Abstract
The Nod-like receptor (NLR) family member, Nlrp6, has been implicated in inflammasome signaling to activate caspase-1, which is essential for the production of mature IL-1β and IL-18. However, a function for Nlrp6 in vivo has never been demonstrated. Due to the relative high expression of Nlrp6 in intestinal tissue, we hypothesized that Nlrp6 has a role in intestinal homeostasis. Indeed, Nlrp6-deficient mice are more susceptible to chemically-induced colitis as well as colitis-induced tumorigenesis than wildtype mice. Nlrp6-deficient mice exhibited significantly more inflammation within the colon than wildtype mice after dextran sulfate sodium treatment. Their inability to resolve inflammation and repair damaged epithelium as efficiently as wildtype mice resulted in prolonged increases in epithelial proliferative activity that likely underlie the increased propensity for tumors in these mice during chronic inflammation. We further show that that the activity of Nlrp6 in hematopoietic cells is critical for protection against inflammation-related colon tumorigenesis. This study highlights the importance of NLR function in maintaining intestinal homeostasis to prevent the development of aberrant inflammation and tumor development within the colon.
Introduction
The Nod-like family of receptors (NLRs) represent a major class of pattern-recognition receptors that play an integral role in host defense (1). These receptors are defined by a tripartite structure typically consisting of an N-terminal PYRIN, CARD, or BIR domain that mediates downstream protein-protein interactions, a central nucleotide-binding oligomerization domain (NOD), and a C-terminal leucine-rich repeat (LRR) that can recognize microbial or endogenous signals. As upstream regulators of key inflammatory signaling pathways, such as NF-κB/MAPK, Type I interferons, and caspase-1, NLRs also evidently participate in crucial functions in addition to pathogen eradication such as in immune and gut homeostasis (2–4). Consequently, impairment in NLR function results in not only decreased immunity against infection, but also can lead to a variety of disease states such as chronic colitis and tumorigenesis, metabolic disorders, and autoimmunity (2, 5, 6).
A subset of NLRs are involved in the activation of caspase-1 through formation of a multimeric complex that has been termed the inflammasome (7). The principal function of the inflammasome is to activate caspase-1 that then leads to cleavage of the pro-forms of the inflammatory cytokines IL-1β and IL-18 to their biologically active forms. Assembly of the inflammasome involves protein-protein interactions that link the NLRs with the adaptor protein ASC and caspase-1. Only a few NLRs have been identified to function in inflammasome signaling, namely Nlrp1, Nlrp3, and Nlrc4, that have physiologic, in vivo relevance (6, 8). More recently, Nlrc5 has been suggested to have inflammasome activity to promote caspase-1 and IL-1β production although an in vivo role for this molecule remains to be determined (9, 10). Nlrp6 is an as yet poorly characterized member of the NLR family defined by an N-terminal PYRIN domain, a central nucleotide binding domain, and C terminal LRR (1). Little is known about the function of Nlrp6. In vitro studies have demonstrated that Nlrp6 colocalizes with ASC presumably through protein-protein interactions with the pyrin domain of both proteins, and that co-expression of NRLP6 with ASC resulted in cooperative production of IL-1β, suggesting that Nlrp6 also participates in inflammasome signaling (11). However, a physiologically relevant function of Nlrp6 has previously not been identified.
We show that in the mouse, Nlrp6 is relatively highly expressed in the intestine, and therefore, we hypothesized that Nlrp6 has a physiologic function within the colon. Using Nlrp6-deficient mice, we demonstrate that Nlrp6 is protective against the development of significant damage and inflammation within the colon during chemically-induced colitis by dextran sulfate sodium (DSS). In a model of inflammation-induced tumorigenesis, Nlrp6-deficient mice developed significantly larger and more tumors compared to wildtype mice. The increase in tumors in Nlrp6-deficient mice correlated with higher levels of intestinal epithelial proliferation and hyperplasia over an extended period of time compared with wildtype mice as well as increases in inflammatory cytokine production that are associated with increased tumorigenicity. Protection against tumorigenesis by Nlrp6 is conferred specifically by hematopoietic cells rather than intestinal epithelial or stromal cells. This is the first study revealing an in vivo role for Nlrp6, particularly in modulating inflammatory responses in the colon to allow recovery from intestinal epithelial damage and limit tumorigenic potential.
Materials and Methods
Mice
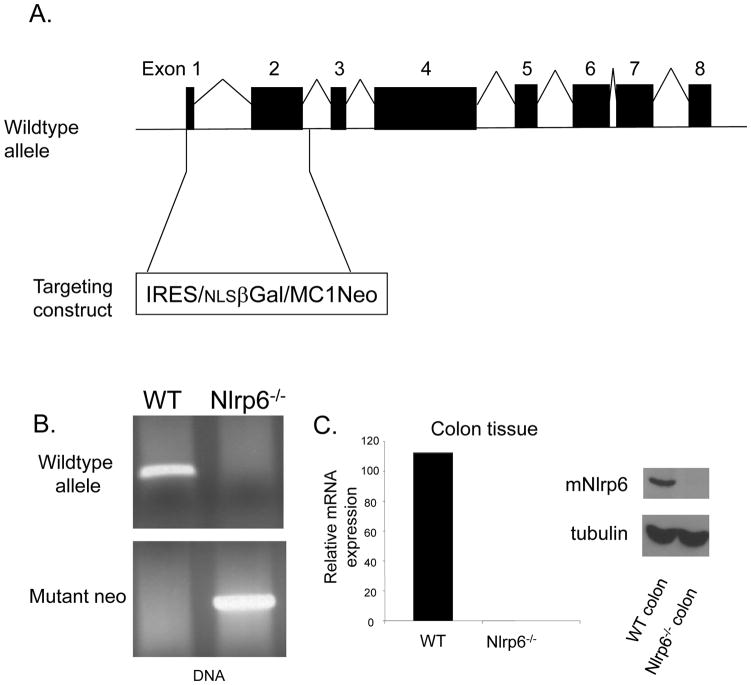
Nlpr6−/− mice were generated by the replacement of exon 1 and 2 of the Nlpr6 gene (N-terminal domain) with the IRES-β-gal-neomycin resistance cassette using a targeting vector (Fig. 2A). The positive embryonic stem cell clone was used to generate chimeric 129/C57BL/6 mice. Chimeric mice were backcrossed onto the C57BL/6 background at least 6 times. Genotyping was performed using primers targeting the neomycin resistance gene and the deleted portion of the targeted Nlrp6-gene (primer sequences available upon request). Wildtype controls were C57BL/6 originally purchased from Jackson Laboratories and bred in-house. Mice were generally 8 to 16 weeks of age and were maintained in a specific pathogen-free facility. Animal studies were conducted under protocols approved by the University of Michigan Committee on the Use and Care of Animals.
