Abstract
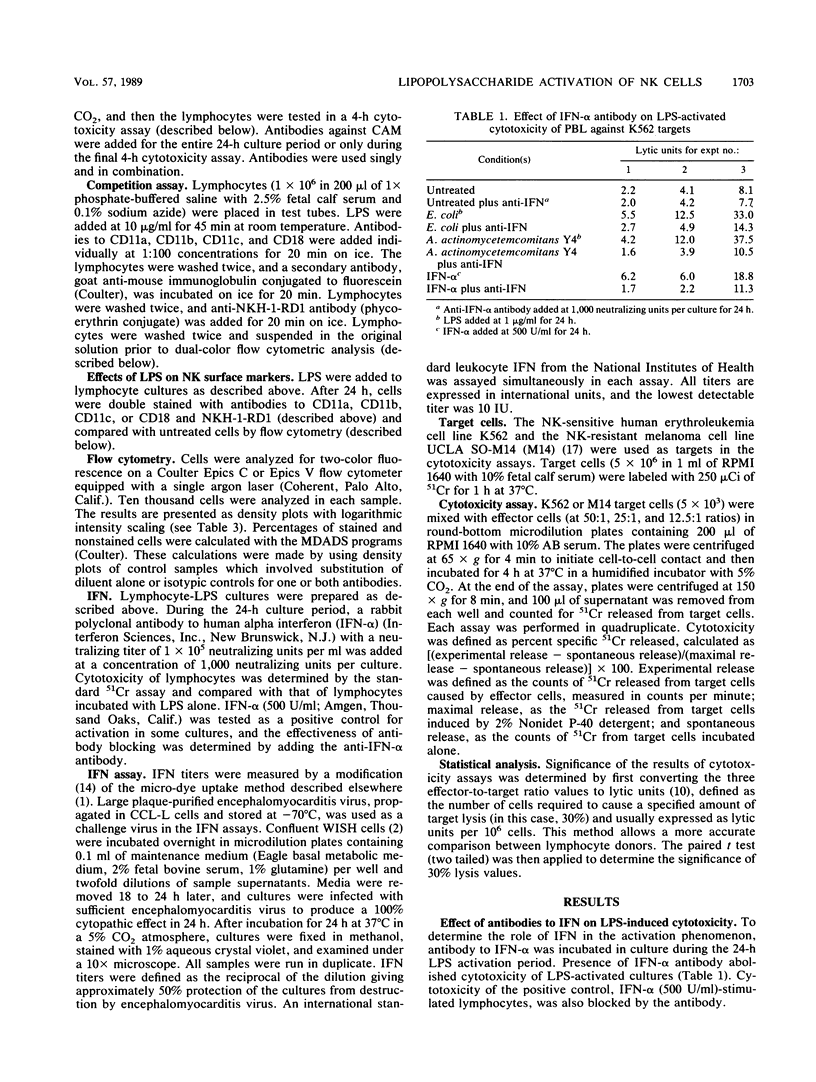
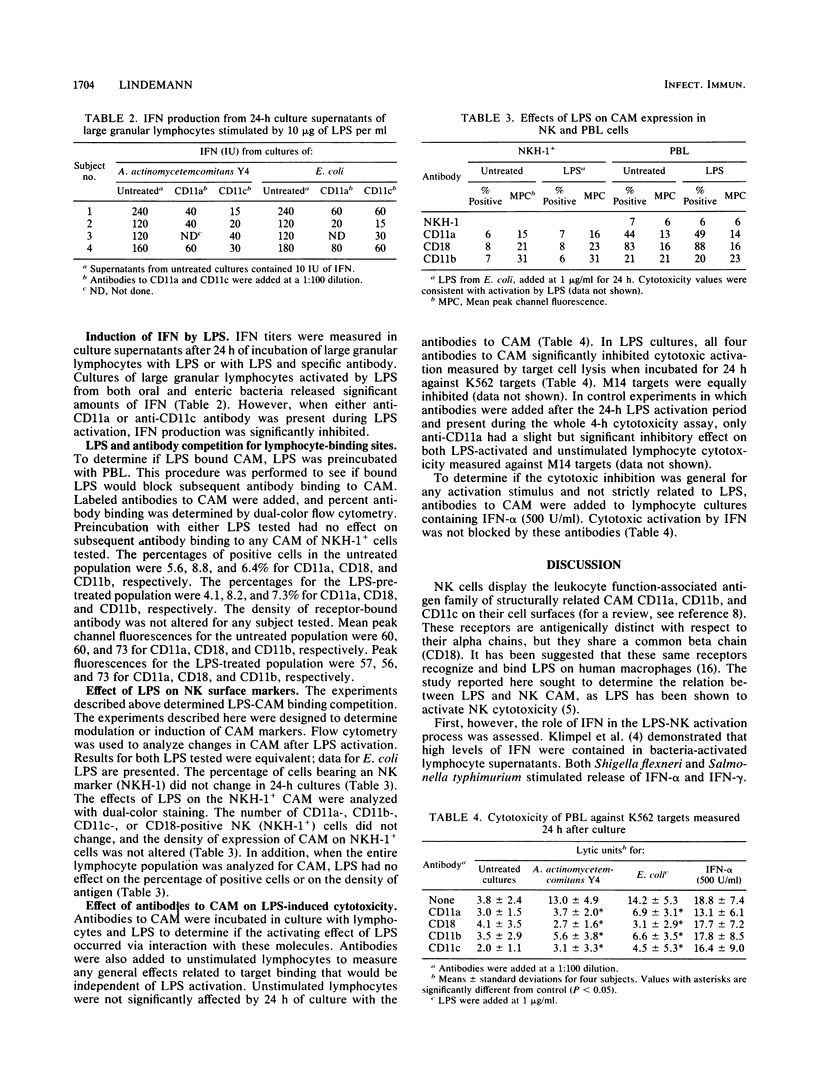
Interaction of lipopolysaccharide (LPS) from enteric and oral bacteria with natural killer (NK) cells enhanced cytotoxicity against NK-sensitive and NK-resistant targets. This activation occurred without expansion of the NK cell population or without changes in the leukocyte function-associated antigen family of cellular adhesion molecule (CAM) expression on NK cells. Significant interferon (IFN) titers were measured in LPS-lymphocyte supernatants, and antibody to IFN-alpha blocked LPS activation. LPS-induced NK cytotoxicity was inhibited by antibodies to individual alpha chains of CAM and, more profoundly, by antibody to the beta chain of CAM. However, LPS, when preincubated with NK cells, did not compete with subsequent anti-CAM antibody binding as detected by flow cytometry. Anti-CAM antibodies had no effect on NK activation by IFN, but antibodies to either CD11a or CD11c abrogated IFN production induced by LPS. These findings suggest that LPS binds NK cells at non-CAM sites, resulting in the release of IFN. IFN then acts in an autocrine manner independent of CAM to enhance NK cytotoxicity. Interaction of anti-CAM antibodies with CAM may provide a negative signal in regulating LPS-induced IFN production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L. The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res. 1961 Feb;23:14–20. doi: 10.1016/0014-4827(61)90059-3. [DOI] [PubMed] [Google Scholar]
- Karavodin L. M., Golub S. H. Universal rosetting reagent for the detection of human cell surface markers. J Immunol Methods. 1983 Jul 29;61(3):293–300. doi: 10.1016/0022-1759(83)90223-5. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Niesel D. W., Asuncion M., Klimpel K. D. Natural killer cell activation and interferon production by peripheral blood lymphocytes after exposure to bacteria. Infect Immun. 