Abstract
In mammals, the hypothalamic-pituitary-adrenal (HPA) axis and immune system play an important role in the maintenance of homeostasis. Dysregulation of either system resulting, for example, from psychosocial or reproductive stress increases susceptibility to disease and mortality risk, especially in aging individuals. In a study of free-ranging rhesus macaques, we examined how female age, reproductive state, social rank, and body condition influence (i) aspects of cytokine biology (plasma concentrations of interleukin-1 receptor antagonist (IL-1ra), IL-6 and IL-8), and (ii) HPA axis activity (plasma and fecal glucocorticoid levels). We also assessed individual differences in cytokine and hormone concentrations over time to determine their consistency and to investigate relations between these two indicators of physiological regulation and demand. Female monkeys showed marked increases in HPA axis activity during pregnancy and lactation, and increased circulating levels of IL-1ra with advancing age. Inter-individual differences in IL-1ra and IL-8 were consistent over successive years, suggesting that both are stable, trait-like characteristics. Furthermore, the concentrations of fecal glucocorticoid hormones in non-pregnant, non-lactating females were correlated with their plasma cortisol and IL-8 concentrations. Some individuals showed permanently elevated cytokine levels or HPA axis activity, or a combination of the two, suggesting chronic stress or disease. Our results enhance our understanding of within- and between-individual variation in cytokine levels and their relationship with glucocorticoid hormones in free-ranging primates. These findings can provide the basis for future research on stress and allostatic load in primates.
Keywords: rhesus macaque, cytokines, cortisol, aging, reproduction, allostatic load
1. INTRODUCTION
The hypothalamic-pituitary-adrenal (HPA) axis and immune system play important roles in the maintenance of homeostasis in the face of perturbations induced by stress or disease. Across a range of taxa, dysregulation of either system resulting from chronic psychosocial or reproductive stress has been shown to increase susceptibility to disease and mortality risk, especially in aging individuals [1–4]. Dysregulation also increases allostatic load, which is the cumulative wear and tear produced by the physiological costs of repeated adjustments to stressful perturbations and to the elevated activity of physiological systems under challenge [5, 6].
To combat the effects of allostatic load, the endocrine and immune systems generally protect the body from stress and illness by exerting reciprocal regulatory influences on one another [7]. Proinflammatory cytokines, such as interleukin 1 (IL-1), IL-6 and IL-8, activate the HPA axis and increase production of cortisol [8], and cortisol protects the body from autoimmune disorders by down-regulating proinflammatory cytokine production [9]. Cortisol and proinflammatory cytokines can be elevated simultaneously [3], such as in laboratory mice (Mus musculus) when chronic social threats increase allostatic load and result in concurrent elevation of both glucocorticoids and proinflammatory cytokines [10]. Simultaneous elevation of glucocorticoids and proinflammatory cytokines indicates a damaged cytokine-glucocorticoid feedback loop, which impairs healing [11, 12] and leads to pathological conditions [13, 14].
Variation across individuals in allostatic load may lead to intra-individual consistency in immune system and HPA axis measures over time. However, aside from evidence in humans that there is a lack of intra-individual consistency in IL-6 over time [15, 16], little is known about intra-individiual consistency or variation in cytokine concentrations. Chronically elevated glucocorticoids, on the other hand, have been shown to accelerate cellular aging [17] and to increase susceptibility to disease and mortality in a variety of species, including humans [18, 19], rhesus macaques (Macaca mulatta) [20], rats (Rattus norvegicus) [21, 22], and the European white stork (Ciconia ciconia) [23].
In humans, upregulation of proinflammatory cytokines due to chronic inflammation is known to accelerate the aging process and is associated with age-related diseases [24]. For example, elevated concentrations of IL-6, which inhibits production of IL-1 and activates production of IL-1 receptor antagonist (IL-1ra) [8], are predictive of mortality risk [25–27], myocardial infarction [28], and sarcopenia [29], and indicate chronic, low-grade inflammation [30]. High concentrations of IL-8 are associated with a variety of inflammatory diseases [31, 32] and psychosocial stress [33–36]. Furthermore, advanced age (>60 years) is associated with increased concentrations of IL-1, IL-6 and IL-8 [31, 37–39]. Unfortunately, studies of human aging may be confounded by the cumulative effects of unhealthy behaviors. Obesity, for example, is more common in older than in younger Americans [40], and has been linked to elevated production of IL-1ra, IL-6 and IL-8 [41, 42]. Consistent with the notion that such factors may be potential confounds, there are differences in the relationship between IL-6 and age between Japanese and American individuals, and these differences have been attributed to larger body mass indices among Americans [43].
Free-ranging, nonhuman primates are excellent animal models for investigating hormonal and immune markers of age-related physiological decline because, unlike their captive conspecifics, they are active, group-dwelling individuals, and unlike humans, they are not affected by confounding lifestyle and cultural variables. Although studies of captive rhesus macaques suggest that levels of IL-6 are a sensitive index of health, aging, and neural atrophy [44, 45], little is known about relations between IL-1ra or IL-8 concentrations and rhesus macaque age, or between IL-6 concentrations and aging in free-ranging rhesus macaques.
The effects of allostatic load and aging on the HPA axis have been documented in baboons (Papio spp.) and rhesus monkeys [46, 47], making them ideal models for research on the relationship between psychosocial and reproductive stress, endocrine and immune function, and health in aging individuals. Among adult female free-ranging rhesus macaques, there is significant variation in adult lifespan, with median lifespan being 15 years and maximum lifespan 32 years [48]; mortality risk is highest during the birth season [49]. Females’ plasma cortisol concentrations following a stressor are correlated across years, indicating that some individuals have a chronically hyperactive HPA axis [50]. Plasma cortisol responses to stress are higher in lactating females than in non-pregnant, non-lactating (NPNL) females [50, 51], and the increase in plasma cortisol response to stress from the NPNL condition to the lactating condition is greater in low-ranking than in high-ranking females [7]. These findings, along with the fact that rhesus macaques reside in highly stratified social groups [52], suggest that differences in survival and longevity among female rhesus macaques may be associated with differences in chronic activation of the immune system and HPA axis, which could be the result of psychosocial and reproductive stress (i.e. allostatic load).
