Abstract
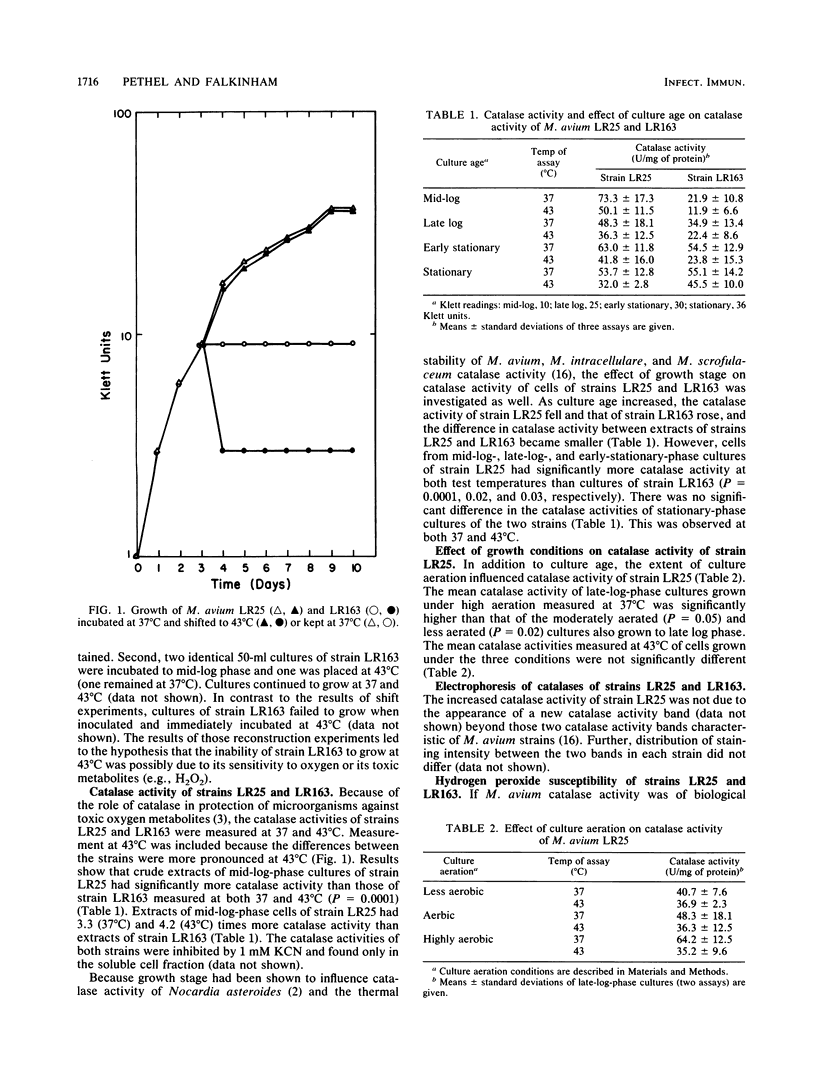
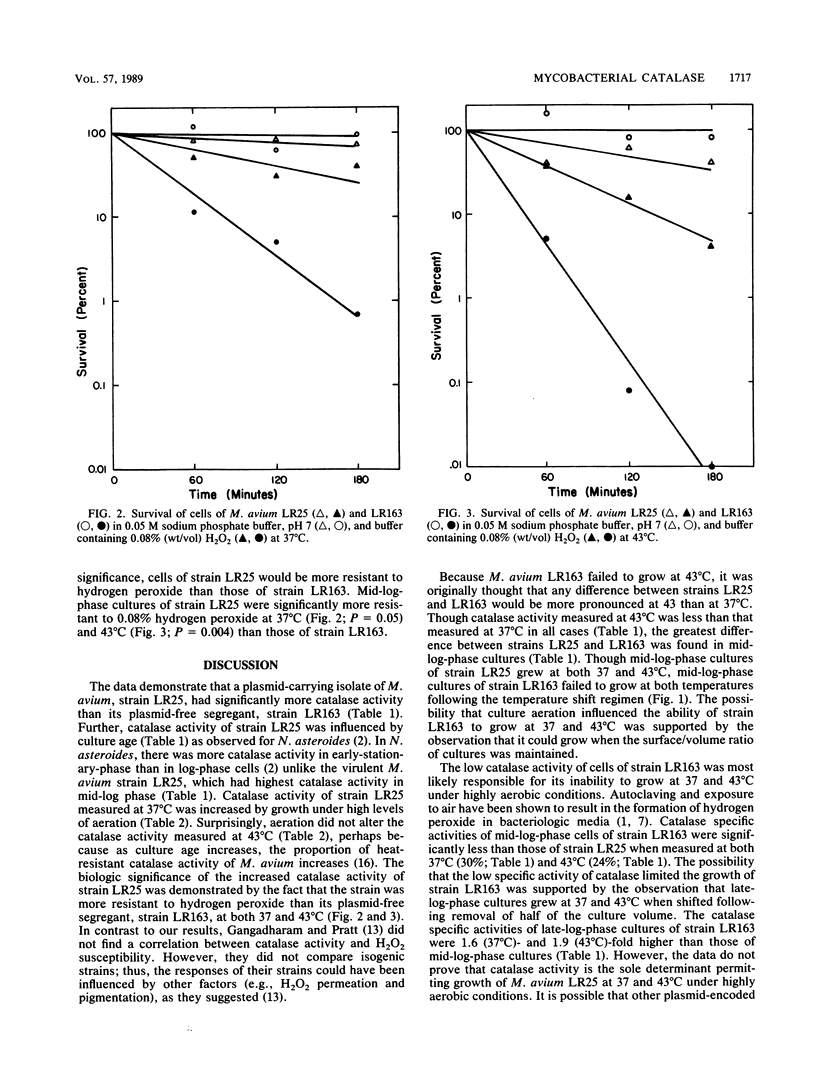
A virulent Mycobacterium avium strain, LR25, which carries three plasmids (18, 28, and 165 kilobases) and grows at 43 degrees C was compared with its plasmid-free, avirulent segregant, strain LR163, to identify the basis for the latter's inability to grow at 43 degrees C. The failure of mid-log-phase cultures of strain LR163 to grow at 43 degrees C was dependent on the presence of high levels of culture aeration. In addition, highly aerated cultures of strain LR163 failed to grow at 37 degrees C. Mid-log-phase cultures of strain LR163 had 30% of the catalase activity of strain LR25 and were more hydrogen peroxide (0.08%, wt/vol) susceptible. Catalase activity of strain LR25 was higher in cultures grown with high aeration than in those grown with almost no aeration. These data support the contention that plasmid-encoded genes influence M. avium catalase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY V. C., CONALTY M. L., DENNENY J. M., WINDER F. Peroxide formation in bacteriological media. Nature. 1956 Sep 15;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Cohn D. L. Opportunistic infections in patients with AIDS: clues to the epidemiology of AIDS and the relative virulence of pathogens. Rev Infect Dis. 1986 Jan-Feb;8(1):21–30. doi: 10.1093/clinids/8.1.21. [DOI] [PubMed] [Google Scholar]
- Brooks R. W., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis. 1984 Oct;130(4):630–633. doi: 10.1164/arrd.1984.130.4.630. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Cave M. D., Bates J. H. Evidence for plasmid-mediated restriction-modification in Mycobacterium avium intracellulare. J Gen Microbiol. 