Abstract
Cell polarity plays a critical role in the development of all metazoans; however, the mechanisms of cell polarity and the specific role of cell polarity pathways in mammalian organisms are still poorly understood. Lethal giant larvae (Lgl) is an apical-basal polarity gene identified in Drosophila, where it functions as a tumor suppressor controlling self-renewal and differentiation of progenitor cells. There are two orthologs of Lgl in mammalian genomes: Llgl1 and Llgl2. While mammalian Lgls are assumed to be tumor suppressor genes, little is known about their function in vivo. Here we report the functional analysis of murine Llgl2. We generated Llgl2−/− mice and found that Llgl2 functions as a polarity protein required for proper branching morphogenesis during placental development. Llgl2−/− pups are born as runts but quickly catch up in size and grow into normal-size adults. Surprisingly, no prominent phenotypes or spontaneous tumors were observed in adult Llgl2−/− mice. Analyses of placental trophoblasts reveal a critical role for Llgl2 in cell polarization and polarized cell invasion. We conclude that mammalian Llgl2 is required for proper polarized invasion of trophoblasts and efficient branching morphogenesis during placental development, but, unlike its Drosophila ortholog, it does not function as a canonical tumor suppressor gene.
INTRODUCTION
Cell polarity and cell-cell adhesion play a critical role in the regulation of normal tissue architecture and function. Disruption of cell adhesion and cell polarity is often associated with neoplastic tumors (4, 19). Lgl (lethal giant larvae) is the first described Drosophila tumor suppressor gene, originally discovered by Bridges in the 1930s and further characterized in modern times by Elisabeth Gateff and Bernard Mechler (10–12, 23). Subsequent functional analysis revealed that Lgl is a polarity protein that is required for apical-basal polarization and asymmetric cell division of Drosophila neuroblasts (27, 29, 30). Lgl mutant progenitors are unable to generate daughter cells that completely withdraw from the cell cycle and differentiate. Instead, they produce progenitors that continue to divide, overproliferate, and kill the animals, exhibiting a phenotype which is remarkably similar to cancer in mammalian organisms (26). Similarly to neuronal tissues, epithelial cells also hyperproliferate in Lgl mutant Drosophila larvae (5).
The potential role of stem cells in cancer initiation in mammalian organisms is a hotly debated topic of modern cancer biology. Since Drosophila Lgl is involved in regulation of stem cell self-renewal and loss of Lgl in Drosophila causes stem cell-derived tumors, it is plausible that mammalian orthologs of Lgl, Llgl1, and Llgl2 are also involved in regulation of stem cell self-renewal and function as tumor suppressors. Indeed, the human Llgl1 can rescue the phenotype of Lgl-mutant Drosophila, indicating the conservation of Lgl function across species (14, 15). We have previously reported that Llgl1−/− mice develop severe brain dysplasia and die at birth from massive hydrocephalus (18). The role of mammalian Llgl2 remained unknown; however, Llgl2-mutant zebrafish (penner mutation) are unable to form hemidesmosomes and display hyperproliferation and disorganization of the basal epidermis (40, 41). While pen/Llgl2 mutants die at 4 to 5 days postfertilization, transplantation of Llgl2−/− cells into normal fish embryos results in development of epidermal tumors, a phenotype that enabled the authors to identify vertebrate Llgl2 as a tumor suppressor gene (31).
To investigate the role of mammalian Llgl2 in vivo, we generated and analyzed Llgl2−/− mice. We found that Llgl2 is required for proper placental development. Llgl2−/− pups are born as runts but quickly catch up in size to control littermates and display no overt phenotypes throughout their subsequent life. Hemidesmosomes are formed and functional, and there is no hyperplasia or tumor development in Llgl2−/− mice. Analysis of the placental defects revealed an important role of Llgl2 in polarized invasion of trophoblasts and branching morphogenesis of the placental labyrinth layer. We conclude that murine Llgl2 is required for proper polarization of trophoblast cells during placental morphogenesis but plays a redundant function in the adult organism. Loss of Llgl2 does not predispose mice to spontaneous tumor development, indicating that mammalian Llgl2 is not a canonical tumor suppressor gene.
MATERIALS AND METHODS
Generation of Llgl2−/− mice and genotyping.
A mutation was generated in the Llgl2 gene via insertion of the gene trap vector pGT0lxf into the intron immediately downstream from the exon with the first ATG site (clone no. XS0846; SIGTR). Chimeric mice were generated using standard ES cell technology (16a). Heterozygous Llgl2tm2Vv+/− mice were crossed to C57BL/6J mice for eight generations and were subsequently interbred to obtain homozygous mutant B6.129P2-Llgl2tm2Vv−/− mice. The mutant mice were genotyped using PCR analysis with 5-CGCCATACAGTCCTCTTCAC-3, 5-CTGCACACTGCGGATTTAGC-3, and 5-CAGCTCTGGGCCTTTTTCAC-3 oligonucleotides, which produce a PCR fragment of 200 bp for the wild type and 350 bp for the mutant allele.
Histology, immunohistochemistry, and electron microscopy.
Freshly collected tissues were fixed overnight in 4% paraformaldehyde, dehydrated in increased concentrations of ethanol, cleared with xylene, and embedded in paraffin. Sections (4 μm) of the paraffinized blocks were made close to the placental midline and stained with hematoxylin and eosin. For immunohistochemistry, tissue sections were rehydrated and stained with anti-Ki67 (Novo Castra), anti-phospho-histone H3 (Cell Signaling), anti-β-catenin (Sigma), anti-PKCzeta (Santa Cruz), anti-connexin 26 (gift from Paul Lampe, FHCRC), anti-β4-integrin (gift from William Carter, FHCRC), or antilaminin (Sigma) antibodies. Anti-E-cadherin antibodies were generated in rabbits by using a glutathione S-transferase (GST)–cytoplasmic-E-cadherin fusion containing the C-terminal 145 amino acids of canine E-cadherin (GB no. XM_536807.2) (Strategic Biosolutions). The antigens were detected using Vectastain ABC kit (Vector Labs) according to manufacturers' specifications. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) stainings were performed using the ApopTag Plus peroxidase kit (Millipore) per the manufacturer's instructions. For immunofluorescent stainings, the primary antibodies were detected using corresponding Texas Red or fluorescein isothiocyanate (FITC)-labeled secondary antibodies (Jackson Lab Immunoresearch). Placental endogenous alkaline phosphatase activity was detected as described previously (33). Stained slides were examined and photographed using an Olympus BX41 microscope and Microfire camera (Optronics) or a Zeiss LSM 510 confocal microscope. To determine the placental labyrinth layer transport surface area, we performed a morphometric analysis of the alkaline phosphatase-positive cells (44). For transmission electron microscopy (EM), samples were fixed in Karnoy's fixative for 2 days at 4°C and processed for Epon embedding. Sections were made close to the placental midline and analyzed with a JEOL 1010 microscope. Digital images were pseudocolored using Adobe Photoshop software.
In situ hybridization.
The plasmids used for synthesis of sense and antisense Llgl2 probes have been previously described (18). Labeled RNA probes were generated using a digoxigenin (DIG) RNA labeling kit (Roche). Frozen sections (14 μm) were rehydrated in phosphate-buffered saline (PBS) and postfixed with 4% paraformaldehyde, treated with proteinase K (1 μg/ml for 15 min at room temperature), acetylated (1 min at room temperature), and hybridized with labeled probes overnight at 58°C. Hybridization buffer contained 0.3 mg/ml yeast tRNA (Sigma-Aldrich), 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (Ambion), 50% formamide, 5× Denhardt's solution (Sigma-Aldrich), 20 mM dithiothreitol (DTT), 0.1% (vol/vol) herring sperm DNA (Sigma-Aldrich), and DIG-labeled RNA probe (5% of the transcription reaction from 1 μg template DNA). After hybridization, slides were first washed with 5× SSC buffer and 2× SSC buffer at 70°C and then with 0.2× SSC buffer at room temperature and then treated with blocking buffer (Roche) for 1 h at room temperature. Slides were then incubated in a 1:200 dilution of horseradish peroxidase (HRP)-conjugated anti-DIG antibody (Roche) for 1 h, washed, and incubated for 10 min with TSA-biotin (Perkin-Elmer). The probes were detected by incubating with a 1:2,000 dilution of streptavidin-Alexa 488 (Molecular Probes) and the cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole).
