Abstract
The growing societal and individual burden of dementia means counting the cases of dementia is critical. There are several approaches and methods that can be used to identify dementia cases. The ascertainment can range from very detailed characterization of the individual (deep) to a brief standardized assessment (wide) that emphasizes individual functioning. The choice of going deep or wide depends on the goal of the ascertainment. These goals are discussed, as are emerging issues that may change the way dementia cases are classified.
1. Introduction
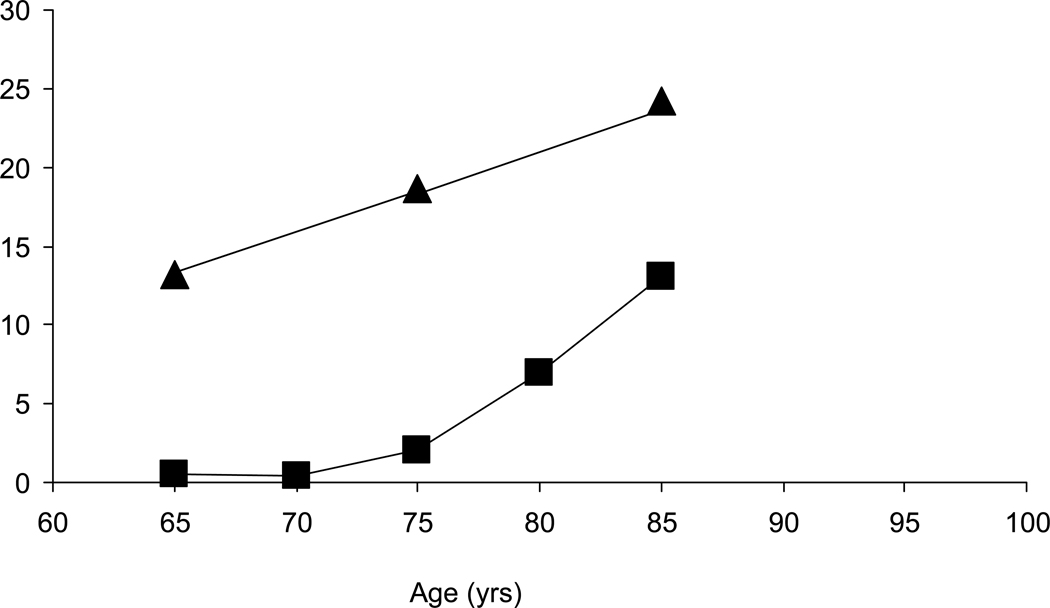
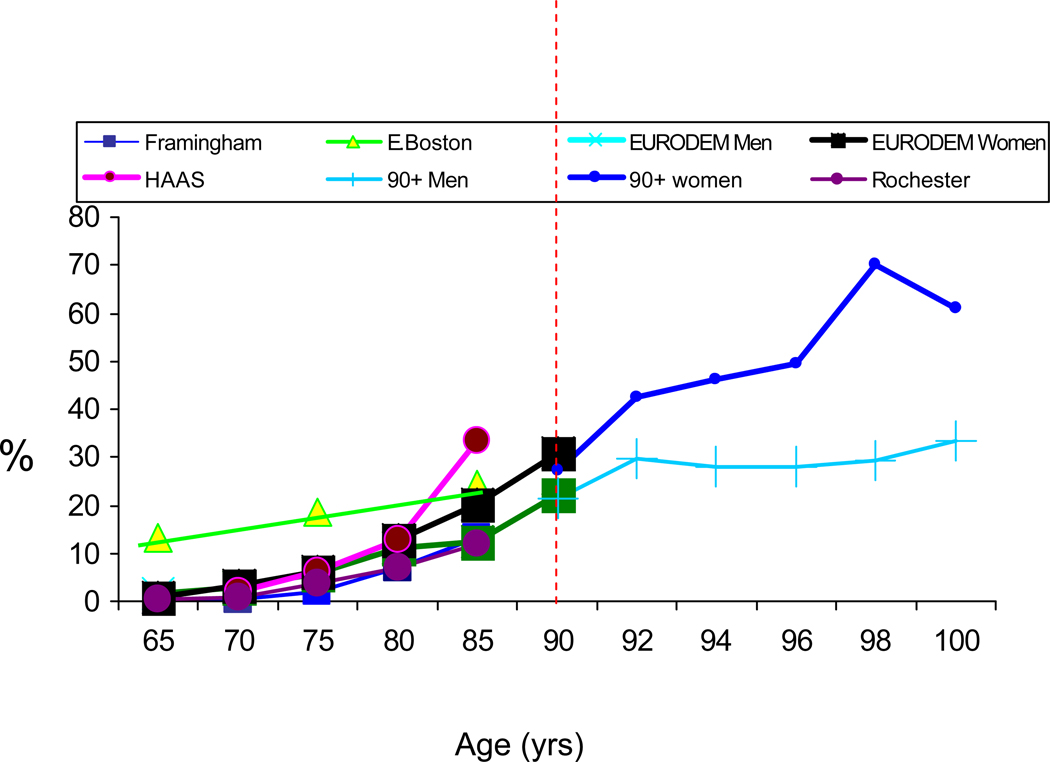
The controversy about the prevalence of dementia, particularly in the United States, began when two studies conducted in Massachusetts, one in East Boston, published in the late 1980s [1] and one in Framingham published in the early 1990s [2], reported a large difference in the prevalence of dementia in community dwelling adults age 65 years or older (Fig. 1). Since then, many more studies have been published [3–6] (Fig. 2), but despite real progress in standardizing the definition of dementia, estimates of the magnitude of the problem are still widely divergent. Most studies show similar trends of an age-dependent exponential increase in prevalence after age 65 years, but specific estimates can translate into differences that represent millions of people with the disease. The uncertainty about the numbers of people with dementia makes it difficult to develop public health policy, plan for prevention or treatment programs, or build and staff care facilities. In the research context, differences in case definition make it difficult to compare results across studies.
Figure 1.
Prevalence of dementia in two early community-based studies [1,2]
Fig. 2.
The main goal of this article is to identify population characteristics and methodologic approaches that may explain the differences in prevalence, and to discuss next steps in applying new approaches to access dementia in the general population. Importantly, the issues considered pertain to identifying dementia cases in the community. These cases may be milder, have more co-morbidity, and functions may be differentially affected compared to cases identified in a clinic. Further, in community-based studies, hundreds or thousands of individuals may be screened for dementia; therefore, assessment procedures must be cost-efficient, must lend themselves to standardization, and must not exert undue burden on the participant.