1981 Dec;127(2):333–338. doi: 10.1099/00221287-127-2-333. [DOI] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Parker B. C., Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980 Jun;121(6):931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- Fry K. L., Meissner P. S., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. VI. Identification and use of epidemiologic markers for studies of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Am Rev Respir Dis. 1986 Jul;134(1):39–43. doi: 10.1164/arrd.1986.134.1.39. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Crawford J. T., Bates J. H. Association of plasmids and virulence of Mycobacterium avium complex. Am Rev Respir Dis. 1988 Jan;137(1):212–214. doi: 10.1164/ajrccm/137.1.212. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. E. Susceptibility of Mycobacterium intracellulare to hydrogen peroxide. Am Rev Respir Dis. 1984 Aug;130(2):309–311. doi: 10.1164/arrd.1984.130.2.309. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meissner P. S., Falkinham J. O., 3rd Plasmid DNA profiles as epidemiological markers for clinical and environmental isolates of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. J Infect Dis. 1986 Feb;153(2):325–331. doi: 10.1093/infdis/153.2.325. [DOI] [PubMed] [Google Scholar]
- Wendt S. L., George K. L., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous Mycobacteria. III. Isolation of potentially pathogenic mycobacteria from aerosols. Am Rev Respir Dis. 1980 Aug;122(2):259–263. doi: 10.1164/arrd.1980.122.2.259. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]