RNA extraction and quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was extracted using TRIZOL (Invitrogen), and cDNA was prepared using the Superscript III first-strand synthesis kit (Invitrogen). Quantitative PCR (qPCR) was performed using Prism 7900HT (Applied Biosystems), platinum qPCR mix (Invitrogen) and the Universal Probe Library kit utilizing the primers, probes, and PCR conditions recommended by the Universal Probe Library assay center (Roche Applied Science). Ribosomal protein Rps16 was used for normalization of qPCR data.
Cell culture and lentivirus production.
The SM10 mouse placental labyrinth trophoblast cells were a gift from Joan Hunt (University of Kansas) and were maintained in RPMI 1650 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 50 μM 2-mercaptoethanol, and 1 mM sodium pyruvate. Human embryonic kidney (HEK) 293FT cells (Invitrogen) were grown in 5% CO2 in Dulbecco's modified essential medium containing 10% FBS and antibiotics. The lentiviruses were produced in HEK293FT cells as described previously (22). The SM10 cells were infected with short hairpin RNA (shRNA) lentiviruses, and stably transduced cells were fluorescence-activated cell sorter (FACS) sorted for green fluorescent protein (GFP) expression. To overexpress human LLGL2, SM10 cells were infected with pLenti6 lentiviruses carrying either lacZ-V5 or hLLGL2-V5 constructs. Stably transduced cells were generated by selection with 3 μg/ml of blasticidin.
DNA constructs.
Full-length human LLGL2 cDNA was PCR amplified from IMAGE clone 3584689 and cloned into the Gateway pCR8/GW/TOPO entry clone (Invitrogen). A lentiviral construct encoding C-terminal V5-tagged human LLGL2 was generated by an LR Gateway reaction from the entry clone into pLenti6/V5-DEST (Invitrogen). Control pLenti6/V5-GW/LacZ vector was obtained from Invitrogen. Lentiviral shRNA construct targeting mouse Llgl2 was generated by inserting 5-GGATCCGGAAGAGAGGTTCACATTGCTTCAAGAGAGCAATGTGAAGCTCTCTTCTTTTTGGAATTC-3 oligonucleotide into BamHI and EcoRI sites of FUGW-H1-GFP-neomycin vector (21).
Western blot analysis and zymography.
Tissues were homogenized and cultured cells were lysed in ice-cold lysis buffer containing 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100 in PBS, along with complete protease and phosphatase inhibitor tablets (Roche). The lysates were centrifuged at 13,000 × g for 10 min at 4°C, and the supernatants were used for Western blot analysis. Protein samples were separated on NuPAGE gradient gels (Invitrogen), transferred to polyvinylidene difluoride (PVDF) membranes (Millipore), and analyzed by Western blotting with anti-human LLGL2 (Abcam), antitubulin (University of Iowa Hybridoma Bank) and β-actin (Sigma), anti-mouse Llgl1 (18), and anti-mouse Llgl2 antibodies. Anti-mouse Llgl2 antibodies were produced in rabbits against the GST fusion containing amino acids 933 to 1027 of mouse Llgl2 (GB no. NM_145438.2) and purified by affinity chromatography. Protease zymography was performed using Novex 10% gelatin gels and protein renaturation and developing buffers as recommended by the manufacturer (Invitrogen). Protease activity was detected by staining with a colloidal blue kit (Invitrogen).
Matrigel invasion and migration assays.
Matrigel invasion and cell migration assays were performed using an 8-μm BD BioCoat Matrigel invasion chamber and cell culture inserts, as described by the manufacturer (BD Biosciences). Cells were plated into the Matrigel invasion chamber or cell culture inserts in the presence of growth medium lacking FBS. Medium containing 10% FBS was used in the lower chamber as a chemoattractant. Cells were allowed to migrate/invade across the membrane for 8 or 24 h, and migrated cells were stained with a DIFF stain kit (IMEB Inc.) per the manufacturer's instructions. Cells at four edges and at the center of each membrane were imaged, and the number of migrated cells was counted using Image J software. The percentage of cell invasion was determined as specified by the manufacturer.
Quantitation of cell polarization.
Scratch wound cell polarization assays were performed as previously described (36). Cell polarization induced by wounding was determined 1 h after scratching an 80% confluent cell monolayer. Cells were fixed and stained with antipericentrin antibodies (Covance) and DAPI nuclear counterstain. The first row of cells showing the centrosome located in front of the nucleus and the first row in the 120° sector facing the wound were counted as oriented; ≥150 cells were counted per coverslip, with four independent experiments per each data point. Statistical tests were performed using a two-tailed t test with equal variances.
RESULTS
Generation of Llgl2−/− mice.
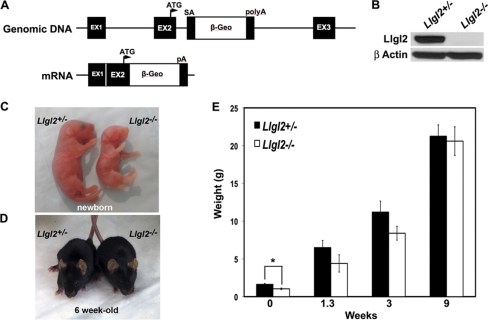
To investigate the role of Llgl2 in vivo, we generated and analyzed Llgl2−/− mice (Fig. 1). The Llgl2 gene was modified in ES cells by the insertion of a retroviral gene trap vector containing strong splice acceptor, β-geo, and polyadenylation sequences (Fig. 1A). The gene trap sequences were inserted into the second intron, immediately downstream from the exon encoding the first methionine and 25 N-terminal amino acids of Llgl2. In the targeted gene configuration, the Llgl2 transcript would terminate with the β-geo polyadenylation sequences and exon 2 of Llgl2 would be spliced with β-geo coding sequence, effectively generating the Llgl2-null allele. ES cell technology was used to generate the Llgl2+/− mice. To obtain pure genetic background, the heterozygous Llgl2+/− animals were crossed for eight generations to C57BL/6J mice. The resulting Llgl2+/− mice were crossed to each other to obtain homozygous mutant Llgl2−/− mice. Western blot analysis with antibodies recognizing murine Llgl2 demonstrated the absence of Llgl2 protein in Llgl2−/− mice (Fig. 1B). In addition, we did not observe the formation of truncated Llgl2 protein fragments in Llgl2−/− tissues, confirming generation of Llgl2 knockout mice (see Fig. 4A).
Fig. 1.
Generation and embryonic growth retardation phenotype of Llgl2−/− mice. (A) Schematic representation of gene trap-targeted Llgl2 allele (genomic DNA), and the predicted resulting transcript (mRNA). ATG, the initiating methionine in exon 2. SA, strong splice acceptor. polyA, strong polyadenylation site. (B) Western blot analyses of kidney protein extracts from Llgl2−/− and Llgl2+/− newborn animals. The blot was analyzed with anti-Llgl2 and anti-β-actin antibodies. (C) Gross appearance of newborn Llgl2−/− and Llgl2+/− littermates. Note that Llgl2−/− newborns are significantly smaller than their heterozygous and wild-type littermates. (D) Gross appearance of adult (6-week-old) Llgl2−/− and Llgl2+/− littermates. Note that Llgl2−/− adults are indistinguishable from their control littermates. (E) Differences in weight between Llgl2−/− and Llgl2+/− littermates. Graph shows the weights of the pups at the indicated time points after birth. Error bars represent standard deviations. Note that significant differences in weight in newborn pups quickly disappear after birth.