1988 Jun;56(6):1436–1441. doi: 10.1128/iai.56.6.1436-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann R. A. Bacterial activation of human natural killer cells: role of cell surface lipopolysaccharide. Infect Immun. 1988 May;56(5):1301–1308. doi: 10.1128/iai.56.5.1301-1308.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann R. A., Economou J. S., Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. J Dent Res. 1988 Aug;67(8):1131–1135. doi: 10.1177/00220345880670081401. [DOI] [PubMed] [Google Scholar]
- Lindemann R. A., Miyasaki K. T., Wolinsky L. E. Induction of activated lymphocyte killing by bacteria associated with periodontal disease. J Dent Res. 1988 May;67(5):846–850. doi: 10.1177/00220345880670051001. [DOI] [PubMed] [Google Scholar]
- Martz E. LFA-1 and other accessory molecules functioning in adhesions of T and B lymphocytes. Hum Immunol. 1987 Jan;18(1):3–37. doi: 10.1016/0198-8859(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Nocera A., Melioli G., Merli A., Santoro F., Zicca A. In vitro production of different interferon types by cloned human NK cells. Clin Exp Immunol. 1985 May;60(2):274–284. [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Tarkkanen J., Saksela E., Lanier L. L. Bacterial activation of human natural killer cells. Characteristics of the activation process and identification of the effector cell. J Immunol. 1986 Oct 15;137(8):2428–2433. [PubMed] [Google Scholar]
- Taylor S., Bryson Y. J. Impaired production of gamma-interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol. 1985 Mar;134(3):1493–1497. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Jong M. T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986 Dec 1;164(6):1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielske J. V., Golub S. H. Fetal calf serum-induced blastogenic and cytotoxic responses of human lymphocytes. Cancer Res. 1976 Oct;36(10):3842–3846. [PubMed] [Google Scholar]