The discussion about measuring dementia in community-dwelling individuals needs to cover the spectrum of goals motivating the research, with the dichotomy of going deep or going wide. At one end of the spectrum, dementia will be assessed in studies interested in etiology—for that goal, assessment needs to go deep. Our increased capacity to study the genetic contributions to disease underscores the need for accurate disease descriptions. At the other end, dementia will be assessed in the surveillance context, with the goal of tracking disease prevalence for public health purposes [7]. When this is the goal, functional impairment, regardless of etiology, becomes an important outcome to measure. Function can be measured in a simple diagnostic process that can be completely standardized and implemented in many thousands of individuals. In studies aimed at functional outcomes, a wide approach is needed.
2. Current paradigm to assess dementia in epidemiologic studies: a challenge
Cited prevalence data are frequently taken from community-based studies aimed to research disease processes modulating the risk for dementia [2–6]. In such studies, protocols are based on face-to-face examinations performed by, or under the supervision of, specialized clinicians, such as neurologists, psychiatrists, geriatricians, and neuropsychologists. Streamlined neurologic examinations, informant interviews, and targeted cognitive testing form the core of data collected to diagnose dementia and its sub-types. International diagnostic guidelines are applied, frequently in the context of a consensus conference of trained specialists. With this level of detail, clinical diagnoses of the major (i.e., highly prevalent) sub-types of dementia—Alzheimer’s disease (AD), vascular dementia, and Parkinson’s dementia—can be made reasonably accurately, but there is often not enough detailed information to accurately identify more rare sub-types of dementia, such as Frontal Temporal Lobe Dementia, Lewy Body Dementia, or Progressive Supranuclear Palsy to name a few. This is a relatively expensive method of case ascertainment, necessitated by the need for well-characterized cases, by no established biomarkers, by no ante-mortem gold standard for diagnosis, and by incomplete, non-standardized data from health and death records.