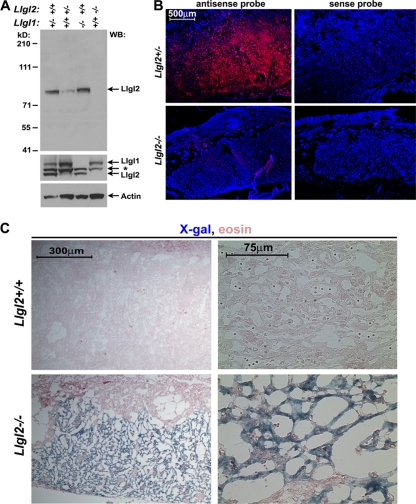
Fig. 4.
Llgl2 is prominently expressed in the placental labyrinth layer. (A) Western blot analyses of total protein extracts from Llgl1−/−, Llgl2−/−, and Llgl2+/− placentas with anti-Llgl2, anti-Llgl1, and anti-β-actin antibodies. Note that anti-Llgl1 antibodies weakly cross-react with Llgl2. Llgl1 is expressed at a low level in placenta, and the blot with anti-Llgl1 antibodies is overexposed. *, background band. (B) In situ hybridization of E13.5 Llgl2−/− and Llgl2+/− sagittal placental sections with antisense and sense (control) probes for Llgl2 (red). Blue is a nuclear DAPI counterstain outlining general tissue morphology. Note the prominent expression of Llgl2 in the placental labyrinth layer. (C) X-Gal staining of E13.5 Llgl2+/+ and Llgl2−/− sagittal placental sections for β-geo activity. Note that while no staining was observed in wild-type placenta, trophoblasts of the labyrinth layer in Llgl2−/− placenta were prominently positive for β-geo activity.
Growth retardation of Llgl2−/− embryos and neonatal pups but normal size of adult Llgl2−/− mice.
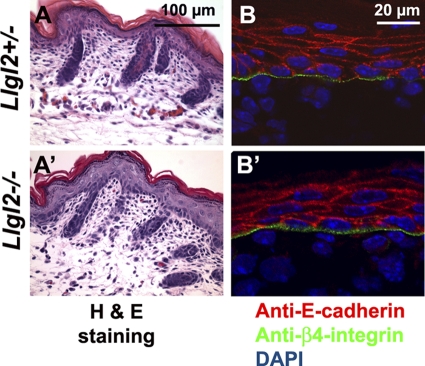
Llgl2−/− newborns were smaller than their wild-type and heterozygous littermates, and many did not survive to weaning (Fig. 1C). Surprisingly, pups that did survive to weaning quickly caught up with their littermates and grew to be normal-sized adults (Fig. 1D and E). Also, reducing the size of the litters containing Llgl2−/− newborns eliminated perinatal death of Llgl2−/− pups, indicating that competition for milk with larger siblings was responsible for their death. Histological examination of major organs from adult Llgl2−/− mice did not reveal any significant abnormalities in these animals (data not shown). This was unexpected, because Lgl2−/− zebrafish display a prominent epidermal phenotype and die within a few days postfertilization. Mutant fish are unable to form hemidesmosomes, which are prominent cell-substratum adhesion structures connecting epidermis with the basement membrane. Ablation of hemidesmosomes in mice results in a dramatic skin-blistering phenotype and neonatal lethality (7). Hence, we analyzed skins of newborn Llgl2−/− pups by histology and staining for hemidesmosomal marker integrin-β4 and found normal hemidesmosomes and overall skin morphology in Llgl2−/− mice (Fig. 2). These data indicate that, unlike zebrafish Lgl2, murine Llgl2 is not required for hemidesmosome formation and function.
Fig. 2.
Normal histology and deposition of hemidesmosomes in the skins of Llgl2−/− pups. (A and A′) Hematoxylin and eosin (H & E) staining of sagittal sections through the back skin of newborn Llgl2−/− and Llgl2+/− pups. (B and B′) Immunofluorescent staining of newborn skin sections from Llgl2−/− and Llgl2+/− pups with anti-E-cadherin (adherens junction marker, red) and anti-β4-integrin (hemidesmosome marker, green) antibodies. Blue is a DAPI nuclear counterstain.
To reveal whether adult phenotypes in Llgl2−/− mice will become apparent with age, we monitored Llgl2−/− mice up to 2 years of age; however, no prominent phenotype developed in these animals (n = 9). Both male and female Llgl2−/− mice were fertile. We conclude that Llgl2 is important for proper embryonic development but is not essential in adult mice.
Placental defects in Llgl2−/− embryos.
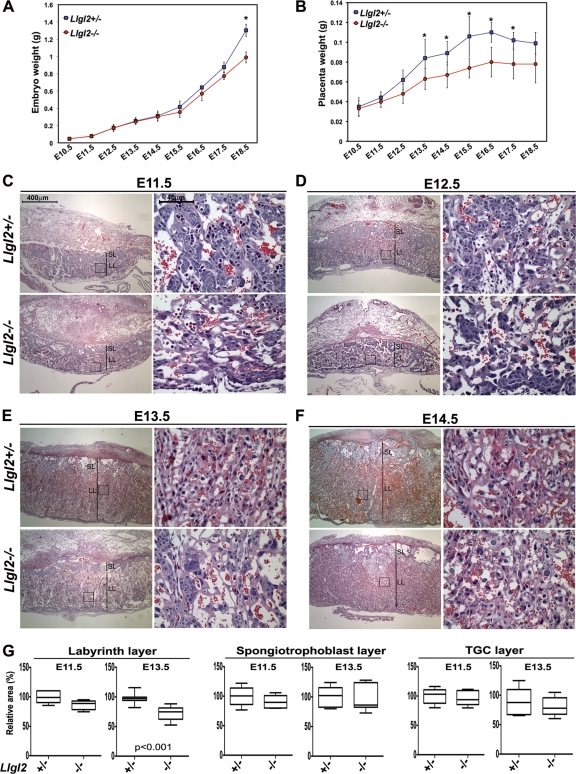
Since Llgl2−/− newborns are born as runts but catch up in size by adulthood, we hypothesized that placental defects may be responsible for the growth retardation of Llgl2-mutant embryos. We measured the weights of both embryos and placentas between embryonic day 11.5 (E11.5) and E18.5 (Fig. 3A and B). Consistent with our hypothesis, we found that the differences in weights between Llgl2+/− and Llgl2−/− placentas preceded the differences in weights between the embryos. E15.5 was the earliest time point when a difference in the weights of Llgl2+/− and Llgl2−/− embryos was observed (Fig. 3A). However, Llgl2−/− placentas were smaller than Llgl2+/− placentas as early as E12.5 (Fig. 3B). Murine placenta contains maternal decidua and three principal placental layers: the outer layer of trophoblast giant cells, the middle layer of spongiotrophoblasts, and the innermost labyrinth layer containing highly branched blood vessels used for the exchange of nutrients between the mother and the embryo (43). To analyze potential placental defects in more detail, we performed histological analysis of Llgl2−/− and Llgl2+/− placentas at E11.5 to E14.5 (Fig. 3C to F). As expected, Llgl2−/− placentas were smaller in size than placentas from Llgl2+/− and wild-type animals. Interestingly, the area of the Llgl2−/− labyrinth layer was significantly decreased, while the areas of the spongiotrophoblast and trophoblast giant cell layers were not changed (Fig. 3G). Since Llgl2−/− females are fertile, we also analyzed Llgl2−/− placentas from embryos carried by Llgl2−/− mothers. However, we did not find differences between these placentas and Llgl2−/− placentas from embryos carried by Llgl2+/− mothers, indicating that maternal Llgl2 does not play an important role in placental development (data not shown). Our data indicate that murine Llgl2 is important for the proper development of the placental labyrinth cell layer.