Figure 2. Generation of Nlr6-deficient mice.
A, Nlrp6−/− mice were generated by replacement of exon 1 and 2 of the Nlrp6 gene with the IRES/βGal/Neomycin resistance gene cassette. B, Genotype of Nlrp6−/− mice confirmed by PCR of genomic tail DNA using primers directed against Nlrp6, and the neomycin resistance gene. C, Confirmation of the absence of Nlrp6 expression within the colon of Nlrp6−/− mice on an mRNA (left) and protein (right) level. Nlrp6 mRNA was measured by quantitative real-time PCR and normalized with respect to actin. Nlrp6 protein expression in colon tissue extracts of wildtype (WT) and Nlrp6−/− mice was assessed by Western blot analysis with tubulin used as a loading control.
Nlrp6 detection
Colons were homogenized in protein extraction buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 0.1% NP40, 0.5 mM DTT, 5% glycerol, and Halt’s protease and phosphatase inhibitor cocktail (Pierce)). After brief sonication, insoluble material was removed by sample centrifugation at 14,000 rpm in a microcentrifuge at 4°C. Supernatants were collected, and protein quantification was performed by standard Bradford assay (Pierce). Proteins were prepared in Laemmli buffer, boiled, and separated by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane and immunoblotted with anti-Nlrp6 (goat polyclonal antibody, clone E20, Santa Cruz). mRNA was collected after colon tissue homogenization using the Macherery-Nagel Nucleospin Kit. cDNA was synthesized using iScript (Biorad) and then used in quantitative PCR reactions with SYBR Green using primers against exon 1/2. Reactions were performed on the ABI 7900HT.
DSS-induced colitis and colitis-associated tumorigenesis
Mice were treated with 7 days of 3.5% DSS (MP Biomedicals, MW: 36–40 kDa) in regular drinking water. To develop colitis-associated tumors, mice were first injected with 10 mg/kg azoxymethane (AOM) (Sigma-Aldrich) intraperitoneally (i.p.) followed 5 days later by a 5 day course of 2% DSS (Fig. 3B). Mice were then allowed to recover for 16 days with regular drinking water. The five days of 2% DSS followed by 16 days of regular drinking water was repeated twice. Mice were sacrificed 21 days after the last cycle of DSS for tumor counting. Colons were harvested, flushed of feces and longitudinally slit open to grossly count tumors with the aid of a magnifier and stereomicroscope.
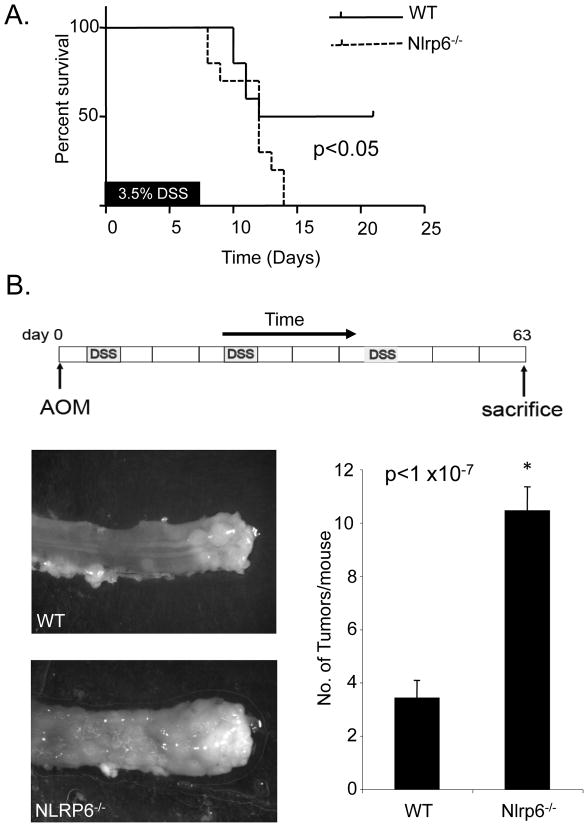
Figure 3. Nlrp6-deficient mice have increased susceptibility to chemically-induced colitis and inflammation-induced tumorigenesis.
A, Ten age- and sex-matched wildtype (WT) and Nlrp6-deficient mice were treated with 7 days of 3.5% DSS and survival of mice was assessed. Data was analyzed by log-rank test. B, Age and sex-matched Nlrp6−/− (N=26 mice) and wildtype (N=25 mice) mice, were treated with AOM intraperitoneally followed by three cycles of 2% DSS. Three weeks after the last cycle of DSS, mice were sacrificed and tumors grossly counted with a stereomicroscope. Photographs are representative of tumors within the distal rectum of Nlrp6−/− and wildtype mice. Data is represented by means +/− S.E.M. *p <1 ×10-7 by Student’s t-test.
Assessment of inflammation
Colons were harvested from mice, flushed free of feces and jelly-rolled for formalin fixation and paraffin embedding. 5 um sections were used for hematoxylin and eosin staining. Histologic assessment was performed in a blinded fashion using a scoring system as described previously (5). Briefly, a 3–4 point scale was used to denote the severity of inflammation (0=none, 1=mild, 2=moderate, and 3=severe), the level of involvement (0=none, 1=mucosa, 2=mucosa and submucosa and 3=transmural) and extent of epithelial/crypt damage (0=none, 1=basal 1/3, 2=basal 2/3, 3=crypt loss, 4=crypt and surface epithelial destruction). Each parameter was then multiplied by a factor reflecting the percentage of the colon involved (0–25%, 26–50%, 51–75%, and 76–100%), and then summed to obtain the overall score. Assessment of colon weight after DSS treatment was performed by measuring the weight of colons (excluding the cecum) after removal of feces and normalizing by the length of colon in age- and sex-matched mice.