In the present study, we investigated whether individual differences in immune system and HPA axis function among free-ranging female rhesus macaques are consistent over time, and whether they may be affected by age, reproductive state, body condition and social rank. We assessed immune and HPA axis function by measuring plasma levels of 3 proinflammatory cytokines (IL-1ra, IL-6 and IL-8), plasma cortisol, and fecal glucocorticoid metabolites. We examined IL-1ra as a proxy for IL-1 because concentrations of IL-1 and IL-1ra are positively correlated [53], and concentrations of the receptor antagonist are higher [54]. We assessed both plasma and fecal glucocorticoids because plasma samples can only be collected from rhesus macaques under the stressful conditions of trapping. Multiple fecal samples, however, can be collected noninvasively from individuals, and each individual’s values can be averaged to assess HPA axis activity under more naturalistic conditions.
We predicted that fecal glucocorticoid levels would be related to reproductive state, but not age, consistent with results from plasma cortisol data [50, 51]. We expected that IL-1ra and IL-6 would be positively correlated since IL-6 activates the production of IL-1ra [8], and that all proinflammatory cytokine levels would be higher in older individuals since inflammation tends to increase with age [24]. We also predicted that plasma cytokine, plasma cortisol and fecal glucocorticoid values collected over a 13-month period would be correlated, demonstrating not only relationships between HPA axis and immune function, but also consistency in these aspects of physiology over time. Consistency in these measures over time would suggest that some individuals experience chronically higher allostatic load than others, and as a consequence, may be subjected to health-related declines and mortality at younger ages. To our knowledge, this is one of the first studies of free-ranging primates to include measures of cytokine function, to examine whether glucocorticoid and cytokine measures are consistent across years, and to assess relations between HPA axis activity and immune function.
2. METHODS
2.1 Field Site and Subjects
Cayo Santiago is a 15.2 ha island located 1 km off the southeastern coast of Puerto Rico. The rhesus macaque colony on this island was established in 1938 with free-ranging monkeys captured in India [55]. Since then, no new individuals have been introduced into the population, except through births. To maintain a stable population size of approximately 1000 monkeys, a portion of the yearlings and two-year olds are transferred off the island each year. Monkeys living on Cayo Santiago forage on natural vegetation, but are also provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders, and in this population the 6-month mating season currently begins in March and is followed by a 6-month birth season that begins in September [49]. The rhesus macaques residing on Cayo Santiago are ideal candidates for studying immune function and HPA axis activity because their living conditions, along with their promiscuous mating system, make them susceptible not only to the psychosocial stressors associated with group living and frequent reproduction but also to inflammation and infectious diseases.
All subjects in this study, which was conducted between January 2007 and February 2008, were female, multiparous, belonged to one of six naturally-formed social groups, and ranged between 7 and 26 years of age (average age: 15.2±5.8 years). Since the sample size of females varied across measures, we report in Table 1 the sample size for each analysis. We classified females who did not have an infant or show signs of pregnancy or miscarriage during the 2007 birth season and the preceding mating season as non-pregnant, non-lactating (NPNL) during the 2007 trapping period, and females who did not have an infant or show signs of pregnancy or miscarriage during the 2008 birth season and preceding mating season as NPNL between April 2007 and the 2008 trapping period. NPNL females may have been cycling during a portion of the time they were classified as NPNL, but we refer to them as NPNL throughout the period defined as we were unable to determine if and when they were cycling. For females who did reproduce within a given birth season, we distinguished between samples collected when females were cycling, pregnant (based on birth date and a 165 day gestation period [56]), and lactating.
Table 1.
Sample description. This table includes the number of females within each reproductive state from which samples were collected. It also includes information on the outliers removed from each analyte and the sample size after the removal of outliers.
| Analyte | Reproductive state | Sample size | Outliers | Sample size after outliers removed |
|---|---|---|---|---|
| FGC | NPNL | 13 | 0 | 13 |
| FGC | Cycling | 10 | 0 | 10 |
| FGC | Pregnant | 25 | 0 | 25 |
| FGC | Lactating | 27 | 1 | 26 |
| 2007 Cortisol | NPNL | 20 | 1 | 19 |
| 2007 Cortisol | Lactating | 25 | 2 | 23 |
| 2008 Cortisol | NPNL | 14 | 0 | 14 |
| 2008 Cortisol | Lactating | 26 | 1 | 25 |
| 2007 IL-1ra | NPNL | 18 | 0 | 18 |
| 2007 IL-1ra | Lactating | 25 | 2 | 23 |
| 2008 IL-1ra | NPNL | 14 | 1 | 13 |
| 2008 IL-1ra | Lactating | 26 | 0 | 26 |
| 2007 IL-6 | NPNL | 18 | 1 | 17 |
| 2007 IL-6 | Lactating | 25 | 2 | 23 |
| 2008 IL-6 | NPNL | 14 | 2 | 12 |
| 2008 IL-6 | Lactating | 26 | 1 | 25 |
| 2007 IL-8 | NPNL | 18 | 2 | 16 |
| 2007 IL-8 | Lactating | 25 | 1 | 24 |
| 2008 IL-8 | NPNL | 14 | 0 | 14 |
| 2008 IL-8 | Lactating | 26 | 2 | 24 |
Subjects, with the exception of 12 individuals for which behavioral data were not available, were classified as high-, middle-, or low-ranking depending on whether their rank was within the top, middle, or bottom third of the dominance hierarchy within their group [57]. Each female was followed twice a week for a minimum of 16 weeks during the study period, and dominance ranks were assigned on the basis of behavioral scores on aggressive (i.e., threats, chases) and submissive (i.e., withdrawals, screams, grimaces) interactions collected by trained observers. In dyadic interactions aggressors were scored as higher ranking than submissive individuals, and dominance was only scored when submissive actions were clearly shown. For a more detailed description of how the dominance hierarchies were constructed, please see Nelson et al. (2010) [57].