Fig. 3.
Llgl2 is required for proper placental morphogenesis. (A) Differences in weight between Llgl2−/− and Llgl2+/− embryos. Graph shows weights of the embryos at the indicated days of embryonic (E) development. Error bars represent standard deviations. Note that weight retardation of Llgl2−/− embryos becomes apparent at E15.5. *, P < 0.01. (B) Differences in weight between Llgl2−/− and Llgl2+/− placentas. Graph shows weights of the placentas at the indicated days of embryonic (E) development. Error bars represent standard deviations. Note that weight retardation of Llgl2−/− placentas becomes apparent at E12.5. *, P < 0.01. (C to F) Hematoxylin and eosin staining of sagittal sections through the central regions of E11.5 (C), E12.5 (D), E13.5 (E), and E14.5 (F) Llgl2−/− and Llgl2+/− placentas. The areas in squares are shown at higher magnification. SL, spongiotrophoblast layer; LL, labyrinth layer. Size bars for low- and high-magnification images in panels C to F are shown in panel C. (G) Quantitation of the relative sizes of the labyrinth, spongiotrophoblast, and trophoblast giant cell (TGC) layers in E11.5 and E13.5 Llgl2−/− and Llgl2+/− placentas. The images of sagittal hematoxylin-and-eosin-stained sections through the central region of placentas were used to measure the relative area of each layer in pixels (n = 6). Box and whisker plots show areas in arbitrary units, with the area in control heterozygous placentas adjusted to 100%. Lines within boxes are median values. The upper and lower borders of the boxes represent 75th and 25th percentiles, respectively. The upper and lower bars represent maximum and minimum values. Statistical significance was assessed by the Mann-Whitney test, and P value is shown only for the data with statistically significant differences.
We next analyzed the expression pattern of Llgl2 in mouse placenta. Western blot analysis revealed a complete loss of Llgl2 protein in Llgl2−/− placentas (Fig. 4A). Llgl1 was expressed at low levels in wild-type placentas, and its levels were not upregulated in Llgl2−/− mutants (Fig. 4A). Our anti-Llgl2 antibodies were unsuitable for immunohistochemical (IHC) staining; therefore, we used in situ hybridization with an Llgl2 antisense probe to analyze the expression pattern of Llgl2 in mouse placenta. We found that Llgl2 is prominently expressed in the labyrinth layer and shows very low levels of expression in the maternal decidual layer (Fig. 4B). Llgl2−/− placentas were used as negative controls to confirm specificity of the in situ hybridization (Fig. 4B). As an alternative approach, we performed X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) stainings of placental sections for β-geo expression, which was controlled by the endogenous Llgl2 promoter. While wild-type placentas did not stain for LacZ, Llgl2−/− placentas displayed prominent LacZ staining in the trophoblasts of the labyrinth layer (Fig. 4C). Therefore, we conclude that Llgl2 is prominently expressed in the trophoblasts of the labyrinth cell layer, which is defective in Llgl2−/− placentas.
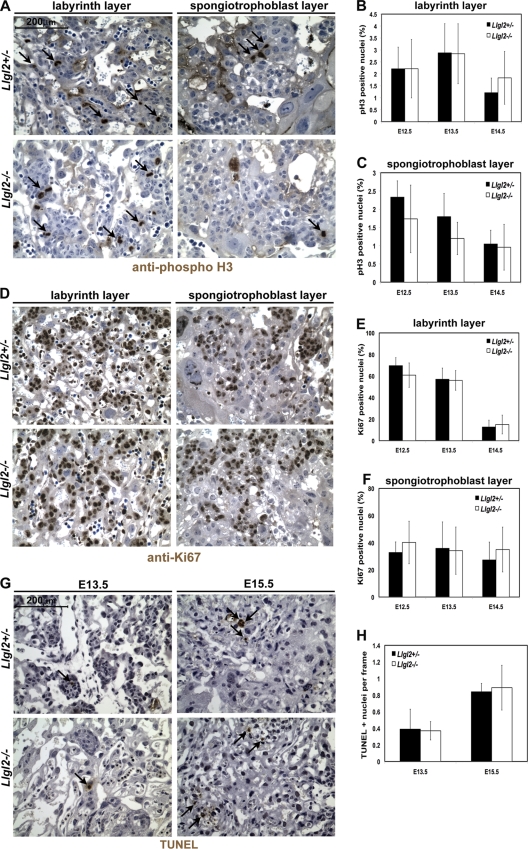
The smaller size of the Llgl2−/− labyrinth cell layer could potentially be due to decreased cell proliferation or increased cell death in the Llgl2−/− placenta. To analyze cell proliferation, we performed IHC with anti-phospho-histone H3 (mitotic cell marker) and anti-Ki67 (cell proliferation marker) antibodies (Fig. 5A and D). No significant differences were detected in the rates of cell proliferation, either in the Llgl2−/− labyrinth or in the spongiotrophoblast cell layer, compared to controls (Fig. 5B, C, E, and F). To analyze the programmed cell death, we performed TUNEL staining on E13.5 and E15.5 placentas from Llgl2−/− and Llgl2+/− mice (Fig. 5G). No significant differences were observed in the number of apoptotic cells between the Llgl2−/− and Llgl2+/− placentas (Fig. 5H). We conclude that ablation of Llgl2 does not result in significant changes in placental cell proliferation or apoptosis.
Fig. 5.
Cell proliferation in Llgl2−/− and Llgl2+/− placentas. (A and D) Stainings of sagittal sections through the central regions of E12.5 Llgl2−/− and Llgl2+/− placentas with anti-phospho-histone H3 (mitotic cell marker) antibody (A) or anti-Ki67 antibody (proliferation marker) (D). Blue is a nuclear hematoxylin counterstain. (B, C, E, and F) Quantitation of stainings shown in panels A (B and C) and D (E and F). pH 3 and Ki67-positive nuclei were calculated as a percentage of the total number of nuclei on three randomly taken images from the labyrinth and spongiotrophoblast layers (n = 3 or greater). The error bars represent standard deviations. (G) TUNEL (apoptosis marker) staining of the labyrinth layers of Llgl2−/− and Llgl2+/− placentas at E13.5 and E15.5. (H) Quantitation of the stainings shown in panel G. The number of TUNEL-positive nuclei in at least 10 independent frames was counted for each section, and the number of TUNEL-positive nuclei per frame was calculated (n = 3). The error bars represent standard deviations.
Llgl2 is required for proper branching morphogenesis during development of the placental labyrinth layer.