Intestinal Permeability
Mice were fasted for four hours with the exception of drinking water prior to the administration of 0.6 mg/kg FITC-dextran (4kD, Sigma). Serum was collected 4 hours later retro-orbitally, diluted 1:3 in PBS and the amount of fluorescence measured using a fluorescent spectrophotometer with emission at 488 nm, absorption at 525 nm.
Cytokine expression
Colonic tissue was homogenized, and total RNA isolated using the Nucleospin RNA kit (Macherey-Nagel). cDNA synthesis was performed using iScript (BioRad), and the cDNA was then used for quantitative PCR using either SYBR Green Master Mix (Applied Biosystems) or Taqman Gene Expression Assays on the ABI 7900HT. Primers sequences are available upon request. Serum was collected retro-orbitally, and IL-18 measurements were performed by ELISA (MBL). IL-18 measurements within colon tissue were performed by harvesting colon tissue and homogenizing in protein extraction buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 0.1% NP40, 0.5 mM DTT, 5% glycerol, and Halt’s protease and phosphatase inhibitor cocktail (Pierce)). After brief sonication, samples were centrifuged at 14,000 rpm in a microcentrifuge at 4°C, and supernatants were collected for IL-18 measurement by ELISA (MBL).
Intestinal epithelial proliferation
Mice were injected with 100 mg/kg BrdU (B.D. Pharmingen) i.p. 2.5 hours prior to sacrifice at various timepoints after treatment with AOM/DSS. Colons were then dissected free, flushed free of feces, jellyrolled, formalin-fixed, and paraffin-embedded. Sections were subsequently stained for BrdU using the BrdU in situ detection kit (BD Biosciences).
Apoptosis
Colon sections from formalin-fixed, paraffin-embedded tissues were assessed for apoptotic cells using the ApoAlert DNA fragmentation assay kit (Clontech).
Generation of bone marrow chimeras
Recipient Nlrp6-deficient and wildtype mice were lethally-irradiated with split dose irradiation to minimize injury to the intestinal epithelium (550 rad separated by 3 hours for a total dose of 1100 rad). 10 × 106 bone marrow cells that had been flushed from the tibias and femurs of donor Nlrp6-deficient and wildtype mice were injected into the lethally-irradiated recipient mice into the tail vein or retro-orbital sinus. Four chimeric groups were generated, wildtype>wildtype, wildtype>Nlrp6−/−, Nlpr6−/−>wildtype, Nlrp6−/−>Nlrp6−/−. Bone marrow reconstitution was verified by flow cytometric analysis of CD45.1 donor and CD45.2 recipient concurrently treated mice. Mice were treated with 3 weeks of antibiotics, and allowed to recover for a minimum of 6 weeks prior to treatment with AOM/DSS to induce tumors.
Statistical Analyses
Data are presented as means +/− SEM. Survival curves was assessed by log-rank test (Prism software, GraphPad). Comparison of tumor counts, intestinal permeability, cytokine measurements, proliferation and apoptosis levels between Nlrp6-deficient and wildtype mice were performed using the Student’s unpaired t-test. P values less than 0.05 were considered statistically significant.
Results
Nlrp6-deficient mice have increased susceptibility to colitis and colitis-associated colon tumorigenesis
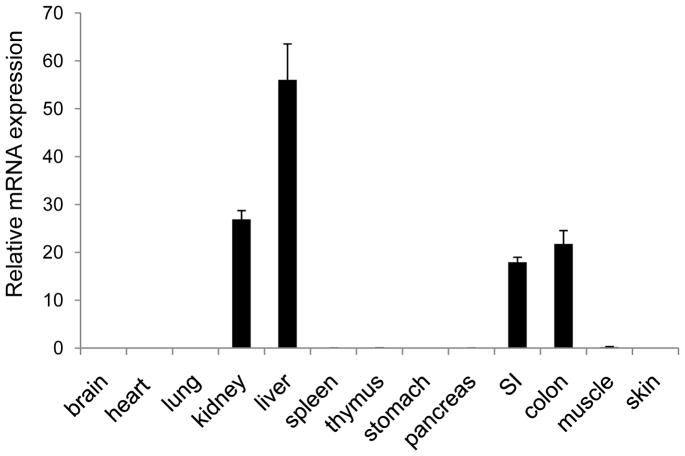
Although Nlrp6 was first studied almost ten years ago, identifying a biological function for Nlrp6 had remained elusive. To help determine a physiologic role for Nlrp6, we performed Nlrp6 mRNA expression profiling of various mouse tissues (Fig. 1). Aside from the liver and kidney, Nlrp6 was also very highly expressed in the small and large intestine, and minimally expressed in other tissues, including the stomach, pancreas, thymus, spleen and lung.
Figure 1. Nlrp6 is relatively highly expressed in the intestine.
mRNA expression of Nlrp6 relative to actin in various tissues isolated from at least 4 mice. Data represented by means +/−S.E.M.
To determine an in vivo role for Nlrp6 within the colon, Nlrp6-deficient mice were subjected to chemically-induced colitis with DSS, or treated with the carcinogen, azoxymethane (AOM), followed by three rounds of 2% DSS, a concentration that minimized treatment-related mortality, to induce colon tumorigenesis, which is a well-established model for colitis-associated colon carcinogenesis. Nlrp6-deficient mice were generated by the removal of exons 1 and 2 (Fig. 2A, B), resulting in ablation of both mRNA and protein expression within the colon (Fig. 2C). With 7 days of high concentration DSS (3.5%), the development of acute colitis resulted in increased mortality in the Nlrp6−/− group compared with wildtype (Fig. 3A). Furthermore, after AOM injection and multiple rounds of 2% DSS that lead to chronic colonic inflammation and tumor formation (Fig. 3B), Nlrp6-deficient mice developed significantly more tumors that were also larger in size compared with that of wildtype mice (Fig. 3B). These results suggest that Nlrp6 has an important function in vivo in regulating acute colitis and protecting against the development of colitis-induced tumors during chronic inflammation.
