Abstract
Background
Numerous investigations demonstrate a novel role of thyroid hormone as a modulator of signal transduction. Protein kinase C (PKC) is critical to the mechanism by which thyroid hormones potentiate both the antiviral and immunomodulatory actions of IFNγ in different cells and regulate the exchange of signalling phospholipids in hepatocytes. Because nothing is known about accumulation of PKC modulator - diacylglycerol in cells treated with T4, we examined the nongenomic effect of thyroid hormones on DAG formation and phospholipase activation in liver cells.
Results
The results obtained provide the first demonstration of phospholipase C, phospholipase D and protein kinase C nongenomic activation and diacylglycerol (DAG) accumulation by L-T4 in liver cells. The experiments were performed in either the [14C]CH3COOH-labeled rat liver slices or isolated hepatocytes pre-labeled by [14C]oleic acid. L-T4 activates the DAG production in a concentration- and time-dependent manner. DAG formation in stimulated cells is biphasic and short-lived event: there is an initial, rapid rise in DAG concentration and then a slower accumulation that can be sustained for a few minutes. The early phase of L-T4 generated DAG only is accompanied by phosphatidylinositol 4,5-bisphosphate level decrease and inositol 1,4,5-trisphosphate formation while the second phase is abolished by PKC inhibitor l,(5-isoquinolinesulphonyl)2methylpiperasine dihydrochloride (H7) and propranolol. The second phase of DAG production is accompanied by free choline release, phosphatidylcholine content drop and phosphatidylethanol (Peth) formation. Inhibitor of phospholipase-C-dependent phosphoinositide hydrolysis, neomycin sulfate, reduced the Peth as well as the DAG response to L-T4.
Conclusions
The present data have indicated the DAG signaling in thyroid hormone-stimulated liver cells. L-thyroxine activates a dual phospholipase pathway in a sequential and synchronized manner: phospholipase C initiates the DAG formation, and PKC mediates the integration of phospholipase D into the signaling response during the sustained phase of agonist stimulation.
Background
Thyroid hormone exerts a broad range of effects on development, growth and metabolism. The actions of thyroid hormone are primarily the result of their interaction with nuclear receptors that bind to regulatory regions of genes (thyroid hormone - response elements) and modify their expression. Nuclear mechanisms of thyroid hormone action have been extensively described [reviewed in 1,2], but an increasing number of nogenomic effects of the hormone at the cellular level have been recognized in the past 10 years [reviewed in 3]. Nongenomic actions of thyroid hormone are by definition independent on nuclear receptors for the hormone and have been described at the plasma membrane, various organelles, the cytoskeleton, and in cytoplasm. The actions include alterations in transport of Ca+2, Na+ and glucose; changes in activities of several kinases, including protein kinase C (PKC), cAMP -dependent protein kinase and mitogen - activated protein kinase. Iodothyronines also can regulate nongenomically through a PKC activation of neutral lipids, phospholipids [4] and phosphatidylinositol 4,5-bisphosphate (PtdIns (4,5)P2) [5] synthesis in rat hepatocytes. It has been determined that in HeLa cells potentiation by thyroxine (T4) interferon -gamma - induced antiviral state requires PKC and phospholipase C (PLC) activities [6]. Direct evidence of the nongenomic PKC activation by thyroid hormones has been found in rabbit erythrocytes [7]. The regulation of PKC is critical to the mechanism by which thyroid hormones rapidly induce phosphorylation and nuclear translocation of mitogen-activated protein kinase and subsequently potentiate both the antiviral and immunomodulatory actions of IFNγ in cultured cells [8].
It is widely demonstrated on various cell types that interaction of Ca+2 - mobilizing hormones and transmitters with the cell surface receptors leads to the phospholipid breakdown under PLC or -D action and accumulation of inosite phosphates and diacylglycerol (DAG). The regulatory molecules generation is accompanied by intracellular free calcium concentration increase and, as a result, by PKC activation.
An addition of the physiological doses of thyroid hormones to the cell suspension rapidly increases the intracellular calcium concentration in rat hepatocytes and single rat heart cell [9,10]. On the other hand, there is no information about accumulation of other PKC modulator - DAG in the cells on T4 administration.