2.2 Plasma Sample Collection and Analysis
Trained staff members captured monkeys in a feeding corral, approximately 100 m2, which was provisioned daily with monkey chow. We collected blood samples for cortisol and cytokine analyses between January 17 and March 13, 2007, and between January 16 and February 7, 2008. Table 1 provides information about the number of lactating and NPNL females trapped each year for these analyses. NPNL females ranged between 8 and 26 years (X=18.2 years, SD=3.9 years), and lactating females ranged between 7 and 22 years (X=16.6 years, SD=4.5 years). There was no significant age difference between groups (t= −1.71, df=82, p=0.092). We had cortisol and cytokine data from both 2007 and 2008 for 22 females.
Trapping generally occurred between 0830 and 1200. Subjects were netted or captured by hand in the corral, transferred to a holding cage (0.62×0.42×0.62 m), and moved to a small field laboratory where they were kept overnight. The morning after capture, the trapping team anesthetized the monkeys with ketamine hydrochloride (approximately 10 mg/kg via IM injection), and then collected intravenous blood samples and recorded body mass and crown-rump length measurements. Body Mass Index (BMI) for each adult female was calculated by dividing mass (kg) by the square of crown-rump length (m2). Mother-infant pairs were separated only for the period of time when mothers were anesthetized. Veterinary technicians collected blood samples from the femoral vein into heparinized vacutainers between 0715 and 1040 (average time of day: 8:18±5.0 min) and 22.1±2.7 min after ketamine administration (range 0–127 min). Linear mixed models revealed there was no relationship between female age and time of sample collection (F[1,73.4]=0.93, p=0.34), or between female age and time lag between ketamine administration and blood draw (F[1,57.2]=0.99, p=0.32). The plasma samples were taken under conditions that were presumably stressful for the monkeys because they were captured and housed in cages overnight prior to sample collection (see [51, 58]). We refrigerated the samples for 20 min and then centrifuged them for 20 min at 1500 rpm. Plasma was aliquoted into microcentrifuge tubes, which were then stored at −80 °C. Frozen samples were shipped on dry ice to the Biomarker Assay Core Lab of the Yerkes National Primate Research Center, where they were assayed for cortisol by radioimmunoassay using a commercial kit (Diagnostic Systems Laboratories, Webster, TX) as described previously [50, 51], and to the Prendergast Laboratory at the University of Chicago, where cytokine concentrations were measured using enzyme-linked immunosorbent assays (ELISAs).
For cytokine assays, plasma samples were thawed at room temperature and diluted (IL-1ra: 1:5; IL-6: undiluted; IL-8: 1:10) in assay buffer. Roughly half of the samples on each plate were collected in 2007, and the other half were collected in 2008. In addition, females representative of each reproductive state and of the age span under study were included on each plate. Concentrations of IL-1ra, IL-6, and IL-8 were measured using three separate ELISAs (Quantikine; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The IL-1ra ELISA had a detection limit (sensitivity) of 6.26 pg/mL, an intra-assay CV of 7.3% and an inter-assay CV of 6.7%. The IL-6 ELISA had a sensitivity of 0.39 pg/mL, an intra-assay CV of 7.8% and an inter-assay CV of 6.5%. The IL-8 ELISA had a detection limit of 3.5 pg/mL, an intra-assay CV of 6.5% and an inter-assay CV of 6.1%. All samples were run in duplicate. To avoid freeze-thaw effects, we completed all 3 cytokine assays for each sample on a single day.
2.3 Fecal Sample Collection, Extraction, and Assay
We collected fecal samples opportunistically from 44 adult females between April 2007 and December 2007. Of those 44 females, we had blood samples from 22 in 2007, and from 36 in 2008. Of the 29 females who gave birth during the 2008 birth season, we collected at least 2 fecal samples from 10 females when they were cycling (mean: 7 samples; range: 2–18), from 25 females when they were pregnant (mean: 7 samples; range: 2–13) and from 27 females when they were lactating (mean: 4 samples; range: 2–11) (Table 1). We also collected fecal samples from 13 females who were NPNL (mean: 18 samples; range: 6–33). NPNL females ranged between 10 and 22 years (X=16.1, SD=4.3), pregnant females studied ranged between 7 and 22 years (X=14.5 years, SD=5.4 years), and lactating females studied ranged between 7 and 22 years (X=12.9 years, SD=5.2 years). There were no significant age differences between these groups (F[2,58]=1.56, p=0.22).
All samples (in total n=606) were collected between 0700 and 1100. We placed samples in labeled Zip-loc bags and stored them in an insulated cooler with frozen ice packs until we could transfer them to a −30 °C freezer at 1430 on the day of collection. We shipped samples on dry ice to the Brookfield Zoo in Riverside, IL, where they were kept in a −30 °C freezer until extraction. 0.50 g of wet fecal matter was extracted overnight (14–18 h) in 5 ml of 80% ethanol. The next day, we centrifuged tubes for 15 min at 1500 rpm. We then added to a polypropylene test tube 1 mL of assay buffer and 1 mL of supernatant from the centrifuged tubes. After capping and vortexing the tubes, we stored them in a −30 °C freezer until we shipped them on dry ice to the endocrine laboratory of the German Primate Center, Göttingen, Germany.