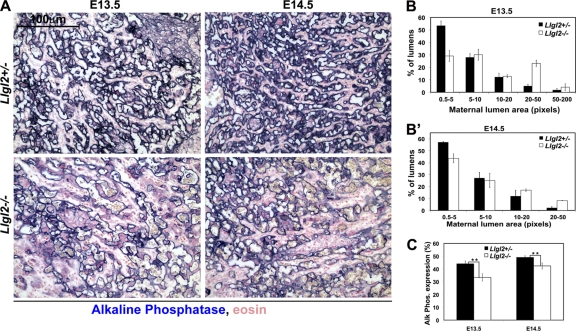
The labyrinth cell layer is formed by extensive branching morphogenesis of the villi, which are generated by fetal trophoblasts and contain fetal endothelial blood vessels. Villi are bathed in the maternal blood, and extensive branching tremendously increases the transport surface area of the villi, which are used to exchange oxygen and nutrients between the maternal and embryonic blood. The trophoblast cells on the surface of the villi line the maternal blood spaces. As these cells express endogenous alkaline phosphatase, the staining for alkaline phosphate activity is routinely used to distinguish the maternal lumens and to measure both the extent of branching and the transport surface area of the labyrinth (1, 44). Alkaline phosphatase staining of E13.5 and E14.5 Llgl2−/− and Llgl2+/− placentas revealed an increase in the size of maternal blood lumens and a decrease in the trophoblast transport surface area in the labyrinth of Llgl2−/− placentas (Fig. 6A to C). In E13.5 Llgl2+/− placentas, ∼54% of the maternal blood lumens are small (0.5 to 5 pixels) and only ∼6% are large (20 to 200 pixels), indicating a well-branched labyrinth (Fig. 6B). In contrast, in E13.5 Llgl2−/− placentas, only ∼30% of the maternal lumens are small (0.5 to 5 pixels) and ∼28% are large (20 to 200 pixels), indicating inefficient branching morphogenesis in the labyrinth layer. Alkaline phosphatase stainings of placental sections at E11.5 did not reveal significant differences in branching, indicating that the branching deficiency is not apparent at the early stages of placental development (data not shown).
Fig. 6.
Decreased branching and trophoblast transport surface area in Llgl2−/− placentas. (A) Alkaline phosphatase staining of sagittal sections through the central region of E13.5 and E14.5 Llgl2−/− and Llgl2+/− placentas. Maternal blood spaces in mouse placenta are lined with a syncytial trophoblast cell layer expressing endogenous alkaline phosphatase activity at the brash border. (B and B′) Image J software quantitation of sizes of maternal blood spaces at E13.5 (B) and E14.5 (B′) outlined by alkaline phosphatase staining shown in panel A. Bars represent the percentage of the lumens with indicated areas. Four randomly selected areas were quantified for each section (n = 3). The error bars represent standard deviations. Note the significant increase in the proportion of large (20 to 200 pixels) maternal blood lumens in Llgl2−/− placentas. (C) Morphometric analysis of the transport surface area outlined by alkaline phosphatase staining shown in panel A. The area of the alkaline phosphatase staining was digitized and the percentage of stained area on four randomly taken images of the labyrinth layer was quantified using Image J software as described previously (44). n = 3. *, P < 0.01.
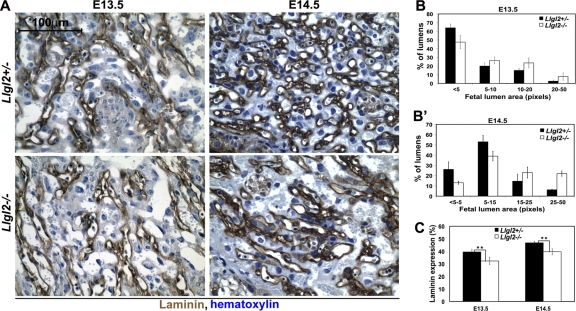
The fetal blood vessels develop within the branched villi. These vessels are lined by endothelial cells, which are connected to the laminin-rich basement membrane separating the endothelial and trophoblast cell layers. Hence, staining for laminin is routinely used to reveal the embryonic blood lumens (44). Analyses of fetal blood lumen sizes and surface areas using staining with antilaminin antibodies revealed an increase in fetal lumen size and a decrease in the surface area of fetal blood vessels at E13.5 and E14.5, but not at E11.5 in Llgl2−/− placentas (Fig. 7 and data not shown). Embryonic vessels are limited to the villi, which are generated by branching morphogenesis of the fetal trophoblasts. Hence, the observed decrease in surface area and increase in size of the embryonic blood spaces are indicative of a secondary defect rather than a primary vascularization defect in Llgl2−/− placentas. We conclude that Llgl2 is required for proper branching morphogenesis executed by fetal trophoblasts during development of the mouse placental labyrinth layer.
Fig. 7.
Decreased vascularization in Llgl2−/− placentas. (A) Laminin staining of sagittal sections through the central region of E13.5 and E14.5 Llgl2−/− and Llgl2+/− placentas. Fetal blood spaces in mouse placenta are lined with a thin layer of endothelial cells residing on a prominent layer of laminin, which is detectable by staining with antilaminin antibodies. (B and B′) Image J software quantitation of sizes of fetal blood spaces at E13.5 (B) and E14.5 (B′) outlined by laminin staining shown in panel A. Bars represent the percentage of the lumens with indicated areas (n = 3). The error bars represent standard deviations. Note the increase in the proportion of large (5 to 50 pixels) fetal blood lumens in Llgl2−/− placentas. (C) Morphometric analysis of laminin-stained area. The area of the laminin staining was digitized and the percentage of stained area on four randomly taken images of the labyrinth layer was quantified using Image J software, as described previously (44). n = 3.
Formation of trilaminar layer in Llgl2−/− placentas.
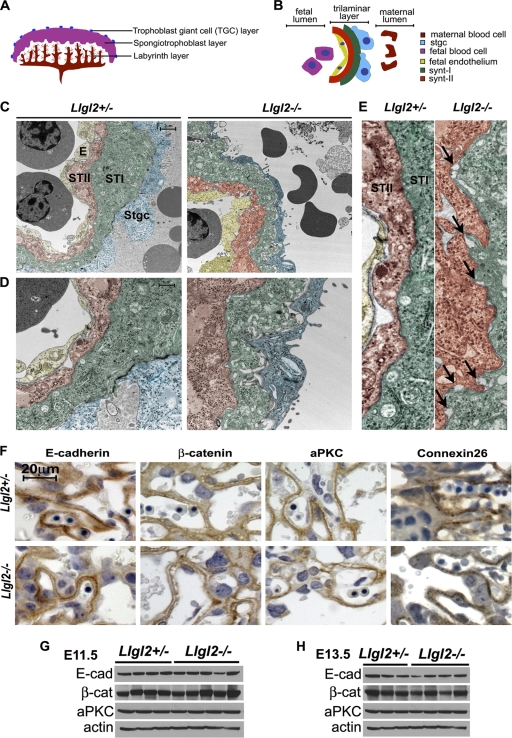
To further characterize the defects in the Llgl2−/− labyrinth layer, we analyzed the interface between the maternal and fetal blood lumens using electron microscopy. In mouse placenta, fetal vessels and maternal blood lumens are separated by a trilaminar trophoblast layer, consisting of a bilayer of syncytiotrophoblasts surrounding the fetal blood vessel and a single layer of mononuclear trophoblasts lining the maternal sinuses (Fig. 8A and B) (43). The syncytiotrophoblast layers I and II (STI and STII) are formed as a result of cell-cell and cell-syncytium fusion events and tightly adhere to each other. They constitute the site of nutrient and gaseous exchange between the maternal and fetal blood. To reveal the primary defects in the Llgl2−/− labyrinth layer, we decided to analyze placentas by electron microscopy at E11.5, the earliest time point displaying the phenotype in mutant animals. These experiments revealed the presence of a well-defined trilaminar layer in both Llgl2−/− and Llgl2+/− placentas (Fig. 8C to E). The STI and STII layers were tightly apposed; however, they contained more frequent gaps and openings in Llgl2−/− placentas (Fig. 8D and E). Cell-cell junctions between STI and STII cell layers contain various cell-cell adhesion structures, which include desmosomes, gap junctions, and adherens junctions. Desmosomes were well visible at the level of electron microscopy, and we found no differences in desmosome formation between Llgl2−/− and Llgl2+/− placentas (Fig. 8D and E and data not shown). To analyze whether other cell-cell adhesion structures are lost or malformed in Llgl2−/− placentas, we performed stainings for markers of gap junctions (connexin 26) and adherens junctions (E-cadherin and β-catenin). No consistent differences were found in either the expression levels or the staining patterns of connexin 26, E-cadherin, and β-catenin between Llgl2−/− and Llgl2+/− placentas (Fig. 8F, G, and H). Since Llgl2 is believed to be a polarity protein involved in the establishment and maintenance of apical-basal cell polarity, we also performed stainings for cell polarity markers atypical PKC and Crumbs 3. Again, no differences were observed in the levels and staining pattern of these proteins (Fig. 8F, G, and H and data not shown). Overall, relatively normal formation of the trilaminar layer and the absence of discernible changes in localization of cell polarity markers indicate the absence of prominent steady-state cell polarity defects in Llgl2−/− placentas.