However, such genomic independent effect on the different types of cells has been determined for steroid hormones [11-13] whose mechanism of action on target cells is known to be similar to that of the thyroid hormones.
In the present study, we have examined the nongenomic effect of thyroid hormones on DAG formation and PKC activation in liver cells. It was determined that L-T4 rapidly induces the biphasic DAG accumulation in liver slices and isolated hepatocytes. The data obtained provide evidence that L-T4 activates PLC and -D in sequential manner that leads to increasing DAG formation during sustained agonist stimulation. The L-T4-induced PLD -PA phosphohydrolase (PAP) pathway of DAG generation in rat hepatocytes is highly specific and PKC - dependent.
Results and Discussion
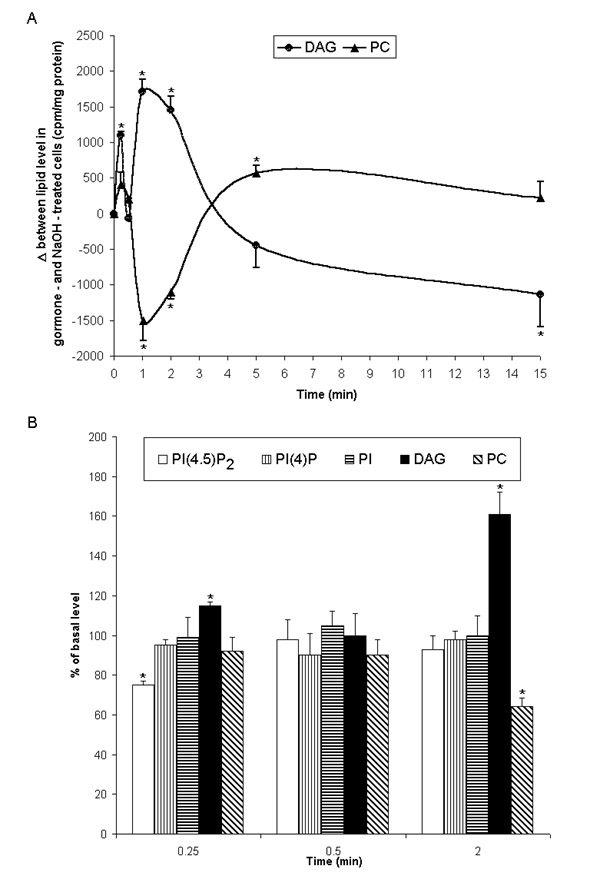
This study was carried out to examine DAG formation and degradation of phospholipids in rat liver cells treated with the thyroid hormones. It was demonstrated that L-T4 induced a biphasic formation of DAG peaking at 15 sec and 1-2 min (Fig. 1A, B). Early phase was abolished by neomycin sulfate, a specific inhibitor of phosphoinositide hydrolysis (Table 1), and accompanied by PtdIns(4,5)P2 level decrease (Fig. 1B). It has also been shown that L-T4 stimulates inositol 1,4,5-trisphosphate (Ins(l,4,5)P3) formations in [3H]inositol pre-labeled hepatocytes. Basal and hormone-induced Ins(l,4,5)P3 levels in liver cells were 840+25 and 1832+42 cpm/107 cells, respectively (time of treatment: 0.25 min, n = 6, P < 0.05).
Figure 1.
Effect of L-T4 on [14C]DAG and [14C]phospholipid level in liver slices and isolated hepatocytes. [14C]sodium acetate labeled liver slices (A) and [14C]oleic acid labeled hepatocytes (B) were treated with 100 nM NaOH (control) or 10 nM L-T4 for indicated times. Lipids were extracted, separated by TLC, and analyzed as described in "Materials and Methods". Basal lipid levels in control cells (Fig. 1B) did not change significantly over the experimental period and were (cpm/107 cells): PI -4721 ± 633, PI(4)P - 4265 ± 264, PI(4,5)P2 - 3605 ± 317, PC - 7662 ± 344, DAG - 6008 ± 106. Results represent the mean ± S.E. of four individual experiments.* P < 0.05 vs. control.
