Abstract
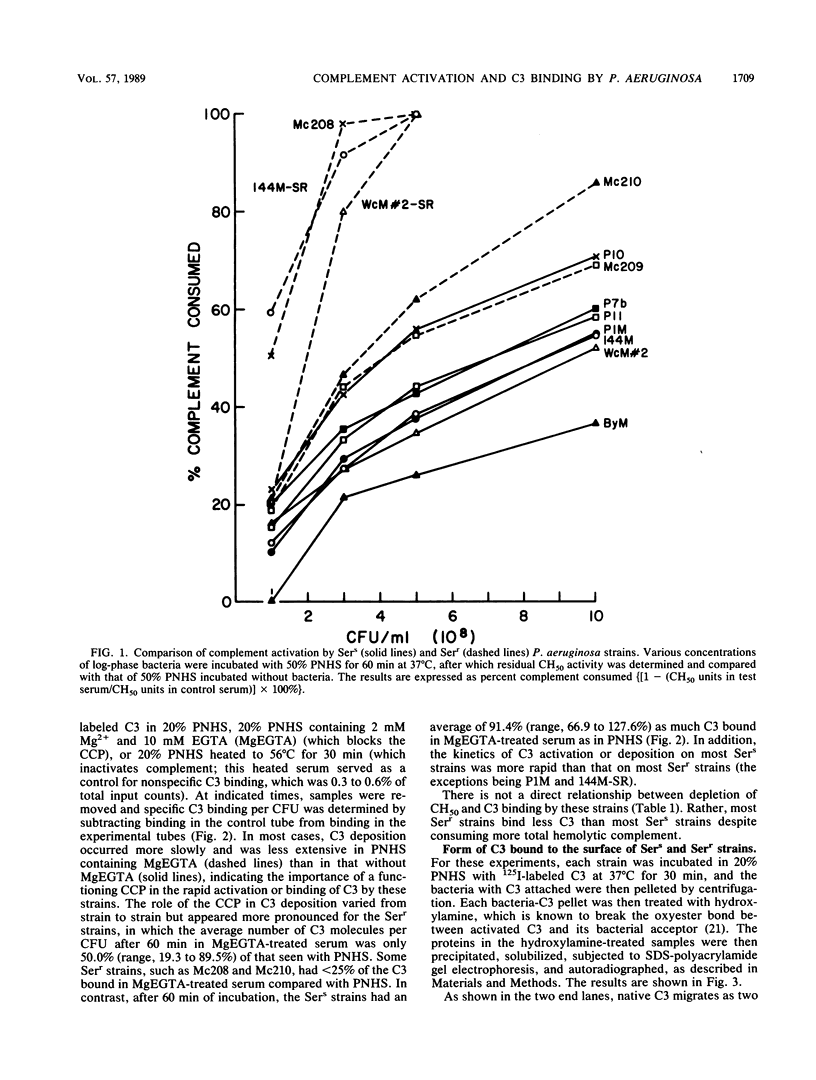
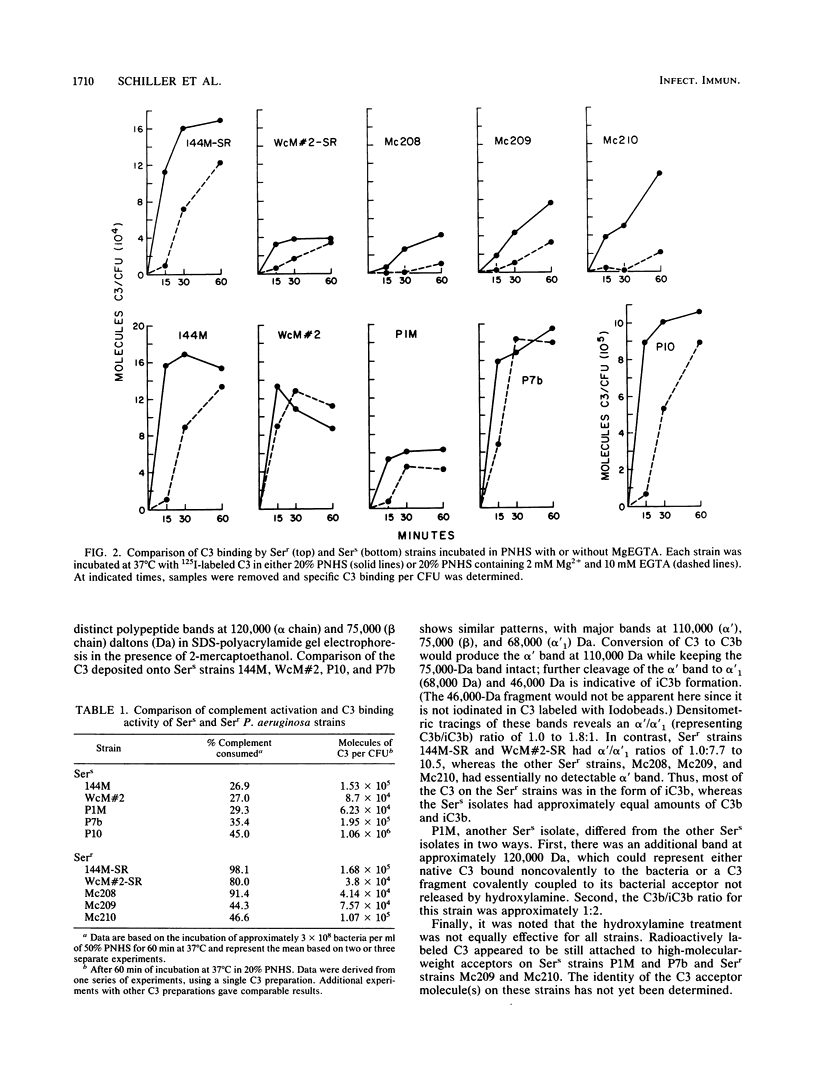
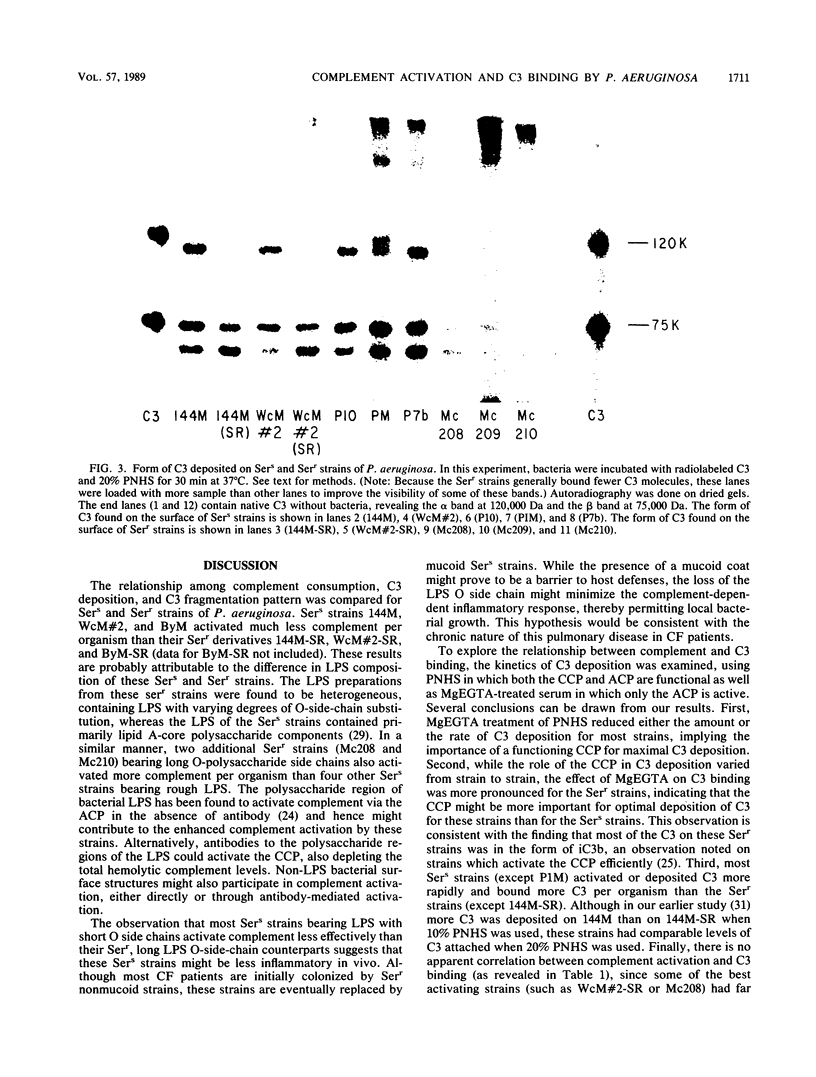
The relationship among complement consumption, C3 deposition, and C3 fragmentation pattern was compared for serum-sensitive (Sers) and serum-resistant (Serr) strains of Pseudomonas aeruginosa. The Sers strains, which were mucoid strains derived from patients with cystic fibrosis, had lipopolysaccharide deficient in O-antigen side chains. These organisms generally activated much less complement per organism than their Serr counterparts, characterized by the presence of lipopolysaccharide with long lipopolysaccharide O side chains. Surprisingly, however, although the Serr strains consumed more total hemolytic complement, less C3 was deposited onto the surface of these strains than onto that of the Sers strains. Maximal C3 binding required the participation of both the classical and alternative complement pathways, although classical complement pathway involvement was more important for Serr strains. Finally, while more than half of the C3 deposited on most Sers strains was in the form of C3b, most of the C3 on the Serr strains was in the form of iC3b, indicating a more rapid and extensive conversion of C3b to iC3b on the surface of these strains. Limited complement activation by Sers mucoid strains of P. aeruginosa may confer a selective survival advantage to these organisms in colonizing the airways of patients with cystic fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Engels W., Endert J., Kamps M. A., van Boven C. P. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1985 Jul;49(1):182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W., Endert J., Van Boven C. P. A quantitative method for assessing the third complement factor (C3) attached to the surface of opsonized Pseudomonas aeruginosa: interrelationship between C3 fixation, phagocytosis and complement consumption. J Immunol Methods. 