Increased tumor burden in Nlrp6-deficient mice is associated with greater inflammation and damage within the colon
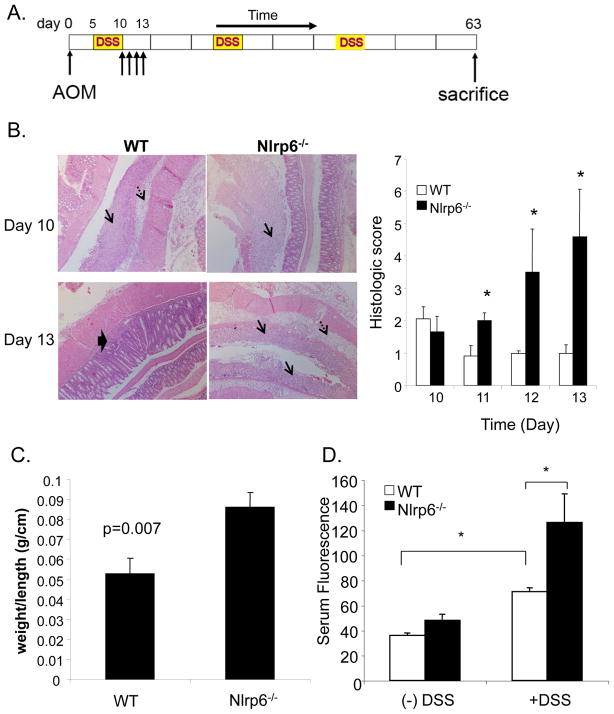
To begin to understand early events that occur in AOM/DSS-treated mice that can precipitate increased tumorigenesis, we evaluated the extent of inflammation and epithelial damage acutely after the first round of DSS (Fig. 4A). Histologic evaluation of the extent of epithelial damage, submucosal edema, and inflammatory cell infiltration revealed that Nlpr6-deficient mice developed worsening, extensive colitis the first week after DSS treatment (days 10–13) that was relatively controlled and limited in wildtype mice (Fig. 4B). The increased inflammation within the colon in the treated Nlrp6-deficient mice was consistent with the heavier weight of Nlrp6-deficient colons afflicted with colitis compared to that of wildtype (Fig. 4C). Furthermore, using FITC-labeled dextran, which is not actively absorbed through the gut and into the bloodstream, we found that Nlrp6-deficient mice had increased serum levels of FITC-dextran after AOM/DSS treatment, indicative of enhanced intestinal permeability and suggestive of increased intestinal damage from DSS-induced epithelial injury (Fig. 4D).
Figure 4. Increased inflammation and damage in Nlrp6-deficient mice.
A, Representative photographs of jelly-rolled colons of Nlrp6−/− and wildtype (WT) mice sacrificed on day 10 and day 13 of the AOM/DSS tumor induction protocol under 250× magnification (left). Single arrows denote mucosal ulceration, dashed arrows point to submucosal edema, and thick arrow indicate regenerating crypts within the mucosa. Histologic scoring based on extent of mucosal ulceration, submucosal edema, and inflammatory cell infiltration was performed (N=5 mice/group/timepoint) treated with AOM/DSS and sacrificed on days 10 through 13 after the first round of DSS (right). C, Colons from sex- and age-matched Nlrp6−/− and wildtype (N=5 mice/group) were harvested on day 13, and weighed. Weights were normalized by length of colon. D, AOM/DSS-treated and untreated Nlrp6−/− and wildtype mice (N=5 mice/group) were given FITC-dextran by oral gavage on day 10. Serum was collected 4 hours later, and fluorescence measured by a fluorescent spectrophotometer. Data are expressed as means +/− S.E.M, *, p< 0.05 using Student’s t-test.
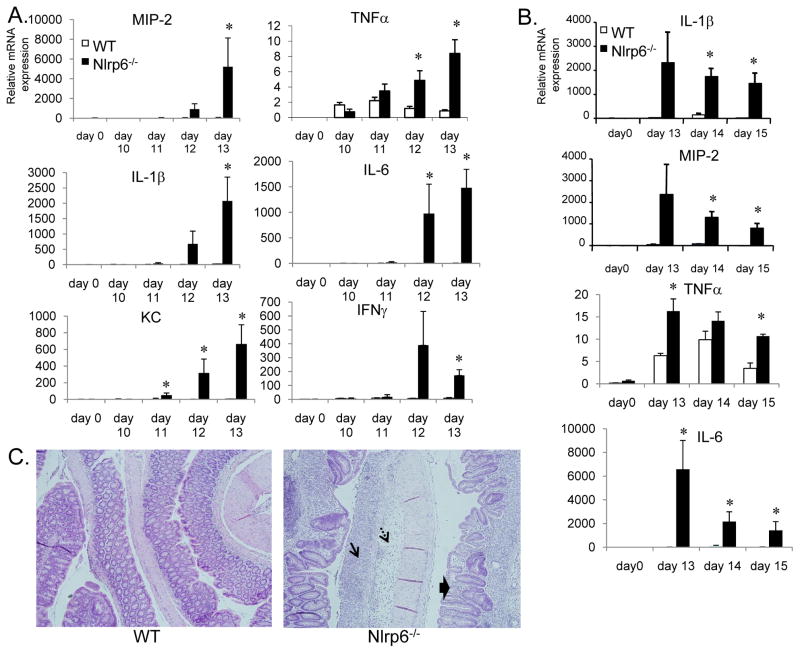
Tumor development as a result of chronic inflammation is typically dependent on the production of inflammatory cytokines that can recruit immune cells and promote the production of mitogenic factors. To determine if the increased epithelial damage and inflammation observed in Nlrp6-deficient mice was associated with increased proinflammatory mediator production, we analyzed the colon for production of various cytokines and chemokines on an mRNA level by quantitative real-time PCR. We observed dramatic increases in the levels of inflammatory cytokines, including TNFα, IL-6 and IL-1β on days 10–13 after the first round of DSS in Nlrp6-deficient that were substantially greater than that observed in wildtype mice (Fig. 5A). Moreover, the production of these inflammatory mediators remained significantly enhanced in Nlrp6-deficient mice beyond day 13 when inflammation within the colons of wildtype mice has mostly resolved and the epithelium has restituted (Fig. 5B, C). In contrast, the colons of Nlrp6-mice remained extensively damaged with significant inflammation present (Fig. 5C).