Table 1.
Effect of neomycin sulfate and propranolol on the L-T4-induced DAG formation in [14]oleic acid pre-labeled hepatocytes.
| Time, | Additions | Control | L-thyroxine | % of control |
| min | ||||
| % total14C-lipids | ||||
| NaOH | 0.96 ± 0.11 | 1.34 ± 0.09* | 140 | |
| 0.25 | NaOH+neomycin sulfate | 1.43 ± 0.37 | 1.38 ± 0.04 | 96.7 |
| NaOH | 1.19 ± 0.06 | 2.02 ± 0.14* | 170 | |
| 2.0 | NaOH+neomycin sulfate | 1.08 ± 0.11 | 1.43 ± 0.09 | 132 |
| NaOH+ propranolol | 1.97 ± 0.21 | 1.77 ± 0.15 | 90 | |
[14C]oleic acid labeled hepatocytes were pre-incubated for 15 min prior to NaOH (l00 nM) or LT4 (l0 nM) addition or pre-incubated for 15 min with neomycin sulfate (1.5 mM) or propranolol (100 nM) prior to stimulation with L-T4 (l0 nM).The data are mean ± S.E. of four separate experiments. * P < 0.05 vs. control.
Many cells produce DAG in a biphasic manner, involving an initial and usually transient rise in DAG that is correlated with phosphoinositide - specific PLC activation followed by a sustained increase in DAG derived from phosphatidylcholine (PC) hydrolysis [14]. It is shown in the present study that the second phase of DAG production in liver slices (Fig. 1A) and isolated hepatocytes (Fig. 1B) coincided with hormone-stimulated PC content decrease. L-T4 - induced DAG formation in hepatocytes was concentration-dependent and highly specific as D-T4 failed to accumulate DAG (Fig. 2A). In rat hepatocytes, it has been reported that various regulatory factors such as phorbol esters, guanine nucleotide-binding proteins, hepatocyte growth factor, vasopressin and insulin caused PC-dependent PLD activation and DAG formation [15-18]. PLD catalyzed hydrolytic cleavage of the terminal diester bound of phospholipids, resulting in the direct formation of PA and the respective base. The PA produced can be converted to DAG by the action of PAP. A unique property of PLD, which provides a specific assay for this enzyme, is the transphosphatidylation reaction to generate the corresponding phosphatidylalcohol in the presence of primary alcohol.
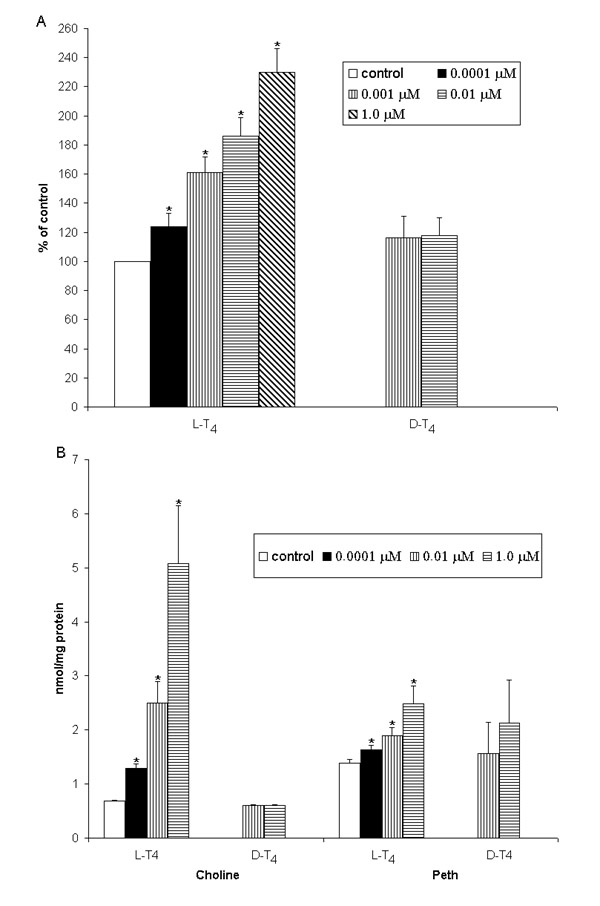
Figure 2.