1985 Jul 16;81(1):43–53. doi: 10.1016/0022-1759(85)90120-6. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fetterhoff T. J., McCarthy R. C. A micromodification of the CH50 test for the classical pathway of complement. J Clin Lab Immunol. 1984 Aug;14(4):205–208. [PubMed] [Google Scholar]
- Friend P. A. Pulmonary infection in cystic fibrosis. J Infect. 1986 Jul;13(1):55–72. doi: 10.1016/s0163-4453(86)92325-x. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J Infect Dis. 1986 Oct;154(4):619–626. doi: 10.1093/infdis/154.4.619. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Rice J., Finlay-Jones J. J., McDonald P. J., Hostetter M. K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988 Apr;157(4):697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- Grossman N., Joiner K. A., Frank M. M., Leive L. C3b binding, but not its breakdown, is affected by the structure of the O-antigen polysaccharide in lipopolysaccharide from Salmonellae. J Immunol. 1986 Mar 15;136(6):2208–2215. [PubMed] [Google Scholar]
- Grossman N., Leive L. Complement activation via the alternative pathway by purified Salmonella lipopolysaccharide is affected by its structure but not its O-antigen length. J Immunol. 1984 Jan;132(1):376–385. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess K., Kern S., Schiller H. H. Blink reflex in trigeminal sensory neuropathy. Electromyogr Clin Neurophysiol. 1984 Mar-Apr;24(3):185–190. [PubMed] [Google Scholar]
- Hostetter M. K., Gordon D. L. Biochemistry of C3 and related thiolester proteins in infection and inflammation. Rev Infect Dis. 1987 Jan-Feb;9(1):97–109. doi: 10.1093/clinids/9.1.97. [DOI] [PubMed] [Google Scholar]