Fig. 8.
Trilaminar cell layer and markers of cell adhesion and cell polarity in Llgl2−/− and Llgl2+/− placentas. (A and B) Schematic representation of mouse placenta (A) and trilaminar cell layer separating maternal and embryonic blood spaces (B). (C to E) Pseudocolored electron micrographs from the labyrinth layers of E11.5 Llgl2−/− and Llgl2+/− placentas. The three layered interhemal barrier at the maternofetal interface indicates the maternal (enucleated) and fetal blood cells (nucleated) separated by the endothelium (E) that lines fetal lumen and two syncytiotrophoblast layers (ST-I and ST-II). Mononuclear sinusoidal giant trophoblast cells (Sgtc) are seen associated with the ST-I cell layer (scale bar, 2 μm). Images are shown at low (C), intermediate (D), and high (E) magnifications. Arrows in panel E show small areas of membrane separation between ST-I and ST-II cell layers in Llgl2−/− placentas. The scale bar in panel C corresponds to 2 μm in panel C, 1 μm in panel D, and 0.5 μm in panel E. (F) Immunohistochemical stainings of sagittal placental sections through the central region of E13.5 Llgl2−/− and Llgl2+/− placentas with anti-E-cadherin, anti-β-catenin, anti-atypical PKC, and anti-connexin 26 antibodies (22). Blue is hematoxylin counterstain. (G and H) Western blot analysis of total protein extracts from E11.5 (G) and E13.5 (H) Llgl2−/− and Llgl2+/− placentas with anti-E-cadherin (E-cad), anti-β-catenin (β-cat), anti-atypical PKC zeta (aPKC), and anti-β-actin antibodies.
Expression of genes implicated in regulation of normal placental development does not change significantly in Llgl2−/− placentas.
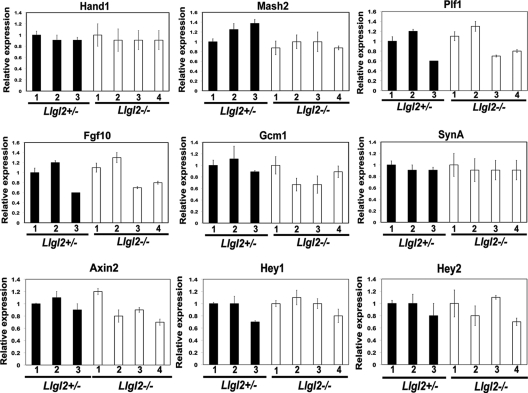
Many genes and signaling pathways have been implicated in mammalian placental development. To examine if these factors may be responsible for defective branching morphogenesis in Llgl2−/− placentas, we analyzed their expression using qRT-PCR (Fig. 9). We did not find significant differences in expression of Hand1 and Mash2 genes, which are required for trophoblast development and differentiation (Fig. 9) (16, 32). Similarly, expression of Gcm1, the gene critical for placental branching morphogenesis, and Proliferin (Plf), a prolactin family hormone stimulating placental angiogenesis, were also unchanged (Fig. 9) (2, 17). Expression levels of SynA, which is necessary for placental syncytiotrophoblast morphogenesis, were also unaffected (8). FGF10-FGFR2 signaling is important for proper placental branching morphogenesis (25, 45); however, we did not find changes in Fgf10 expression levels in Llgl2−/− placentas (Fig. 9). Concordantly, the activities of MAPK and the Akt signaling pathway, which are the prominent targets of FGF signaling, were not significantly changed in Llg2−/− placentas (data not shown). Next, we analyzed the expression levels of Hey1 and Hey2, which are the endogenous targets of Notch signaling in placenta and are required for proper placental vasculogenesis (9). In addition to this, Lgl regulates Notch signaling in Drosophila, and Llgl1−/− embryos display increased Notch signaling in the developing brain (18). However, Hey1 and Hey2 expression levels remained unchanged in Llgl2−/− placentas, indicating that Llgl2 does not impact the Notch signaling pathway in this organ (Fig. 9). Finally, qRT-PCR analysis of Axin2, an endogenous target of the canonical Wnt signaling pathway, also did not reveal changes in expression between Llgl2−/− and control placentas (Fig. 9) (48). We conclude that Llgl2 is not a significant regulator of FGF, Notch, or Wnt signaling pathways in placental development and, therefore, these signaling pathways are unlikely to be responsible for the labyrinth phenotype in Llgl2−/− placentas.
Fig. 9.
Expression levels of genes implicated in regulation of normal placental development in Llgl2+/− and Llgl2−/− placentas. Total RNA was extracted from E11.5 Llgl2−/− and Llgl2+/− placentas, and the expression levels of indicated genes were analyzed by qRT-PCR. Ribosomal Rsp16 was used as a normalization control. Bar graphs show mean values for three Llgl2+/− and four Llgl2−/− placentas ± standard deviation (SD). In each case, expression level in the first Llgl2+/− sample was arbitrarily adjusted to 1.
Llgl2 is necessary for induced polarization and directional invasion of placental trophoblasts.
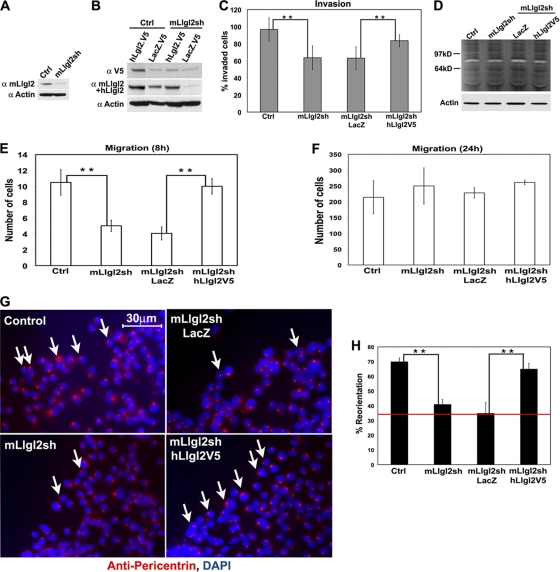
Outgrowth of the labyrinth layer and branching morphogenesis of embryonic trophoblasts constitute a highly dynamic process, which is impossible to capture by the analysis of steady-state images from mutant placentas. To reveal the role of Llgl2 in trophoblasts, we used the mouse placental labyrinthine trophoblast cell line SM10 (37, 38). Expression of Llgl2 in these cells was knocked down (KD) using Llgl2 lentiviral shRNA construct, and this knockdown was rescued by lentiviral reexpression of human V5-tagged LLGL2 (Fig. 10A and B). We used these cells to analyze the potential role of Llgl2 in the regulation of trophoblast migration, invasion, and induced polarization.
Fig. 10.