Dose-dependent action of thyroid hormone on DAG (A), choline and Peth (B) formation in hepatocytes. [14C]oleic acid pre-labeled cells were treated with 100 nM NaOH (control) or thyroid hormones for 2 min (A). The DAG level in control cells: 4707 ± 544 cpm/107 cells. To determine Peth formation in intact hepatocytes(B), 1.5% ethanol was added 15 min before hormone addition. Cells were treated with hormones for 2 min. Reaction was stopped by addition of ice-cold methanol to the culture dishes. Lipid extraction was performed as described in [17]. Peth was separated by silica gel plates in a solvent system of upper phase of ethyl acetate/2,2,4-trimethylpentane/acetic acid/water (13:2:3:10, v/v). The Peth area identified by co-migration with Peth standard was scraped off the plates and the radioactivity was determined by a liquid scintillation counter. The intensity of the lipid spots was estimated by densitometry using a LKB Ultroscan laser densitometer. Free choline content was determined in intact control and hormone-treated cells by the method described in [18]. Results are mean ± S.E. of five individual experiments. * P < 0.05 vs.control.
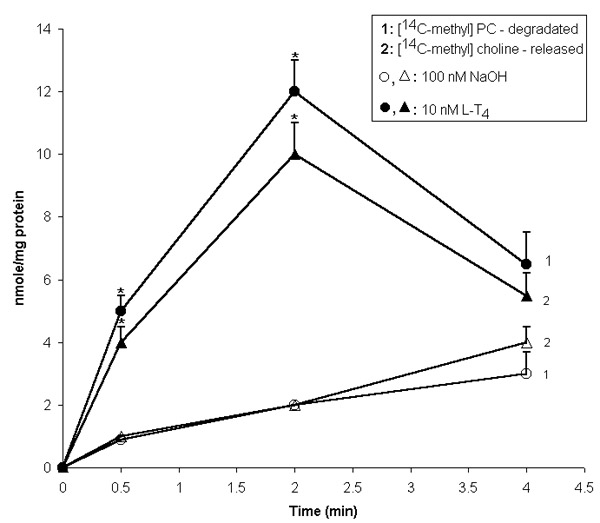
In the present study, to determine the PLD activity, the formation of phosphatidylethanol (Peth) was measured in intact (Fig. 2B) and [14C]oleic acid pre-labeled cells (Table 2) in the presence of 1.5% ethanol. L-T4 stimulatory effect on Peth formation was specific and concentration-dependent (Fig. 2B). To determine if PC could be the substrate for the PLD in L-T4 - stimulated cells the effect of hormone on isolated plasma membranes has been investigated in the presence of [14C-methyl]PC (Fig. 3). It was found that L-T4 rapidly stimulated [14C]PC degradation and free [14C]choline accumulation. Besides, L-T4 increased the level of choline in intact liver cells in a concentration -dependent manner (Fig. 2B). Early work [19] has shown that L-T3 rapidly induced choline and PA accumulation in liver slices. To assess the possibility of PA conversion to DAG in hormone-stimulated liver cells we have used propranolol, which is known to block the conversion of PA to DAG by inhibiting PAP. 100 nM propranolol was added 15 min prior to the hormone to [14C]oleic acid pre-labeled cells. Propranolol completely abolished L-T4 (10 nM for 2 min) stimulatory effect on DAG accumulation (Table 1). Inhibitor of polyphosphoinositide-specific PLC, neomycin sulfate, nullified the first rise of DAG production and decreased the second one in hormone stimulated cells. In rat hepatocytes, commonly used PKC inhibitors, 1,(5- isoquinolinesulphonyl)2methylpiperasine dihydrochloride (H7), Ro31-8425 and calphostin C, inhibited HGF-, vasopressin- and insulin -induced PC-specific PLD, suggesting that PLD activity is regulated by PKC [16, 18]. Phorbol esters are highly effective stimuli of PLD activity in intact cells [reviewed in 15]. The results presented show that the pretreatment of [14C]-labeled hepatocytes with protein kinase inhibitor - H7 decreased L-T4 effect on [14C]Peth and [14C]DAG formation (Table 2). Pre-incubation of liver cells with L-T4 leads to translocation of PKC from cytosol to membrane fraction and its activation. D-T4 had no effect on enzyme activity of the fractions investigated. The PKC activity in membranes isolated from control (100 nM NaOH-treated cells) and D-T4(10 nM)-treated hepatocytes was 1040 ± 111 and 865 ± 61 pmol/mg protein/min, respectively, and 1593 ± 167 and 937 ± 120 pmol/mg protein/min in cytosol of control and hormone-treated cells, respectively. However, the enzyme activity in L-T4-induced cells was 9498 ± 224 pmol/mg protein/min (for membrane; Pcontrol-L-T4 < 0,05, n = 4) and 633 ± 20 pmol/mg protein/min (for cytosol).