Figure 5. Increased proinflammatory mediator production and delayed resolution of inflammation in Nlrp6-deficient mice.
A, mRNA was isolated from age- and sex-matched wildtype and Nlrp6-deficient on days 0 (N=3 mice/group), 10–13 (N=5 mice/group/timepoint) after treatment with AOM/DSS. mRNA expression of various proinflammatory mediators relative to β-actin was measured by quantitative real-time PCR. B, mRNA expression of proinflammatory mediators on days 13 to 15 after the first round of DSS in the AOM/DSS tumor induction protocol. Data expressed as means +/− S.E.M., *, p< 0.05 by Student’s t-test. C, Representative photographs of hematoxylin and eosin-stained colon sections from Nlrp6−/− and wildtype (WT) mice on day 15. Single arrow shows area of mucosal ulceration; dashed arrows point to submucosa edema. Thick arrows show regenerating crypts in a background of inflammation with inflammatory cell infiltration.
Nlrp6-deficient mice develop increased epithelial proliferation that is prolonged
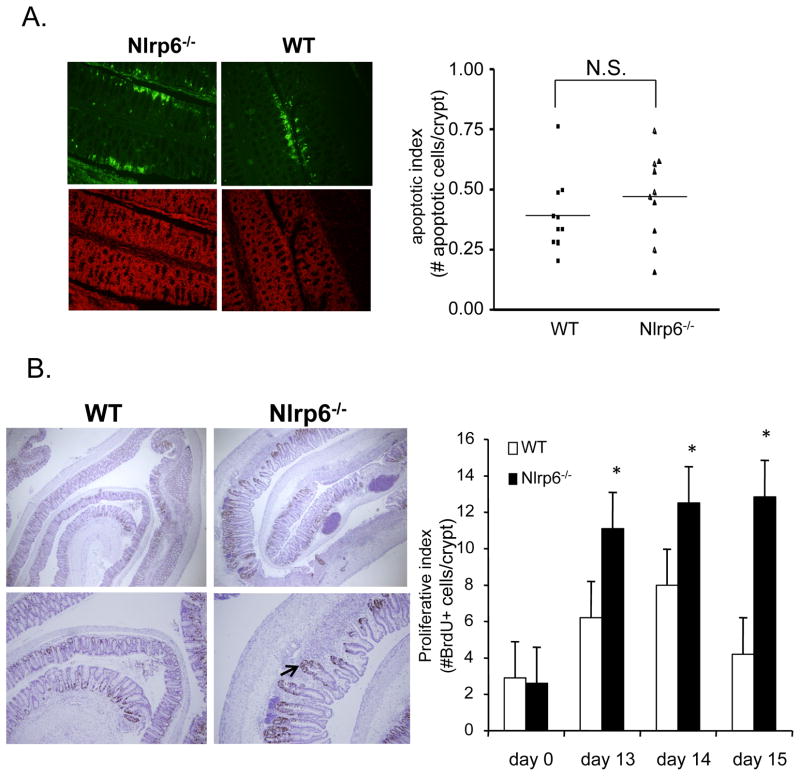
To determine whether there were differing levels of intestinal epithelial apoptosis or proliferation that can explain the increased tumor development in Nlrp6-deficient mice, we first assessed apoptotic activity by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). During the first round of DSS treatment, we saw no significant differences in the amount epithelial apoptosis in the colons of Nlrp6−/− and wildtype mice (Fig. 6A). However, in the week following the first round of DSS when the epithelium has mostly healed in wildtype mice, there is a sustained increase in intestinal epithelial proliferative activity in Nlrp6-deficient mice (Fig. 6B). In addition, in a background of inflammation, there are areas of dysplastic changes within the regenerating epithelium (Fig. 6B). These results suggest that the increased tumor development observed in Nlrp6-deficient mice is due to the enhanced and prolonged proliferative activity as compared with that in wildtype mice.
Figure 6. Increased epithelial proliferation in inflamed colons of Nlrp6-deficient mice.
A, 10 age- and sex-matched mice were treated with AOM/DSS and sacrificed on day 8. Colons were harvested, jelly-rolled, formalin-fixed and paraffin-embedded. Levels of intestinal epithelial apoptosis was assessed by TUNEL assay using a fluorescent microscope. Representative photographs under 500× magnification of colon sections from Nlrp6−/− and wildtype mice (N=10 mice/group) stained for TUNEL activity (apoptotic cells labeled green, top) with corresponding sections counterstained with propidium iodide (red, bottom) (left). Apoptotic index represents number of apoptotic cells within the surface epithelium per crypt (~500 total crypts counted) (right). B, Age- and sex-matched Nlrp6−/− and wildtype mice (N=5 mice/group) were injected with BrdU intraperitoneally 2.5 hours prior to sacrifice on days 13–15 (N=5 mice/group/timepoint) and day 0 (N=3 mice/group). Proliferative activity of colonic epithelium was assessed by counting number of Brdu+ cells per crypt (~100 properly aligned crypts counted). Photographs are representative sections stained for BrdU at 250× magnification(top, left) and 500× (bottom, left). Arrow points to a regenerating crypt with disorganized architecture. Data expressed as means +/− S.E.M in bar graphs (right). *, p<0.05 by Student’s t-test. N.S., not significant.
