros V., Chen X. 2007. The regulation of genes and genomes by small RNAs. Development 134:1635–1641 [DOI] [PubMed] [Google Scholar]
- 2. Banegas I., et al. 2006. Brain aminopeptidases and hypertension. J. Renin Angiotensin Aldosterone Syst. 7:129–134 [DOI] [PubMed] [Google Scholar]
- 3. Bartel D. P. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beitzinger M., Peters L., Zhu J. Y., Kremmer E., Meister G. 2007. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 4:76–84 [DOI] [PubMed] [Google Scholar]
- 5. Blank U., et al. 2008. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev. Biol. 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brodersen P., Voinnet O. 2009. Revisiting the principles of microRNA target recognition and mode of action. Nat. Rev. Mol. Cell Biol. 10:141–148 [DOI] [PubMed] [Google Scholar]
- 7. Ding Y., Chan C. Y., Lawrence C. E. 2005. RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble. RNA 11:1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Easow G., Teleman A. A., Cohen S. M. 2007. Isolation of microRNA targets by miRNP immunopurification. RNA 13:1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eberhart J. K., et al. 2008. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat. Genet. 40:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eggan K., et al. 2001. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl. Acad. Sci. U. S. A. 98:6209–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo H., Ingolia N. T., Weissman J. S., Bartel D. P. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashida Y., Nishibu T., Inoue K., Kurokawa T. 2009. A useful approach to total analysis of RISC-associated RNA. BMC Res. Notes 2:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hendrickson D. G., Hogan D. J., Herschlag D., Ferrell J. E., Brown P. O. 2008. Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. PLoS One 3:e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hornstein E., Shomron N. 2006. Canalization of development by microRNAs. Nat. Genet. 38(Suppl.):S20–S24 [DOI] [PubMed] [Google Scholar]
- 15. Hsu R. J., Yang H. J., Tsai H. J. 2009. Labeled microRNA pull-down assay system: an experimental approach for high-throughput identification of microRNA-target mRNAs. Nucleic Acids Res. 37:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karaplis A. C., et al. 1994. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8:277–289 [DOI] [PubMed] [Google Scholar]
- 17. Karginov F. V., et al. 2007. A biochemical approach to identifying microRNA targets. Proc. Natl. Acad. Sci. U. S. A. 104:19291–19296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katagiri T., et al. 2002. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7:949–960 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T., et al. 2002. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129:2977–2986 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T., et al. 2008. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. U. S. A. 105:1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi T., et al. 2005. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Invest. 115:1734–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanske B., et al. 1996. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273:663–666 [DOI] [PubMed] [Google Scholar]
- 23. Lee Y. S., Dutta A. 2007. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 21:1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayr C., Hemann M. T., Bartel D. P. 2007. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315:1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLeod M. J. 1980. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22:299–301 [DOI] [PubMed] [Google Scholar]
- 26. Minina E., Kreschel C., Naski M. C., Ornitz D. M., Vortkamp A. 2002. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3:439–449 [DOI] [PubMed] [Google Scholar]
- 27. Miyaki S., et al. 2010. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 24:1173–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murtaugh L. C., Zeng L., Chyung J. H., Lassar A. B. 2001. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev. Cell 1:411–422 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura Y., et al. 2007. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J. Clin. Invest. 117:3075–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicolas F. E., et al. 2008. Experimental identification of microRNA-140 targets by silencing and overexpressing miR-140. RNA 14:2513–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ørom U. A., Lund A. H. 2010. Experimental identification of microRNA targets. Gene 451:1–5 [DOI] [PubMed] [Google Scholar]
- 32. Ovchinnikov D. A., Deng J. M., Ogunrinu G., Behringer R. R. 2000. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26:145–146 [PubMed] [Google Scholar]
- 33. Park C. Y., Choi Y. S., McManus M. T. 2010. Analysis of microRNA knockouts in mice. Hum. Mol. Genet. 19:R169–R175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Retting K. N., Song B., Yoon B. S., Lyons K. M. 2009. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136:1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodríguez C. I., et al. 2000. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25:139–140 [DOI] [PubMed] [Google Scholar]
- 36. Schier A. F., Talbot W. S. 2005. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39:561–613 [DOI] [PubMed] [Google Scholar]
- 37. Tallquist M. D., Soriano P. 2003. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development 130:507–518 [DOI] [PubMed] [Google Scholar]
- 38. Tuddenham L., et al. 2006. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 580:4214–4217 [DOI] [PubMed] [Google Scholar]
- 39. Valencia-Sanchez M. A., Liu J., Hannon G. J., Parker R. 2006. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20:515–524 [DOI] [PubMed] [Google Scholar]
- 40. Wienholds E., et al. 2005. MicroRNA expression in zebrafish embryonic development. Science 309:310–311 [DOI] [PubMed] [Google Scholar]
- 41. Yoon B. S., et al. 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:5062–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L., et al. 2007. Systematic identification of C. elegans miRISC proteins, miRNAs, and mRNA targets by their interactions with GW182 proteins AIN-1 and AIN-2. Mol. Cell 28:598–613 [DOI] [PMC free article] [PubMed] [Google Scholar]