Table 2.
Effect of H7 on the L-T4-induced DAG and Peth formation in [14]oleic acid pre-labeled hepatocytes.
| Lipid | Additions | Control | L-T4 | % of control |
| cpm/1 07 cells | ||||
| NaOH+ethanol | 2170 ± 362 | 5000 ± 638* | 230 | |
| [14C]Peth | ||||
| H7 + NaOH + ethanol | 1847 ± 296 | 2915 ± 44* | 158 | |
| NaOH | 10797 ± 715 | 16515 ± 1077* | 153 | |
| [14]DAG | ||||
| H7+NaOH | 7359 ± 746 | 9051 ± 1290 | 123 | |
[14C]oleic acid labeled hepatocytes were pre-incubated for 15 min with H7 (50 μM) or NaOH (l00 nM) alone prior to stimulation with L-T4 for 2 min. 1.5% ethanol was added 15 min before L-T4 addition or together with H7 (50 μM) or NaOH (100 nM) for determination of Peth formation in the cells. The data are mean ± S.E. of four separate experiments. * P < 0.05 vs. control.
Figure 3.
Effect of L-T4 on [14C-methyl]PC metabolism in isolated liver cell plasma membranes. [14C-methyl]PC metabolism in isolated plasma membranes was investigated as described in "Materials and Methods". Results are mean ± S.E. of four individual experiments. * P < 0.05 vs. control.
Conclusions
The investigations made indicate that in liver cells L-T4 rapidly stimulates the hydrolysis of polyphosphoinositides by PLC with the resultant production of the second messengers inositol triphosphate as well as DAG and PKC activation. The major new finding of this study was that in hepatocytes L-T4 stimulated PC cleavage by PLD. As in the other cells, operated by Ca2+-mobilizing receptors, PLD contributes to DAG formation in L-T4-stimulated hepatocytes. DAG formed by PA breakdown could further activate PKC in hormone-treated cells. Inhibitor of PLC-dependent phosphoinostide hydrolysis, neomycin sulfate, completely abolishes the first phase of DAG production and reduces the PLD-dependent DAG response to L-T4, indicating that PLD is activated during the PLC-dependent signaling in liver cells. These data indicate that L-T4 activates a dual phospholipase pathway in a sequential and synchronized manner. PLC initiates the increase in Ins(l,4,5)P3 and DAG formation and PKC mediates integration of PLD into the signaling response during the sustained phase of agonist stimulation. The effect of L-T4 on PKC, PLD activation and DAG accumulation is highly specific and too rapid (from seconds to a few minutes) to be compatible with mRNA and protein synthesis. These results provide the first evidence concerning L-T4 nongenomic stimulation of phospholipid hydrolysis by phospholipases and DAG accumulation in liver slices and isolated hepatocytes.