Fecal extracts (diluted 1:100 in assay buffer prior to assay) were analyzed for immunoreactive 11β-hydroxyetiocholanolone, a group-specific measurement of 5β-reduced 3α,11β-dihydroxylated cortisol metabolites (3α,11β--dihydroxy-CM, hereafter fecal glucocorticoid or FGC) using an enzymeimmuno assay described in detail by Heistermann et al. [59]. The assay has been validated and successfully applied to monitor glucocorticoid output in a wide range of primate species, including various species of macaques (e.g. Macaca sylvanus: [60, 61]; Macaca assamensis: [62]; Papio hamadryas anubis: [63]; Propithecus verreauxi: [64]; Macaca fascicularis: [60, 65]; Pygathrix nemaeus: [59]). Sensitivity of the assay at 90% binding was 2.4 pg. Intra-assay variation, calculated from repeated measures of high and low concentrated quality controls (n=16), was 6.5% (high) and 8.7% (low) while measures of inter-assay variation (n=23) were 11.6% (high) and 14.4% (low). All samples were run in duplicate, and all assay results were standardized for differences in extraction volume and fecal weight and are presented as ng hormone/g wet fecal weight.
We confirmed the validity of our FGC assay for assessing glucocorticoid output in rhesus macaques by undertaking a biological validation. For this, we collected fecal samples prior to and following anesthetization (a well-known stressor eliciting an increase in cortisol secretion [66]) for routine physical examinations of female rhesus macaques housed at the Harlow Primate Laboratory in Madison, WI. We collected morning samples from 6 individuals during the 4 days preceding anesthetization. On the day of anesthetization and physical exam and for the 3 days that followed, we collected every fecal sample deposited. The 3α,11β--dihydroxy-CM assay consistently showed higher concentrations of FGCs after the procedure than before (t= −2.94, df=5, p=0.03), with FGC values across all females beginning to be elevated 26 h following the sedation and handling, peaking at 38 h, and returning to baseline by 48 h. The data confirm the predicted pattern, both in terms of elevated FGC concentrations in response to the stressor as well as in terms of the delay time in fecal hormone excretion and thus clearly indicate the biological validity of our fecal hormone measure as a general index of arousal and disturbance in rhesus macaques.
2.4 Data Analysis
Hormone results reported in this paper are restricted to those concerning relationships between cortisol and cytokines and with respect to FGC concentrations because Maestripieri et al. [51] and Hoffman et al. [50] have already reported on the plasma cortisol data with respect to reproductive state, age, social rank and BMI from this dataset (see Introduction).
For all data, we considered values that were more than 2 standard deviations above or below the mean of the dataset for that variable to be outliers and so excluded them from analyses (Table 1). After outliers were removed, FGC and cortisol values were normally distributed, but plasma cytokine values were normally distributed only after log-transformation. Thus, all results reported utilize raw FGC and cortisol values and log-transformed plasma cytokine values.
We used Linear Mixed Models (LMM) for most analyses, which allowed us to control for multiple observations of the same individual by including individual identity (all models) and year (all models involving cytokines, or plasma cortisol and cytokines) as random factors.
Cytokines
We performed partial Pearson’s correlations to determine whether the 2007 cytokine values for the monkeys were correlated with their 2008 cytokine values while controlling for female age, BMI, and the time of day when blood was collected. Collection time was included as a control variable because although there was no individual consistency across females trapped in 2007 and 2008 in the amount of time that passed between ketamine administration and blood collection (r=0.03, p=0.91, n=22), there was a positive correlation between 2007 and 2008 blood collection times (r=0.47, p=0.03, n=22). We included in separate models 2 of the 3 cytokines (fixed covariates) to determine if any of the cytokines predicted the other cytokine values (response variables). We used LMMs to assess the effects of reproductive state and dominance rank (fixed factors) and age and BMI (fixed covariates) on cytokine concentrations (response variables).
Glucocorticoids
For FGC concentrations, we calculated the average FGC value for each female within each reproductive state. For NPNL females, we determined that FGC values collected did not differ significantly according to season (F[2,19.71]=0.04, p=0.96). Therefore, we averaged FGC values collected across the entire study period for NPNL females. Similarly, we averaged FGC concentrations across all females’ cycling samples because sample collection date did not affect FGC values for these samples (F[2,3.33]=1.42, p=0.36). Because FGC values differed across periods within pregnancy (between trimesters) and lactation (between months) (pregnancy: F[2,38.86]=11.94, p<0.001; lactation: F[2,33.68]=5.18, p=0.01), we controlled for this influence of reproductive stage by averaging within periods and then across periods of pregnancy and lactation.
To determine whether FGC concentrations predicted cortisol concentrations, we used LMMs, with FGC concentration as a fixed factor and plasma cortisol as the response variable. Because reproductive state affects plasma cortisol concentrations [50, 51], we analyzed lactating and NPNL females’ glucocorticoid values separately. Analyses comparing FGC and plasma cortisol values were one-tailed since we had a priori reasons to believe that these measures would correlate positively. We used LMMs to determine whether reproductive state and social rank (fixed factors) and age and BMI (fixed covariates) affected FGC values (response variable). We then performed paired t-tests to determine how FGC values differed between reproductive states. Because of multiple testing (three tests for pairwise comparisons of three reproductive states), we performed a Bonferroni correction for paired comparisons between reproductive states, such that p-values less than 0.05/3=0.017 were considered significant. We performed partial Pearson’s correlations to determine whether FGC values collected in one reproductive state were correlated with FGC values collected within another while controlling for female age and BMI.
Cytokines and Glucocorticoids
We used LMMs to determine whether cortisol and FGC values (fixed covariates) predicted cytokine values (response variables).
Unless otherwise stated, all tests were two-tailed, and we considered probabilities <0.05 to be statistically significant. Analyses were conducted in SPSS 18.0 (SPSS Inc., Chicago, IL).
3. RESULTS
There were no effects of social rank or BMI on any of the cytokines or on the FGCs (for all p>0.10), so these variables were excluded from subsequent analyses.