- Hostetter M. K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986 Apr;153(4):682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I., Cooper N. R., Müller-Eberhard H. J., Pangburn M. K. Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry. 1981 Jul 21;20(15):4458–4467. doi: 10.1021/bi00518a034. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Grossman N., Schmetz M., Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J Immunol. 1986 Jan;136(2):710–715. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Mäkelä P. H., Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J Immunol. 1982 Mar;128(3):1229–1235. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J Exp Med. 1985 Jun 1;161(6):1414–1431. doi: 10.1084/jem.161.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A., Pitt T., Roberts D., Hodson M. E., Batten J. C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983 May;127(5):605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L. Characterization of the susceptibility of Pseudomonas aeruginosa to complement-mediated killing: role of antibodies to the rough lipopolysaccharide on serum-sensitive strains. Infect Immun. 1988 Mar;56(3):632–639. doi: 10.1128/iai.56.3.632-639.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Hatch R. A. The serum sensitivity, colonial morphology, serogroup specificity, and outer membrane protein of Pseudomonas aeruginosa strains isolated from several clinical sites. Diagn Microbiol Infect Dis. 1983 Jun;1(2):145–157. doi: 10.1016/0732-8893(83)90044-5. [DOI] [PubMed] [Google Scholar]
- Schiller N. L., Joiner K. A. Interaction of complement with serum-sensitive and serum-resistant strains of Pseudomonas aeruginosa. Infect Immun. 1986 Dec;54(3):689–694. doi: 10.1128/iai.54.3.689-694.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