Llgl2 is necessary for induced polarization and polarized migration and invasion of placental trophoblasts. (A and B) Western blot analyses of total protein extracts from SM10 trophoblast cells stably transduced with lentiviral control (Ctrl) or mouse Llgl2-targeting (mLlgl2sh) shRNAs and expressing LacZ (LacZ.V5) or human LLGL2 (hLlgl2.V5) proteins. Proteins analyzed with anti-V5 (α V5), anti-mouse or -human Llgl2 (α mLlgl2+hLlgl2), and anti-β-actin (α Actin) antibodies. (C) Quantitation of Matrigel cell invasion assays of SM10 cells transduced with lentiviral control (Ctrl) or mouse Llgl2-targeting (mLlgl2sh) shRNAs and expressing LacZ or human LLGL2 (hLlgl2V5). Bar graph shows mean values of invaded cells ± standard deviation (SD). **, P < 0.01. (D) Protease zymography of SM10 cells transduced with lentiviral control (Ctrl) or mouse Llgl2-targeting (mLlgl2sh) shRNAs and expressing LacZ or human LLGL2 (hLlgl2V5). Total cell proteins were separated on gelatin gels, renatured, exposed to gelatin substrate, and stained by Coomassie. Clear bands indicate position and activity of gelatinases. Proteins were analyzed in parallel by Western blotting with anti-β-actin (Actin) antibodies. (E and F) Quantitation of short-term (E, 8 h) and long-term (F, 24 h) transwell cell migration assays of SM10 cells transduced with lentiviral control (Ctrl) or mouse Llgl2-targeting (mLlgl2sh) shRNAs and expressing LacZ or human LLGL2 (hLlgl2V5). Bar graph shows mean values of migrated cells ± SD. **, P < 0.01. (G) Centrosome reorientation toward the scratch wound in SM10 cells transduced with lentiviral control (Ctrl) or mouse Llgl2-targeting (mLlgl2sh) shRNAs and expressing LacZ or human LLGL2 (hLlgl2V5) at 1 h after wounding. Immunofluorescent staining of the wound edge with antipericentrin antibodies (red). DAPI is a nuclear counterstain (blue). Arrows point to cells properly oriented toward the scratch wound. (H) Quantitation of centrosome reorientation shown in panel G. The graph shows mean values ± SD of centrosomes polarized toward the wound cells. n ≥ 150. **, P < 0.01. Red line shows the value for random orientation of centrosomes.
Migration and invasion of placental trophoblasts play a critical role in placental development. We found significant differences in the rates of cell migration and Matrigel invasion between Llgl2KD and control cells (Fig. 10C, E, and F). The cultured placental trophoblasts are invasive in Matrigel invasion assays. Llgl2KD cells displayed significantly decreased rates of Matrigel invasion, and these differences were rescued by reexpression of human LLGL2 (Fig. 10C). Expression of extracellular matrix metalloproteinases (MMPs) plays a critical role in Matrigel invasion (39, 42). We analyzed the activity of MMPs using zymogram assays; however, no differences in MMP activities were observed between Llgl2KD and control cells (Fig. 10D). In addition to the defects in Matrigel invasion, Llgl2KD cells displayed significantly reduced rates of migration in short-term transwell migration assays (Fig. 10E). These differences disappear after long-term exposure, when cells have had enough time to equilibrate between the inner and outer surfaces of the transwell membrane (Fig. 10F). These data indicate that Llgl2 is not required for cell migration per se but is necessary for directional cell migration and invasion.
The ability of cells to polarize in response to stimuli plays a critical role in regulation of directional cell migration and invasion. We hypothesized that Llgl2 may be required for timely cell polarization and reorientation toward a stimulus, and the failure of cells to polarize may be responsible for the defects in invasion and short-term migration observed in Llgl2KD cells. To address this issue, we performed scratch wound cell polarization assays (36). We found that within 1 h after monolayer wounding, 68% of control cells polarized toward the wound (Fig. 10G and H). In contrast, only 40% of Llgl2KD cells were polarized. Importantly, these differences in induced cell polarization were rescued by reexpression of human LLGL2 (Fig. 10G and H). We conclude that Llgl2 is required for dynamic repolarization of placental trophoblasts. The deficiency in the ability of cells to polarize in a timely manner may be responsible for the decreased rates of branching morphogenesis in Llgl2−/− placenta.
DISCUSSION
Lgl is an important apical-basal cell polarity gene in Drosophila. To reveal the in vivo role of mammalian orthologs of Drosophila Lgl, we generated and analyzed mouse mutants for Llgl1 and Llgl2. We previously reported that Llgl1−/− mice display a prominent brain phenotype and show disruption of cell polarity in neural progenitor cells (18). We report here that Llgl2−/− mice display a very specific defect in placental development and show that Llgl2 is required for branching morphogenesis of the placental labyrinth layer.
While Llgl2 is necessary for proper placental development, it is not required either in the developing embryo or in the adult mice. The lack of significant abnormalities in Llgl2−/− mice is a surprising finding of this study. Indeed, the mutation of Lgl2 in zebrafish results in a dramatic phenotype in embryonic epidermis, which shows a loss of hemidesmosomes and displays prominent hyperproliferation (40, 41). Moreover, Lgl2 overexpression experiments in Xenopus suggested an important role for Lgl2 in the maintenance of cell polarity of early blastomeres and in proper regulation of neurogenesis (6, 34). Our results indicate that Llgl2 serves a nonessential or redundant function in mice. We believe that its function is more likely to be redundant, since a closely related mouse homolog of Llgl2, Llgl1, is prominently expressed in all mouse organs and may compensate for the loss of Llgl2. Indeed, the phenotype in Llgl1−/− mice is restricted to the developing brain, which is the only developing organ where Llgl2 is not expressed and, hence, cannot compensate for the loss of Llgl1 (18). Moreover, the cell polarity phenotype in MDCK cells and the mitotic spindle disorganization phenotype in HEK293 cells are exhibited only when both Llgl1 and Llgl2 genes are simultaneously knocked down (46). These findings suggest that Llgl1 and Llgl2 are likely to play a redundant role and the lack of the prominent phenotype in Llgl2−/− mice is due to compensation by Llgl1. Interestingly, we found that Llgl1 is also expressed in placenta, and, therefore, the placental phenotype in our Llgl2−/− animals cannot be explained by the absence of Llgl1 expression in this organ. One possibility is that the overall levels of Llgl proteins are important for normal placental development and depletion of Llgl2 results in a placental phenotype, which may become even more dramatic in double-mutant Llgl1/Llgl2 embryos. Future generation and analysis of Llgl1/Llgl2 double mutant mice will be informative for understanding the in vivo function of mouse Llgl proteins.
Llgl2−/− mice display a very specific defect in branching morphogenesis of the placental labyrinth layer. Many genes and signaling pathways have been implicated in regulation of proper placenta development (43). The phenotypes of mouse mutants with placental defects vary tremendously. The most severely affected mutants display a compacted labyrinth layer with very little branching, usually resulting in embryonic lethality (3, 8, 13, 43, 47). In contrast, partial deficiency in the branching of the labyrinth layer leads to undersized newborns which either die postnatally or, similarly to Llgl2−/− pups, survive to be normal adults (20, 33, 35, 45). qRT-PCR analyses of the expression levels of many genes critical for placental development failed to reveal consistent differences between heterozygous and Llgl2−/− placentas. Instead, we found a specific defect in the ability of placental trophoblasts to repolarize in response to stimuli.
Our work reveals an important role of mammalian Llgl2 in cell polarity, and this is consistent with the notion of Lgls as cell polarity genes in other model organisms. Since we did not observe steady-state cell polarity defects in Llgl2−/− placentas, it is likely that the Llgl2 function in cell polarity is somewhat redundant even in the placenta and that the requirement of Llgl2 is revealed only when cells have to quickly repolarize in response to the modified external cues. The ability to quickly readjust the cell polarity axis is likely to play a very important role during the highly dynamic process of branching morphogenesis, which is associated with rapid remodeling of cell-cell and cell-substratum adhesion junctions. Therefore, it is possible that the inability to promptly respond to very dynamic and ever-changing cell polarization cues can cause the general decrease in placental branching morphogenesis in Llgl2−/− embryos.