3.1 Cytokines
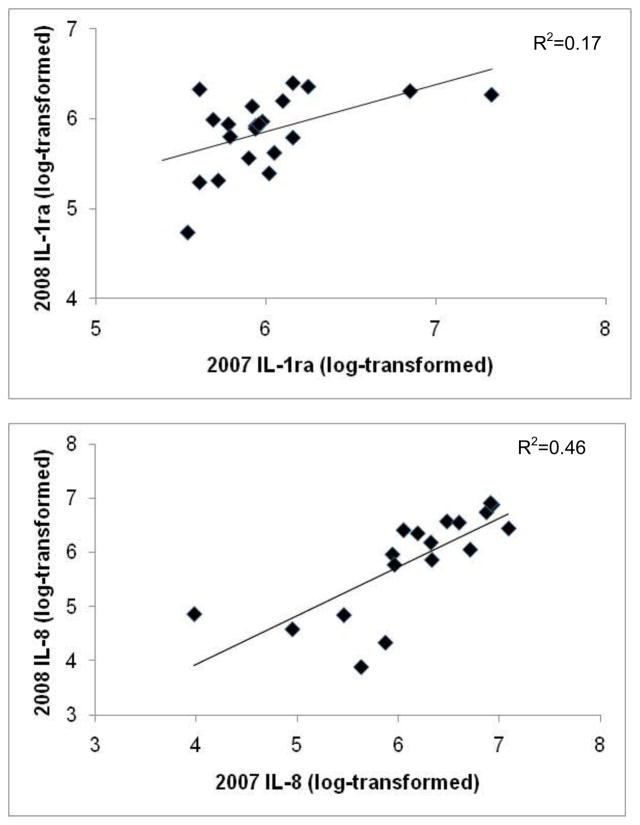
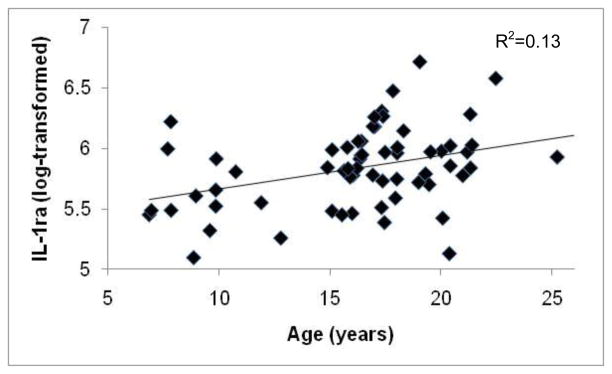
For individual monkeys with data for both years, there were significant positive correlations between plasma concentrations of IL-1ra and IL-8 measured in 2007 and 2008 (IL-1ra: r=0.50, p=0.04, n=16; IL-8: r=0.75, p=0.001, n=13; Figs. 1a & 1b), but not for IL-6: r=0.13, p=0.65, n=12. Thus, individual differences in IL-1ra and IL-8 concentrations, but not in IL-6, were stable across the two years. IL-1ra values were related to IL-6 values (F[1,4.14]=9.65, p=0.03 ), but IL-8 values were not associated with the other cytokines (all results p>0.10). There were no significant effects of female reproductive condition on plasma cytokine concentrations, or significant associations between age and IL-6 or IL-8 (all results p>0.10), but the relationship between age and IL-1ra was significant (F[1,76.80]=3.83, p=0.05; Fig. 2).
Figure 1.
Relationship between free-ranging, female rhesus macaque plasma levels of (a) IL-1ra and (b) IL-8 across the 2 study years. All cytokine values were log-transformed.
Figure 2.
Relationship between the age of adult female rhesus macaques and plasma concentrations of IL-1ra (log-transformed).
3.2 Glucocorticoids
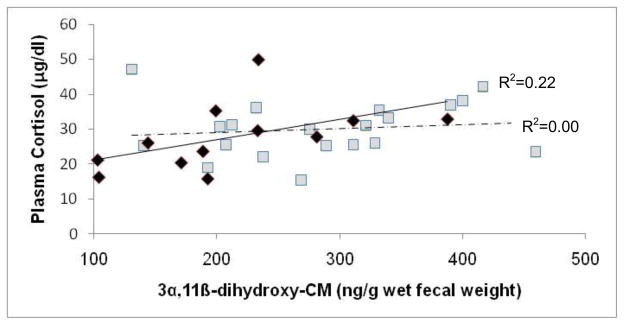
There was a significant relationship between FGC and plasma cortisol concentrations for NPNL females, but not for lactating females (NPNL females: F[1,9.84]=3.54, p=0.045, one-tailed; lactating females: F[1,21]=0.73, p=0.20, one tailed; Fig. 3). FGC concentrations varied significantly with respect to female reproductive state (F[2,35.52]=36.04, p<0.001), but not in relation to age (p>0.10). Within-subject analyses with paired t-tests showed that females had higher FGC concentrations when they were pregnant than cycling (pregnant: X=387.8 ng/g, SD=105.3; cycling: X=174.8 ng/g, SD=57.7; t= −6.07, df=7, p=0.001, Bonferroni correction) or lactating (lactating: X=289.5 ng/g, SD=82.5; t= −4.81, df=19, p<0.001, Bonferroni correction); females also had higher FGC concentrations when lactating than cycling (t= −4.16, df=7, p=0.004, Bonferonni correction).There was not a significant correlation between females’ cycling and pregnancy FGC concentrations, or between their cycling and lactating values (all results p>0.10). There was, however, a strong, positive correlation between females’ pregnant and lactating FGC concentrations (r=0.60, p=0.01, n=14).
Figure 3.
Relationship between concentrations of fecal glucocorticoid (FGC) metabolite 3α,11β--dihydroxy-CM and plasma cortisol for non-pregnant, non-lactating rhesus macaques (diamonds and solid regression line) and lactating rhesus macaques (squares and dotted regression line).