Materials and Methods
Materials
[14C]oleic acid (58 mCi/mmol), myo- [3H]inositol (60 Ci/mmol) and [14C-methyl]phosphatidylcholine (58 mCi/mmol) - Amersham Corp., [γ-32P]ATP (l000 Ci/mmol) and [14C]CH3COOH (25 mCi/mmol) - BPO Isotop (Russia); DEAE-52-cellulose from Whatman (England); silica gel from Woelm (Germany).Phosphatidylserine was isolated from ox brain; other lipid standards, histone (typeIII), H7, propranolol and neomycin were obtained from Sigma (USA). Other chemicals used were of chemically pure grade.
Cell Suspension Experiments
Hepatocytes were isolated from the adult 90-day-old male Wistar rats which had a free access to food and water and were kept at 24°C on a cycle of 12 h light/12 h darkness by the method described in [5]. Preparation of hepatocytes was started between 9:00 and 10:00 a.m. Cells (107/ml) were labeled by incubation in Eagle medium containing 10% fetal calf serum, 100 units/liter streptomycin, 100 units/liter penicillin, 13 mg/ml gentamycin and 2.5 μCi/ml of [14C]oleic acid for 3 h in 95% 02/5% CO2 atmosphere at 37°C. Before hormones addition, cells were washed twice with a Krebs-Henseleit buffer pH 7.4, containing 2 mM CaCl2, 25 mM HEPES, 0.1% BSA, and then preincubated at 37°C for 1 h. After adding L-T4 or D-T4 or NaOH only to the cell suspension (3 × 106 cells/ml), the probes were incubated for different periods. Reactions were stopped with ice-cold mixture of chloroform and methanol (2:1, v/v). The PLD activity in response to T4 was determined by measuring the formation of Peth as was previously described [20]. The free choline content in intact cells was analyzed by the method [21].
Experiments with Liver Slices
The 1 mCi of [14C]CH3COOH was intraperitoneally injected four times every 30 minutes for 2 hours to rats [21]. The liver was perfused with 0.9% NaCl, then removed and washed in Krebs-Henseleit buffer, pH 7.4, containing 2 mM CaCl2 and 0.2% BSA. Slices of liver were preincubated in Krebs-Henseleit buffer for 10 minutes before hormones addition. Reaction was stopped with ice-cold mixture of chloroform and methanol (2:1, v/v). The lipids were extracted and analyzed as described below.
Isolation and Incubation of Liver Cell Plasma Membranes
Plasma membranes were prepared using differential centrifugation through various concentration of sucrose and characterized by their specific marker enzymes as described [22]. For PC hydrolysis, [14C-methyl]PC (0.25 μCi) was mixed with dipalmitoylphosphatidylcholine suspended in 0.05% Triton X-100 (w/v) to 10 mM by sonication and used as the substrate to give about 105 cpm/assay. Isolated plasma membranes were incubated in medium (200 μl) containing 50 mM sodium HEPES, pH 7.5, 2 mM CaCl2, 3 mM MgCl2, 70 mM KCl and [14C-methyl]PC at 37°C for the indicated time. The reaction was terminated by adding of 200 μl of 12.5% trichloroacetic acid (w/v) and 70 μl of bovine serum albumin solution (2 mg/ml). After vortexing and centrifugation at 12,000 g for 5 min the aqueous phase was separated and the released 14C-labeled choline was analyzed by TLC. The solvent system was: 0.5% NaCl/methanol/ethanol/conc. NH4OH (50:20:30:5, v/v). The aqueous product was identified by comparison with choline standard and analyzed by scintillation counting. The [14C]lipids were extracted and analyzed as described below.
Extraction and Separation of Lipids
Lipids were extracted according to Folch et al [23] and phosphoinositides as described in [24]. The chloroform phase was collected and dried under N2 at 37°C. The lipids were redissolved in chloroform/methanol (1:2, v/v) and applied on TLC plates. For isolation of DAG the solvent system: hexane/diethyl ether/acetic acid (80:20:2, v/v) was used; for PC separation -chloroform/methanol/acetic acid/water (25:15:4:2, v/v) and for ItdIns(4,5)P2 -chloroform/methanol/NH4OH (50:40:10, v/v). Appropriate standards were applied on each plate for quantification. The gel spots containing lipids were scraped and transferred to scintillation vials. Radioactivity was measured by scintillation counter.