Nlrp6-deficient mice have decreased IL-18 production during the acute inflammatory response
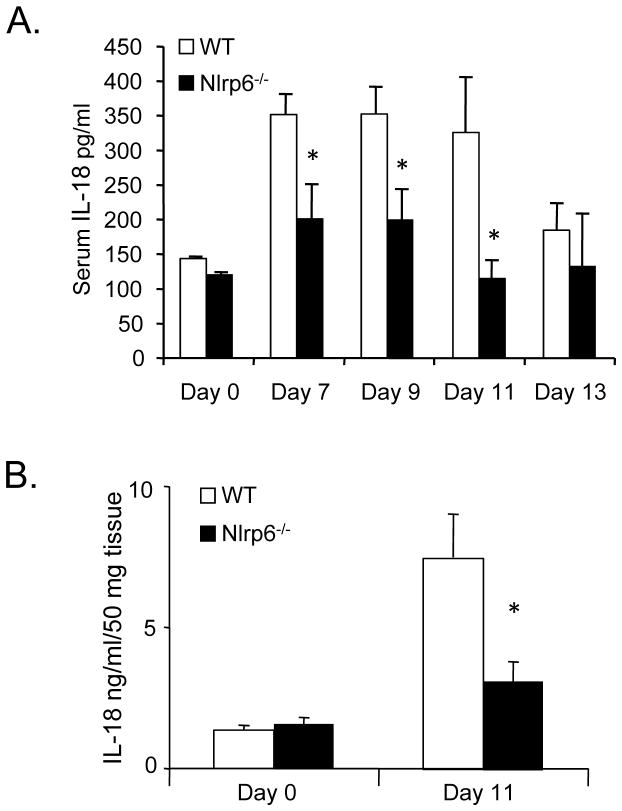
The activity of the inflammasome, particularly in the production of IL-18, was recently shown to be important in intestinal repair and tumorigenesis (4, 12–14). Mice deficient in the Nlrp3 inflammasome, for example, were demonstrated to be highly susceptible to both DSS-induced colitis and AOM/DSS-induced tumorigenesis (4, 12, 13). In both scenarios, the increased intestinal inflammation and tumorigenesis was related to impaired production of IL-18 as observed in ASC-deficient mice that had a similar phenotype as Nlrp3-deficient mice. IL-18 is a pro-inflammatory cytokine that enhances the production of IFNγ and Th1 responses (15–17). However, during DSS-induced colitis, IL-18 was also shown to have a protective effect important for the repair of the intestinal mucosa (4, 14). Given previous in vitro studies suggesting a link between Nlrp6 and the inflammasome, and for the recently identified role of IL-18 in regulating colitis-associated tumorigenesis, we investigated whether AOM/DSS-treated Nlrp6-deficient mice had alterations in IL-18 production during and after the first round of DSS compared with wildtype.
We analyzed levels of IL-18 present in the serum (Fig. 7A) and colon tissue (Fig. 7B) of Nlrp6-deficient and wildtype mice after AOM/DSS treatment. Consistent with a presumed role for Nlrp6 in inflammasome signaling and caspase-1 activation, we saw decreased levels of serum IL-18 in Nlrp6-deficient mice on days 6–11 during and after DSS treatment. Furthermore, IL-18 was also significantly reduced within the colon tissue of Nlrp6-deficient mice on day 11 after DSS treatment despite increases in other proinflammatory mediators (Fig. 5A, B). The reduction in IL-18 in the colon tissue was not due to lower levels of pro-IL-18 as mRNA levels were not significantly different between wildtype and Nlrp6-deficient mice (Supplemental Fig. 1A). This indicates an association between delayed epithelial reconstitution and prolonged inflammation with an impairment in IL-18 production in Nlrp6-deficient mice.
Figure 7. Decreased Il-18 production in Nlrp6-deficient mice during inflammation-induced tumorigenesis.
Serum was collected from age- and sex-matched untreated (N=3 mice/group) and AOM/DSS-treated Nlrp6−/− and wildtype mice on days 7 to 13 (N=5 to 9 mice/group/timepoint). IL-18 was measured by ELISA. B, Colon tissue protein extracts were made from untreated (N=4 mice/group) and AOM/DSS-treated mice (N=5 mice/group) sacrificed on day 11. IL-18 was measured by ELISA and normalized by colon weight. Data expressed as means +/− S.E.M., *, p<0.05 by Student’s t-test.
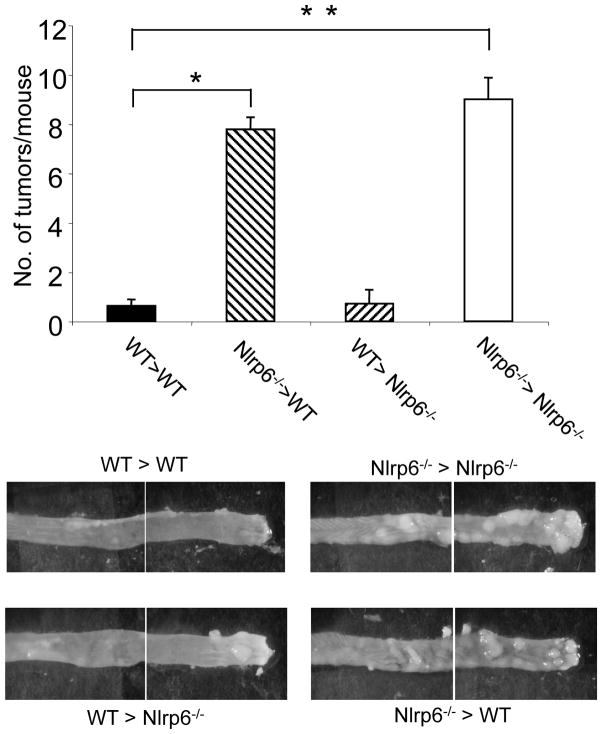
Nlrp6 function in hematopoietic cells is important for protection against colon tumorigenesis
To further understand the role of Nlrp6 within the colon, we wished to determine which cellular compartment (i.e., hematopoietic versus epithelial/stromal) was important for Nlrp6 protection against colon tumorigenesis during chronic inflammation. Nlrp6 is relatively highly expressed in isolated colon epithelial cells, but also detectable in lamina propria cells, including cells of the granulocyte and monocyte lineage (Supplemental Fig. 2). To determine whether Nlrp6 function in hematopoietic or epithelial/stromal cells was important for inflammation-related tumor suppression, we generated bone marrow chimeric mice in which lethally-irradiated Nlpr6-deficient or wildtype mice were reconstituted with either Nlrp6-deficient or wildtype bone marrow and then treated with AOM and DSS to induce tumor formation (Fig. 8). As expected, wildtype recipient mice that were adoptively transferred with wildtype bone marrow had few tumors (WT > WT) and Nlrp6-deficient mice that were reconstituted with Nlrp6 bone marrow (Nlrp6−/−>Nlrp6−/−) had significantly more tumors. Lethally-irradiated wildtype mice that were transplanted with Nlrp6-deficient bone marrow (Nlpr6−/−>WT) had similar numbers of tumors as Nlrp6-deficient mice Nlrp6−/−>Nlrp6−/−), which suggests that wildtype epithelial and stromal cells have no role in limiting tumor development. In contrast, Nlrp6-deficient recipients that received wildtype bone marrow (WT>Nlrp6−/−) were significantly protected against tumorigenesis to a similar extent as wildtype animals (WT>WT). Altogether, these results strongly suggest that a deficiency in Nlrp6 function in hematopoietic-derived cells rather than radio-resistant epithelial/stromal cells is important for Nlrp6-mediated protection against colitis-induced tumorigenesis.