3.3 Cytokines and Glucocorticoids
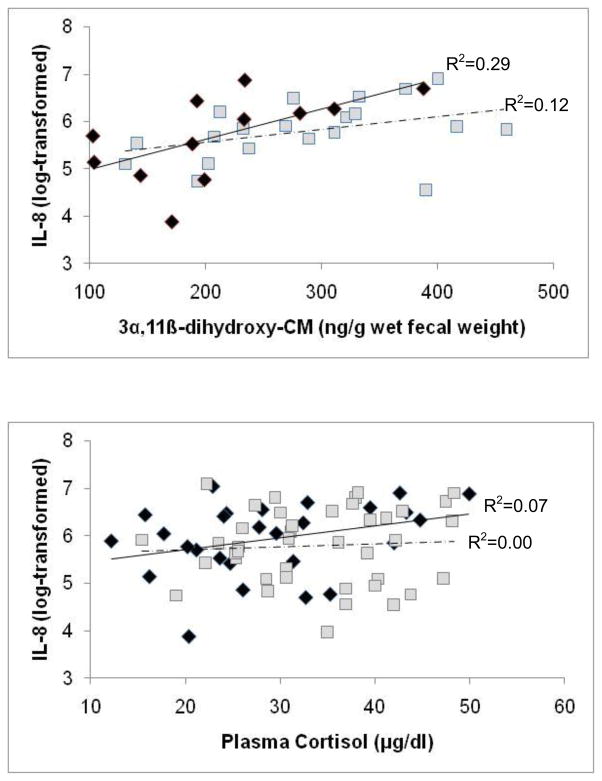
Because female reproductive state had such a strong effect on glucocorticoid concentrations, the relationships between cytokine and glucocorticoid values were analyzed separately for NPNL and lactating females (no cytokine data were available for pregnant females). There was a significant positive correlation between FGC and IL-8 values for both NPNL and lactating females (NPNL females: F[1,12.91]=6.48, p=0.03; lactating females: F[1,19.38]=6.10, p=0.02; Fig. 4a), while the relationship between plasma cortisol and IL-8 values approached significance for NPNL females (F[1,26]=4.03, p=0.06), but not for lactating females (F[1,41]=1.80, p=0.19; Fig. 4b). Neither the IL-1ra nor the IL-6 values were significantly associated with FGC or plasma cortisol values in either NPNL or lactating females (all results p>0.10).
Figure 4.
Relationship between log-transformed concentrations of free-ranging, female rhesus macaque plasma IL-8 and (a) fecal glucocorticoid (FGC) metabolite 3α,11β-dihydroxy-CM and (b) plasma cortisol. Diamonds and the solid regression line represent NPNL females, and squares and the dotted regression line represent lactating females.
4. DISCUSSION
In our study of free-ranging rhesus macaque females, we found that age and reproductive state significantly affected measures of immune and endocrine function, with older females having higher IL-1ra concentrations than younger females, and with females having higher glucocorticoid concentrations when pregnant and lactating than when non-pregnant, non-lactating (NPNL). The data also provide evidence for consistency of individual differences in cytokine and glucocorticoid concentrations over time, as well as positive associations between glucocorticoid and IL-8 concentrations. These findings suggest that some individuals experience higher allostatic load than others, perhaps as a result of greater reproductive output over time, which may affect susceptibility to disease and may at least partially explain the variation in age at death previously reported for this population [48].
Female age was positively associated with IL-1ra concentrations, but we did not find a relationship between age and IL-6 or IL-8 concentrations. The increase in IL-1ra concentrations by age indicates that older females experience greater inflammation and immune system activity, which may contribute to age-associated increases in interbirth intervals and declines in maternal condition, infant mass, and offspring survival that have been reported for this population [48]. Our sample size may have been insufficient to detect an age-related increase in IL-6 or IL-8 concentrations, or it may be that age-associated increases in IL-6 and IL-8 concentrations that have been reported in human studies are confounded by age-associated increases in obesity [40–42]. Unlike in humans, where BMI typically increases with age, BMI declines with age in female rhesus macaques on Cayo Santiago [48].
Consistent with plasma cortisol measures reported elsewhere [50, 51], fecal glucocorticoid (FGC) values differed across reproductive states. Furthermore, females’ FGC values when pregnant correlated with their FGC values when lactating, and were significantly higher when females were in these reproductive states than when they were cycling. Because gestation in rhesus macaques is approximately 165 days [56], the most intense period of lactation lasts roughly 3 months [67], and most females give birth annually [48], females are likely to experience elevated concentrations of glucocorticoids for much of their adult life. Such long-term hyperactivation of the HPA axis can impact allostatic load and survival [1]. Furthermore, since chronic hyperactivation of the HPA axis increases susceptibility to illness [18–21], chronically elevated glucocorticoids observed during pregnancy and lactation might at least partially explain why female mortality rates in this population are highest during the birth season [49].
We found that inter-individual differences in 2 of the 3 immune measures tested, as well as glucocorticoid concentrations, were highly consistent over time, with females’ 2007 IL-1ra and IL-8 values predicting their respective 2008 values. Individuals’ 2007 IL-6 values were not predictive of their 2008 values, consistent with previous research on humans [15, 16]. There was, however, a positive association between IL-1ra and IL-6 values, which was expected since IL-6 activates the production of IL-1ra [8]. FGC values were predictive of plasma cortisol values for NPNL females but not for lactating females, perhaps because lactating females experience more intense physiological changes than NPNL females outside of the mating season. FGC values collected from lactating females were averaged across the first 3 months post-partum, but by the time plasma samples were collected, most females’ infants were at least 3 months old. At this point in an infant’s development, suckling intensity declines [67], which may cause females’ cortisol concentrations to start returning to pre-pregnancy (i.e. cycling) levels. Although previous studies in various mammalian species have demonstrated that fecal and plasma glucocorticoid values are positively correlated [68–70], this is the first study to show that these values are correlated even when relatively long intervals (2–9 months) separate the collection of fecal and plasma samples.