[3H]Inositol Labeling of Hepatocytes and Determination of Inositol Phosphates Release in Stimulated Cells
Freshly isolated hepatocytes were plated in Petri dishes (9 × 106 cells/dish) in Williams E medium containing 10% fetal calf serum, 20 units/I penicillin, 13 mg/ml gentamycin and 4 mg/ml dexametasone, 10 μCi myo- [3H]inositol [25]. After 24-h incubation, the cells were washed three times with inositol-free Williams E medium, containing 0.1% BSA, then preincubated in the presence of 20 mM LiCl2 for 15 min, and incubated for 0.25 min in the presence of 0.1 μM of L-T4. Reactions were performed at 37°C and stopped at the indicated time by removing the medium and washing three times with the same inositol-free medium. The samples preparation and inositol phosphates analysis were done as described previously [24].
Protein kinase C Enzyme Assay
Activities of protein kinase C in the cytosol and in the crude membrane fraction of hormone-treated (10 nM for 5 min) and control (100 nM NaOH) hepatocytes were determined after partial purification of the enzyme by chromatography on DEAE-cellulose [26]. The activity of protein kinase C was determined by the transfer of 32P from [γ-32P]ATP into HI histone in the presence of phosphatidylserine and Ca2+ (0.2 mM).This approach measures all the isoforms of PKC. The enzyme activity was expressed as pmoles of phosphate transferred from [γ-32P]ATP into HI histone per minute. The protein content was determined by the method of Bradford [27].
Abbreviations
T4 - thyroxine, PKC - protein kinase C, PtdIns(4,5)P2 - phosphatidylinositol 4,5-bisphosphate, PLC - phospholipase C, PLD - phospholipase D, DAG - diacylglycerol, PA - phosphatidic acid, PC -phosphatidylcholine, Ins(l,4,5)P3 - inositol 1,4,5- trisphosphate, Peth - phosphatidylethanol.
Contributor Information
Nataliya S Kavok, Email: babenko@univer.kharkov.ua.
Oksana A Krasilnikova, Email: babenko@univer.kharkov.ua.
Nataliya A Babenko, Email: babenko@univer.kharkov.ua.
References
- Brent GA, More DD, Larsen PR. Thyroid hormone regulation of gene expression. Annu Rev Physiol. 1991;53:17–35. doi: 10.1146/annurev.ph.53.030191.000313. [DOI] [PubMed] [Google Scholar]
- Laser MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB. Nongenomic action of thyroid hormone. Thyroid. 1996;16:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- Babenko NA, Kavok NS. Effect of thyroid hormones, phorbol esters, and sphingosine on the incorporation of linoleic acid into lipids in the liver of white rats. Biokhimiya. 1995;60:1545–1550. [PubMed] [Google Scholar]
- Krasilnikova OA, Babenko NA. Role of thyroid hormones in regulation of phosphatidic acid, phosphatidylinositole, and polyphosphoinositide synthesis in liver cells. Biokhimiya. 1996;61:1008–1014. [PubMed] [Google Scholar]
- Lin HY, Thacorf HR, Davis FB, Davis PJ. Potentiation by thyroxine of interferon-gamma-induced antiviral state requires PKA and PKC activities. Am J Physiol. 1996;271(4 Pt1):C1256–C1261. doi: 10.1152/ajpcell.1996.271.4.C1256. [DOI] [PubMed] [Google Scholar]
- Lawrence WD, Schoenl M, Davis PJ. Stimulation in vitro of rabbit erythrocyte cytosol phospholipid-dependent protein kinase activity. A novel action of thyroid hormone. J Biol Chem. 1989;264(9):4766–4768. [PubMed] [Google Scholar]
- Lin HY, Davis FB, Gordinier JK, Martino LJ, Davis PJ. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am J Physiol. 2000;276(5Pt1):1014–1024. doi: 10.1152/ajpcell.1999.276.5.C1014. [DOI] [PubMed] [Google Scholar]