Branching morphogenesis is one of the fundamental processes employed by the developing embryo to increase the surface area of an organ. In addition to placental development, this morphogenetic event plays an important role in the development of lung, kidney, pancreas, vasculature, breast, prostate, and salivary glands. Despite its important role in metazoan development, the mechanisms responsible for branching morphogenesis are not well understood, and it is an active area of recent research (24, 28). Since Llgl2 is one of the canonical apical-basal cell polarity genes, our data suggest that apical-basal cell polarity mechanisms may play an important role in the regulation of mammalian branching morphogenesis. Future analysis of Llgl1/Llgl2 double-mutant mice will help to determine whether Lgl proteins are necessary for branching morphogenesis in other organs of the developing mammalian embryo.
ACKNOWLEDGMENTS
We thank all members of our laboratory for suggestions and comments, Nanyan Jiang for blastocyst injection of ES cells, Olga Klezovitch for help with the maintenance of mutant mice, and William Carter, Paul Lampe, and the Developmental Studies Hybridoma Bank for gifts of antibodies.
This work was supported by NCI grants R01 CA098161 and R01 CA131047 to V.V.
Footnotes
Published ahead of print on 23 May 2011.
REFERENCES
- 1. Adamson S. L., et al. 2002. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250:358–373 [DOI] [PubMed] [Google Scholar]
- 2. Anson-Cartwright L., et al. 2000. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 25:311–314 [DOI] [PubMed] [Google Scholar]
- 3. Barak Y., et al. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585–595 [DOI] [PubMed] [Google Scholar]
- 4. Bilder D. 2004. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18:1909–1925 [DOI] [PubMed] [Google Scholar]
- 5. Bilder D., Li M., Perrimon N. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289:113–116 [DOI] [PubMed] [Google Scholar]
- 6. Chalmers A. D., et al. 2005. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132:977–986 [DOI] [PubMed] [Google Scholar]
- 7. Dowling J., Yu Q. C., Fuchs E. 1996. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dupressoir A., et al. 2009. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. U. S. A. 106:12127–12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer A., Schumacher N., Maier M., Sendtner M., Gessler M. 2004. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 18:901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gateff E. 1978. The genetics and epigenetics of neoplasms in Drosophila. Biol. Rev. Camb. Philos. Soc. 53:123–168 [DOI] [PubMed] [Google Scholar]
- 11. Gateff E. 1978. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200:1448–1459 [DOI] [PubMed] [Google Scholar]
- 12. Gateff E., Schneiderman H. A. 1969. Neoplasms in mutant and cultured wild-tupe tissues of Drosophila. Natl. Cancer Inst. Monogr. 31:365–397 [PubMed] [Google Scholar]
- 13. Giroux S., et al. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369–372 [DOI] [PubMed] [Google Scholar]
- 14. Grifoni D., et al. 2007. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 26:5960–5965 [DOI] [PubMed] [Google Scholar]
- 15. Grifoni D., et al. 2004. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene 23:8688–8694 [DOI] [PubMed] [Google Scholar]
- 16. Guillemot F., et al. 1995. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat. Genet. 9:235–242 [DOI] [PubMed] [Google Scholar]
- 16a. Hogan B., Beddington R., Costantini F., Lacy E. 1994. Manipulating the mouse embryo. A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17. Jackson D., Volpert O. V., Bouck N., Linzer D. I. 1994. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science 266:1581–1584 [DOI] [PubMed] [Google Scholar]
- 18. Klezovitch O., Fernandez T. E., Tapscott S. J., Vasioukhin V. 2004. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18:559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee M., Vasioukhin V. 2008. Cell polarity and cancer—cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121:1141–1150 [DOI] [PubMed] [Google Scholar]
- 20. Li Y., Behringer R. R. 1998. Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat. Genet. 20:309–311 [DOI] [PubMed] [Google Scholar]
- 21. Lien W.-H., Gelfand V. I., Vasioukhin V. 2008. αE-catenin binds to dynamitin and regulates dynactin-mediated intracellular traffic. J. Cell Biol. 183:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lois C., Hong E. J., Pease S., Brown E. J., Baltimore D. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868–872 [DOI] [PubMed] [Google Scholar]
- 23. Mechler B. M., McGinnis W., Gehring W. J. 1985. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metzger R. J., Klein O. D., Martin G. R., Krasnow M. A. 2008. The branching programme of mouse lung development. Nature 453:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Natanson-Yaron S., et al. 2007. FGF 10 and Sprouty 2 modulate trophoblast invasion and branching morphogenesis. Mol. Hum. Reprod. 13:511–519 [DOI] [PubMed] [Google Scholar]
- 26. Neumuller R. A., Knoblich J. A. 2009. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 23:2675–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohshiro T., Yagami T., Zhang C., Matsuzaki F. 2000. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408:593–596 [DOI] [PubMed] [Google Scholar]
- 28. Onodera T., et al. 2010. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science 329:562–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng C. Y., Manning L., Albertson R., Doe C. Q. 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408:596–600 [DOI] [PubMed] [Google Scholar]
- 30. Prehoda K. E. 2009. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb. Perspect. Biol. 1:a001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reischauer S., Levesque M. P., Nusslein-Volhard C., Sonawane M. 2009. Lgl2 executes its function as a tumor suppressor by regulating ErbB signaling in the zebrafish epidermis. PLoS Genet. 5:e1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riley P., Anson-Cartwright L., Cross J. C. 1998. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 18:271–275 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez T. A., et al. 2004. Cited1 is required in trophoblasts for placental development and for embryo growth and survival. Mol. Cell. Biol. 24:228–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabherwal N., et al. 2009. The apicobasal polarity kinase aPKC functions as a nuclear determinant and regulates cell proliferation and fate during Xenopus primary neurogenesis. Development 136:2767–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saxton T. M., et al. 2001. Gene dosage-dependent functions for phosphotyrosine-Grb2 signaling during mammalian tissue morphogenesis. Curr. Biol. 11:662–670 [DOI] [PubMed] [Google Scholar]
- 36. Schlessinger K., McManus E. J., Hall A. 2007. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 178:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma R. K. 1998. Mouse trophoblastic cell lines. I: relationship between invasive potential and TGF-beta 1. In Vivo 12:431–440 [PubMed] [Google Scholar]
- 38. Sharma R. K. 1998. Mouse trophoblastic cell lines. II: relationship between invasive potential and proteases. In Vivo 12:209–217 [PubMed] [Google Scholar]
- 39. Solberg H., Rinkenberger J., Dano K., Werb Z., Lund L. R. 2003. A functional overlap of plasminogen and MMPs regulates vascularization during placental development. Development 130:4439–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sonawane M., et al. 2005. Zebrafish penner/lethal giant larvae 2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis. Development 132:3255–3265 [DOI] [PubMed] [Google Scholar]
- 41. Sonawane M., Martin-Maischein H., Schwarz H., Nusslein-Volhard C. 2009. Lgl2 and E-cadherin act antagonistically to regulate hemidesmosome formation during epidermal development in zebrafish. Development 136:1231–1240 [DOI] [PubMed] [Google Scholar]
- 42. Teesalu T., Masson R., Basset P., Blasi F., Talarico D. 1999. Expression of matrix metalloproteinases during murine chorioallantoic placenta maturation. Dev. Dyn. 214:248–258 [DOI] [PubMed] [Google Scholar]
- 43. Watson E. D., Cross J. C. 2005. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20:180–193 [DOI] [PubMed] [Google Scholar]
- 44. Wu L., et al. 2003. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421:942–947 [DOI] [PubMed] [Google Scholar]
- 45. Xu X., et al. 1998. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125:753–765 [DOI] [PubMed] [Google Scholar]
- 46. Yamanaka T., et al. 2006. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J. Cell Sci. 119:2107–2118 [DOI] [PubMed] [Google Scholar]
- 47. Yan L., et al. 2003. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev. Biol. 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu H. M., et al. 2005. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]