Some individuals experienced heightened HPA axis and immune system activity simultaneously. There were strong relationships between FGC and IL-8 values for both NPNL and lactating females, and the relationship between plasma cortisol and IL-8 values approached significance for NPNL females. These results are consistent with research in humans showing that chronically elevated glucocorticoid concentrations increase vulnerability to viral infections and decrease antibody production [18], though our data do not allow us to infer causation. Reyes and Coe [8, 71] demonstrated experimentally that IL-6 stimulated the release of cortisol in rhesus macaques, but in the current study, there was no relationship between either IL-1ra or IL-6 and cortisol, perhaps because cytokines and glucocorticoids were assessed in individuals that were healthy and not subjected to experimental immune challenges.
Collectively, our results provide some of the first evidence from free-ranging primates for consistent variation between individuals in glucocorticoid and cytokine activity, and for relationships between these two measures of endocrine and immune function. The results of our study can provide the basis for further research on the relationship between psychosocial and reproductive stress, allostatic load, and health in aging free-ranging populations. Future studies are required to test the hypothesis that chronic stress associated with low social status and frequent reproduction may have cumulative effects on allostatic load, and that high allostatic load results in greater risk of disease and reduced longevity in aging females. Additionally, further investigation is needed on the specific endocrine and immune mechanisms through which psychosocial and reproductive stress impacts health and survival in female primates.
Highlights.
We tested how age and psychosocial factors affect immune and HPA axis activity.
We tested inter-individual differences in cytokine and hormone levels over time.
Circulating levels of IL-1ra increased with advancing age.
Inter-individual differences in IL-1ra and IL-8 were stable over successive years.
Some individuals showed permanently elevated cytokine levels or HPA axis activity.
Acknowledgments
We thank Richelle Fulks for assistance with data collection, the staff of the Caribbean Primate Research Center for logistical support and assistance with animal capture and handling, and Jerome Galang for assistance with the cytokine assays. This study was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico. This research was supported by NIH grant R21-AG029862 to D.M. This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romero LM, Wikelski M. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc Natl Acad Sci U S A. 2001;98:7366–70. doi: 10.1073/pnas.131091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero LM, Wikelski M. Stress physiology as a predictor of survival in Galapagos marine iguanas. Proceedings of the Royal Society B-Biological Sciences. 2010;277:3157–62. doi: 10.1098/rspb.2010.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansel A, Hong SZ, Camara RJA, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–21. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Pride RE. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta) Biology Letters. 2005;1:60–3. doi: 10.1098/rsbl.2004.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS, Stellar E. Stress and the individual - Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 6.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress - Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 7.Goshen I, Yirmiya R. Interleukin-1 (IL-1): A central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Reyes TM, Coe CL. The proinflammatory cytokine network: interactions in the CNS and blood of rhesus monkeys. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1998;274:R139–R44. doi: 10.1152/ajpregu.1998.274.1.R139. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos GP, Gold PW. The concepts of stress and stress system disorders - Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–52. [PubMed] [Google Scholar]
- 10.Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Neurol Clin. 2006;24:483–91. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–46. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altemus M, Rao B, Dhabhar FS, Ding WH, Granstein R. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–17. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–9. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, et al. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–93. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Cava F, Gonzalez C, Pascual MJ, Navajo JA, Gonzalez-Buitrago JM. Biological variation of interleukin 6 (IL-6) and soluble interleukin 2 receptor (sIL2r) in serum of healthy individuals. Cytokine. 2000;12:1423–5. doi: 10.1006/cyto.2000.0714. [DOI] [PubMed] [Google Scholar]
- 16.Rao KMK, Pieper CS, Currie MS, Cohen HJ. Variability of plasma Il-6 and cross-linked fibrin dimers over time in community-dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–5. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 17.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Rozlog LA, Kiecolt-Glaser JK, Marucha PT, Sheridan JF, Glaser R. Stress and immunity: Implications for viral disease and wound healing. J Periodontol. 1999;70:786–92. doi: 10.1902/jop.1999.70.7.786. [DOI] [PubMed] [Google Scholar]
- 19.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome - Nested case-control study. Circulation. 2002;106:2659–65. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 20.Kulstad JJ, McMillan PJ, Leverenz JB, Cook DG, Green PS, Peskind ER, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neurol. 2005;64:139–46. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 22.Cavigelli SA, Ragan CM, Michael KC, Kovacsics CE, Brliscke AP. Stable behavioral inhibition and glucocorticoid production as predictors of longevity. Physiol Behav. 2009;98:205–14. doi: 10.1016/j.physbeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc Natl Acad Sci U S A. 2007;104:8880–4. doi: 10.1073/pnas.0700232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Research Reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 26.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease - Effects of an early invasive or noninvasive strategy. Jama-Journal of the American Medical Association. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 27.Drost AC, Burleson DG, Cioffi WG, Jordan BS, Mason AD, Pruitt BA. Plasma cytokines following thermal-injury and their relationship with patient mortality, burn size, and time postburn. Journal of Trauma-Injury Infection and Critical Care. 1993;35:335–9. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 29.Jensen GL. Inflammation: Roles in aging and sarcopenia. Journal of Parenteral and Enteral Nutrition. 2008;32:656–9. doi: 10.1177/0148607108324585. [DOI] [PubMed] [Google Scholar]
- 30.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13:389–91. [PubMed] [Google Scholar]
- 31.Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol. 2009;22:23–34. doi: 10.1177/0891988708328216. [DOI] [PubMed] [Google Scholar]