- Hummerich H, Soboll S. Rapid stimulation of calcium uptake into rat liver by L-triiodothyronine. Biochem J. 1989;258:363–367. doi: 10.1042/bj2580363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamax RB, Gobbold PH, Cuthbertson KSR, Robertson WR. Acute effects of thyroid hormones on cytoplasmic [Ca2+] in single rat heart cells. J Endocrinol. 1989;121:57. [Google Scholar]
- Facchinetti M, de Boland AR. Calcitriol transmembrane signaling regulation of rat muscle phospholipase D activity. J Lipid Research. 1998;39:197–204. [PubMed] [Google Scholar]
- Tien X-Y, Brasitus TA, Qasawa BN, Norman AW, Sitrin MD. Effect of 1,25(OH)2D3 and its analogues on membrane phosphoinositide turnover and [Ca2+] in Caco-2 cells. Am J Physiol. 1993;265:G143–G148. doi: 10.1152/ajpgi.1993.265.1.G143. [DOI] [PubMed] [Google Scholar]
- Beno DWA, Brady LM, Bissonnette M, Davis BH. Protein kinase C and mitogen-activated protein kinase are required for 1,25-dihydroxyvitamin D3-stimulated Egr induction. J Biol Chem. 1995;270:3642–3647. doi: 10.1074/jbc.270.8.3642. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hidrolisis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Gustavsson L, Moehren G, Torres-Marqez ME, Benistant C, Rubin R, Hoek JB. The role of cytosolic Ca2+, protein kinase C and protein kinase A in hormonal stimulation of phospholipase D in rat hepatocytes. J Biol Chem. 1994;269:849–859. [PubMed] [Google Scholar]
- Donchenko JB, Zannetti A, Baldini PM. Insulin-stimulated hydrolysis of phosphatidylcholine by phospholipase C and phospholipase D in cultured rat hepatocytes. Biochim Biophys Acta. 1994;1222:492–500. doi: 10.1016/0167-4889(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. Phospholipase D activation in growth factor-stimulated rat hepatocytes mediates the expressions of c-jun and c-foc: involvement of protein tyrosine kinase, protein kinase C, and Ca2+ Hepatology. 1996;24:1274–1281. doi: 10.1053/jhep.1996.v24.pm0008903410. [DOI] [PubMed] [Google Scholar]
- Babenko NA, Phylonenko NS, Viliyasenor VZ, Nikitin VN. Thyroid hormones stimulate the phospholipase D activity in hepatocytes of rats of different age and thyroid state. Reports USSR Acad Sci. 1991;320:745–748. [Google Scholar]
- Kumada T, Miyata H, Nozawa Y. Involvement of tyrosine phosphorylation in IgE receptor-mediated phospholipase D activation in rat basophilic leukemia (RBL-2H3) cells. Biochem Biophys Res Commun. 1993;191:1363–1368. doi: 10.1006/bbrc.1993.1367. [DOI] [PubMed] [Google Scholar]
- Kates M. Techniques of lipidology: Isolation, analysis and identification of lipids. New York: American Elsevier Publishing Company. 1972.
- Provost JJ, Fudge J, Isaraelit S, Siddiqi AR, Exton JH. Tissue and subcellular distribution of phospholipase D. Biochem J. 1996;319:285–291. doi: 10.1042/bj3190285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipid from animal tissues. J Biochem. 1957;226:497–509. [PubMed] [Google Scholar]
- Andrews WV, Conn PM. Measurement of inositol phospholipid metabolites by one-dimensional thin-layer chromatography. In Methods in Enzymology New York: Academic Press, Inc. 1987;141:156–168. doi: 10.1016/0076-6879(87)41064-1. [DOI] [PubMed] [Google Scholar]
- Jones MJ, Murray W. Evidence that ceramide selectively inhibits protein kinase C-α tranclocation and modulates bradykinin activation of phospholipase D. J Biol Chem. 1995;270:5007–5013. doi: 10.1074/jbc.270.10.5007. [DOI] [PubMed] [Google Scholar]
- Kikawa U, Takai Y, Minakuchi R, mohara S, Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase of rat brain. Subcellular distribution, purification and properties. J Biol Chem. 1982;257:13341–13348. [PubMed] [Google Scholar]
- Bradford M. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1972;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]