Figure 8. Nlrp6 function in hematopoietic cells is important for protection against inflammation-induced tumorigenesis.
Bone marrow chimeras were generated by lethally-irradiating Nlrp6−/− and wildtype (WT) recipient mice and transplanting bone marrow by tail vein injection from either wildtype or Nlpr6−/− mice donor mice to generate 4 groups of mice: WT>WT (wildtype bone marrow into wildtype recipients) (N=8 mice), Nlrp6−/−>WT (N=9 mice), WT>Nlrp6−/− (N=7 mice), Nlrp6−/−>Nlrp6−/− (N=9 mice). Chimeric mice were treated with AOM/DSS and tumors counted after sacrifice (top). Data expressed as means +/−S.E.M., *,**p<0.05. Representative photographs of the mid-colon and distal colon sections for the four groups of chimeric mice after AOM/DSS treatment as viewed under a stereomicroscope (bottom).
Discussion
In this study, we address for the first time a functional in vivo role for the NLR receptor Nlrp6. Using Nlrp6-deficient mice in a model of chemically-induced colitis with DSS, and colitis-associated cancer with AOM/DSS, we show that Nlrp6 has an integral part in the maintenance of intestinal homeostasis such that in the absence of Nlrp6, there is increased susceptibility to chemically-induced injury and inflammation within the colon, resulting in extensive damage, delayed epithelial restitution, and sustained production of proinflammatory mediators that eventually lead to tumorigenesis. There are two possible, non-exclusive, explanations for the increased tumor burden observed in Nlrp6-deficient mice. One, the overwhelming inflammation that occurs as a result of DSS-induced injury is conducive to tumorigenesis with the production of cytokines that can promote cellular survival and growth of tumor cells (18). In particular, cytokines such as TNFα and IL-6 were markedly elevated in Nlrp6-deficient during the acute inflammatory response to DSS. Both cytokines are tumor-promoting and activate NF-κB and STAT3 signaling pathways that are commonly dysregulated during carcinogenesis, and it has been shown that blockade of TNFα or genetic ablation of IL-6 results in decreased colitis-associated cancer in the AOM/DSS mouse model(19–21). Enhanced chemokines too can promote tumorigenesis through the recruitment of additional inflammatory cells as well as through direct promotion of growth and angiogenesis as has been demonstrated with MIP-2 (22, 23). Therefore, the sustained induction of proinflammatory mediators in Nlrp6−/− mice may well explain the increased tumor development that occurs with chronic inflammation.
A second possible explanation for the increased tumorigenesis in Nlrp6−/− mice is the sustained increase in proliferative activity in the Nlrp6−/− colon during regeneration. Although Nlrp6−/− mice fail to repair the injured epithelium as efficiently as wildtype mice, epithelial restitution does occur to a certain extent with proliferating, regenerating crypts present at a later period after DSS-induced injury. The delay in full restitution of the epithelium may reflect an impairment in repair in Nlrp6-deficient mice, but may also be due to the greater extent of damage and inflammation that occurs in the absence of Nlrp6. Further studies into the production of factors important in intestinal repair in Nlrp6-deficient mice may help distinguish these possibilities. Regardless, once exposed to the carcinogen AOM, Nlpr6-deficient mice are more likely to develop tumors compared with wildtype mice since these mice exhibit higher levels of intestinal epithelial proliferative activity over a longer period of time. Furthermore, the presence of dysplastic changes in the regenerating epithelium may reflect increased susceptibility to dysregulated tumor growth. These changes are already evident after the first round of DSS and therefore, repeated cycles of DSS would likely increase the potential for tumor development even further as is observed in patients with frequent, longstanding relapses from inflammatory bowel disease (24).