- 32.Boekholdt SM, Peters RJG, Hack CE, Day NE, Luben R, Bingham SA, et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women - The EPIC-Norfolk prospective population study. Arteriosclerosis Thrombosis and Vascular Biology. 2004;24:1503–8. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda H, Ichinose T, Kusama T, Sakurai R, Anndow K, Akiyoshi N. Stress assessment in acute care department nurses by measuring interleukin-8. Int Nurs Rev. 2008;55:407–11. doi: 10.1111/j.1466-7657.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 34.Marsland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behavior and Immunity. 2007;21:218–28. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Weik U, Herforth A, Kola-Bachofen V, Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosom Med. 2008;70:906–12. doi: 10.1097/PSY.0b013e3181835bf3. [DOI] [PubMed] [Google Scholar]
- 36.Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–35. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population - The MEMO-Study. Neurobiol Aging. 2008;29:937–44. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Roubenoff R, Harris TB, Abad LW, Wilson PWF, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: Effect of age and inflammation. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1998;53:M20–M6. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 39.Wei J, Xu HM, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy-subjects. Life Sci. 1992;51:1953–6. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging US population. Obesity. 2007;15:2855–65. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 41.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Interleukins. 2006:443–77. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 42.Sempere L, Martinez J, de Madaria E, Lozano B, Sanchez-Paya J, Jover R, et al. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–64. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 43.Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, et al. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav Immun. doi: 10.1016/j.bbi.2010.11.013. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MJ, Aiken JM, Havighurst T, Hollander J, Ripple MO, Weindruch R. Adult-onset energy restriction of rhesus monkeys attenuates oxidative stress-induced cytokine expression by peripheral blood mononuclear cells. J Nutr. 1997;127:2293–301. doi: 10.1093/jn/127.12.2293. [DOI] [PubMed] [Google Scholar]
- 45.Willette AA, Bendlin BB, McLaren DG, Canu E, Kastman EK, Kosmatka KJ, et al. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:987–94. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 47.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: Relevance to human health interventions. Science. 2004;305:1423–6. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behavioral Ecology. 2010;21:972–8. doi: 10.1093/beheco/arq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology. 2008;62:1711–8. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman CL, Ayala JE, Mas-Rivera A, Maestripieri D. Effects of reproductive condition and dominance rank on cortisol responsiveness to stress in free-ranging female rhesus macaques. Am J Primatol. 2010;72:559–65. doi: 10.1002/ajp.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maestripieri D, Hoffman CL, Fulks R, Gerald MS. Plasma cortisol responses to stress in lactating and nonlactating female rhesus macaques. Horm Behav. 2008;53:170–6. doi: 10.1016/j.yhbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melnick DJ, Pearl MC. Cercopithecines in multimale groups: Genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 121–34. [Google Scholar]
- 53.Arend WR. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 54.Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, et al. Production of interleukin-1-receptor antagonist during experimental endotoxemia. Lancet. 1991;338:1423–4. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- 55.Rawlins RG, Kessler MJ. The Cayo Santiago Macaques: History, Behavior, and Biology. Albany, NY: SUNY Press; 1986. [Google Scholar]
- 56.Ardito G. Checklist of data on gestation length of primates. J Hum Evol. 1976;5:213–22. [Google Scholar]
- 57.Nelson E, Hoffman CL, Gerald MS, Shultz S. Digit ratio (2D:4D) and dominance rank in female rhesus macaques (Macaca mulatta) Behavioral Ecology and Sociobiology. 2010;64:1001–9. [Google Scholar]
- 58.Laudenslager ML, Rasmussen KL, Berman CM, Lilly AA, Shelton SE, Kalin NH, et al. A preliminary description of responses of free-ranging rhesus monkeys to brief capture experiences: Behavior, endocrine, immune, and health relationships. Brain Behavior and Immunity. 1999;13:124–37. doi: 10.1006/brbi.1998.0548. [DOI] [PubMed] [Google Scholar]
- 59.Heistermann M, Ademmer C, Kaumanns W. Ovarian cycle and effect of social changes on adrenal and ovarian function in Pygathrix nemaeus. International Journal of Primatology. 2004;25:689–708. [Google Scholar]
- 60.Heistermann M, Palme R, Ganswindt A. Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol. 2006;68:257–73. doi: 10.1002/ajp.20222. [DOI] [PubMed] [Google Scholar]
- 61.Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biology Letters. 2007;3:231–3. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostner J, Heistermann M, Schulke O. Dominance, aggression and physiological stress in wild male Assamese macaques (Macaca assamensis) Horm Behav. 2008;54:613–9. doi: 10.1016/j.yhbeh.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 63.Higham JP, MacLarnon AM, Heistermann M, Ross C, Semple S. Rates of self-directed behaviour and faecal glucocorticoid levels are not correlated in female wild olive baboons (Papio hamadryas anubis) Stress-The International Journal on the Biology of Stress. 2009;12:526–32. doi: 10.3109/10253890902756565. [DOI] [PubMed] [Google Scholar]
- 64.Fichtel C, Kraus C, Ganswindt A, Heistermann M. Influence of reproductive season and rank on fecal glucocorticoid levels in free-ranging male Verreaux’s sifakas (Propithecus verreauxi) Horm Behav. 2007;51:640–8. doi: 10.1016/j.yhbeh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis) Physiol Behav. 2009;98:168–75. doi: 10.1016/j.physbeh.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Whitten PL, Stavisky R, Aureli F, Russell E. Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes) Am J Primatol. 1998;44:57–69. doi: 10.1002/(SICI)1098-2345(1998)44:1<57::AID-AJP5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 67.Johnson RL, Malik I, Berman CM. On the quantification of suckling intensity in primates. Am J Phys Anthropol. 1998;105:33–42. doi: 10.1002/(SICI)1096-8644(199801)105:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 68.Cavigelli SA. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed femurs, Lemur catta. Anim Behav. 1999;57:935–44. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- 69.Mateo JM, Cavigelli SA. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool. 2005;78:1069–84. doi: 10.1086/432855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheriff MJ, Krebs CJ, Boonstra R. Assessing stress in animal populations: Do fecal and plasma glucocorticoids tell the same story? Gen Comp Endocrinol. 2010;166:614–9. doi: 10.1016/j.ygcen.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Reyes TM, Coe CL. Interleukin-1 beta differentially affects interleukin-6 and soluble interleukin-6 receptor in the blood and central nervous system of the monkey. J Neuroimmunol. 1996;66:135–41. doi: 10.1016/0165-5728(96)00038-0. [DOI] [PubMed] [Google Scholar]