The precise molecular mechanism by which Nlrp6 protects against the uncontrolled colitis and tumorigenesis remains unclear. Recently, there have been several studies to suggest the importance of inflammasome components and the production IL-18 in reducing colitis and colitis-associated colon carcinogenesis (12–14). Both caspase-1- and ASC-deficient mice develop increased tumors and colitis as well as Nlrp3 and Nlrc4, although there have been conflicting data with respect to Nlrp3 and Nlrc4 (4, 12–14, 25). In addition, IL-18−/− and IL-18R−/−mice have previously been shown to have increased colitis-associated tumors (26). These findings are somewhat counterintuitive in that that IL-18 stimulates the production of IFNγ and Th1 responses that can promote colitis as observed with IL-18 producing transgenic mice (27). However, administration of recombinant IL-18 was sufficient to rescue caspase-1-deficient mice from severe colitis and increased intestinal permeability (14). Thus, it has been proposed that although IL-18 is generally proinflammatory, it is also important for mucosal healing and intestinal barrier function to prevent excessive bacterial translocation and inflammation (28). How IL-18 signaling achieves this remains largely unknown, and may be related to signaling through the adaptor protein MyD88, which is important in intestinal repair (29–31). Also, as IFNγ is important for antitumor activity through the activation of cytotoxic T and NK cells (32, 33), it has been suggested that IL-18 may limit tumor development through production of IFNγ and activation of STAT1(13). Based on our studies, Nlrp6-deficient mice produce lower levels of IL-18 during DSS treatment, but whether this impairment in IL-18 production underlies increased tumor development remains to be determined. We do not observe decreases in IFNγ production during the acute inflammatory response, and therefore IL-18-mediated immune surveillance mechanisms would be unlikely to explain the increased tumors in Nlrp6-deficient mice. Also, although IL-18 production by the epithelium was implicated in promoting intestinal repair in acute DSS-induced colitis, we do not observe a contribution by the epithelial compartment in tumor suppression (4, 14). As rescue of ASC- or caspase-1-deficient mice from tumors has never been demonstrated with IL-18 administration, it remains unclear whether IL-18 is truly the key factor in tumor suppression. Whether the compromise in IL-18 production in Nlrp6-deficient mice sufficiently explains the increased tumor burden compared with wildtype is currently being examined. Nonetheless, the decreased IL-18 production early on during the first course of DSS treatment suggests a role for Nlrp6 in inflammasome signaling. Human NLRP6 in fact has been presumed to be involved in inflammasome signaling since it was previously reported to co-localize with ASC in characteristic punctuate structures within the cytoplasm (11). Consistently, this association was dependent on the N-terminal PYRIN domain of NLRP6 which would allow protein-protein interactions with the PYRIN domain of ASC (11). Furthermore, co-expression of ASC and NLRP6 in COS-7L cells in vitro resulted in increased IL-1β production that was caspase-1-dependent and required the presence of the Nlrp6 PYRIN domain (11). Thus, one can speculate that Nlrp6 participates in inflammasome signaling, and in this way, protects against intestinal inflammation and tumorigenesis. It is curious, however, that despite a reduction in IL-18 in colon tissue extracts in Nlrp6-deficient mice early on during AOM/DSS treatment, there is no observable reduction in mature IL-1β (Supplemental Fig. 1B) at least by immunoblotting, suggesting that in Nlrp6-deficient mice, production of mature IL-1β and IL-18 is differentially regulated. It is possible that redundant inflammasome or inflammasome-independent pathways (34, 35) contributed to IL-1β production in Nlrp6-deficient mice.
We show that Nlrp6 function in hematopoietic-derived rather than in epithelial/stromal cells is essential for protection against tumorigenesis. This is consistent with previous studies showing that the myeloid compartment is important for the production of cytokines such as IL-6 and TNFα during colitis-associated carcinogenesis, which are increased in Nlrp6−/−-treated mice (36). It is therefore possible that one function of Nlrp6 is to limit aberrant production of inflammatory cytokines. Previous studies of inflammasome activity, at least via Nlrp3, also demonstrated an importance for the hematopoietic compartment in suppressing tumors(12), and one possibility is that Nlrp6 functions similarly as Nlrp3 to regulate inflammasome activity in hematopoietic cells specifically. At this time, we do not believe that Nlrp6 specifically interacts with Nlrp3 to activate the inflammasome as bone marrow-derived macrophages from Nlrp6-deficient mice have normal production of IL-1β in response to lipopolysaccharide (LPS) and ATP (data not shown), a known stimulator of Nlrp3 inflammasome activity (37) although Nlrp6 expression in untreated bone marrow-derived macrophages is absent (data not shown). Therefore, Nlrp6 likely acts independently of the Nlrp3 inflammasome.
We have now identified a functional role for Nlrp6 in vivo, specifically in the protection of intestinal homeostasis to prevent the development of inflammation-induced tumors within the colon. Further mechanistic studies to confirm and elucidate the role of Nlrp6 in inflammasome signaling will need to be done, and specifically, whether Nlrp6 acts directly through ASC or through other NLR members to activate caspase-1. In addition, as a member of the NLR family, it remains to be determined how Nlrp6 acts as a pattern-recognition receptor, that is, whether it responds to specific microbial-specific (PAMPs) or endogenous signals. Answers to these questions would be critical for fully understanding how the various NLRs interact to regulate intestinal inflammation, healing, and carcinogenesis as well as identify additional targets for therapeutic purposes in the treatment of either IBD or colon carcinogenesis.
Supplementary Material
Acknowledgments
We would like to thank Millennium Pharmaceuticals Inc. for providing mutant mice. We would also like to thank Michael Shaw and Luigi Franchi for technical assistance, helpful discussions, and critical review of manuscript.
Footnotes
This work was supported by the Tom Liu Memorial Fund and the Jeffrey Colby Colon Cancer Research Fund to G.N. and G.Y.C. and National Institutes of Health Grants K08 CA133185 and American Recovery and Reinvestment Act Supplement to the University of Michigan’s Cancer Center Support Grant P30 CA46592-22S3 to G.Y.C.
References
- 1.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 4.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis BK, Wen H, Ting JP. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 9.Kumar H, Pandey S, Zou J, Kumagai Y, Takahashi K, Akira S, Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J Immunol. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- 10.Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper MA, Duncan JA, Ting JP. Cutting Edge: NLRC5-Dependent Activation of the Inflammasome. J Immunol. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, DiStefano PS, Bertin J. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530:73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 12.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 16.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 20.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Kollmar O, Scheuer C, Menger MD, Schilling MK. Macrophage inflammatory protein-2 promotes angiogenesis, cell migration, and tumor growth in hepatic metastasis. Ann Surg Oncol. 2006;13:263–275. doi: 10.1245/ASO.2006.03.096. [DOI] [PubMed] [Google Scholar]
- 24.Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis. 2010;28:619–624. doi: 10.1159/000320276. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikura T, Kanai T, Uraushihara K, Iiyama R, Makita S, Totsuka T, Yamazaki M, Sawada T, Nakamura T, Miyata T, Kitahora T, Hibi T, Hoshino T, Watanabe M. Interleukin-18 overproduction exacerbates the development of colitis with markedly infiltrated macrophages in interleukin-18 transgenic mice. J Gastroenterol Hepatol. 2003;18:960–969. doi: 10.1046/j.1440-1746.2003.03097.x. [DOI] [PubMed] [Google Scholar]
- 28.Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 2010;32:300–302. doi: 10.1016/j.immuni.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–245. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 33.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 34.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA, Dinarello CA. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 35.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.