3. What contributes to the variability in prevalence figures?
Differences in prevalence estimates depend on sample characteristics such as age, competitive risks for death, ethnicity and culture, region of residence, socioeconomic status, life style, and access to health care. There are also differences in study design [8,9], sampling strategy, and the technology used to evaluate subjects that may contribute to variability. Although there are few data, it is also possible there are secular trends that change the expression of dementia [5], as well as changes in how physicians are trained to diagnose dementia. Identification of dementia in the very old may also introduce variability in diagnoses. Finally, we typically don’t know subject characteristics and susceptibility to dementia of those who die before they are evaluated, or those who refuse to participate. Many of these issues will be discussed in detail in the subsequent articles of this special issue of Alzheimer’s and Dementia.
One part of the diagnostic spectrum that leads to variability in case ascertainment is the area somewhere between clinical dementia and lesser cognitive impairment. The differences in distinguishing the two conditions partly reflect the usual variability in clinical judgment. This variability can be minimized by training and standardizing clinic staff and using standard cognitive and functional assessments; however, such approaches are used to various degrees in different studies, and have not been harmonized in the international forum. Another source of variability in the diagnostic process is how a change in function is defined and assessed. A dementia diagnosis is, by definition, a diagnosis of change, but, particularly at baseline, it is difficult to assess just how much change a person has experienced. Change is usually established by proxy interview, which can vary in reliability, and follow-up exams to measure progression are often not feasible.
4. New developments that impact on identifying a dementia case in the community
4.1. Mild cognitive impairment
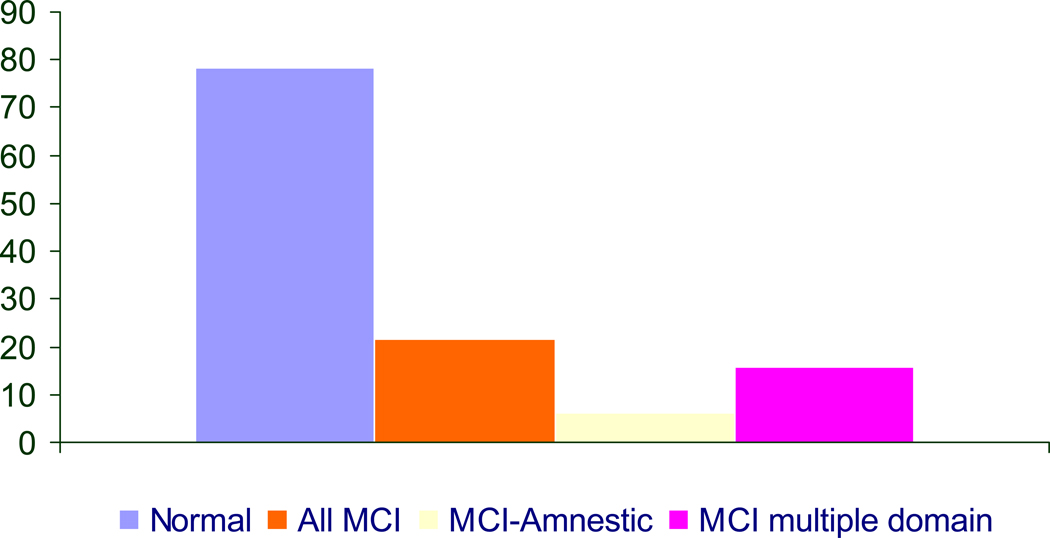
A prodromal phase of dementia, characterized by mild cognitive impairment (MCI), has long been a topic of discussion [10]. Recent efforts have added additional evidence for such a phase, and there is increasing acceptance of this concept. Specifically there has been progress in standardizing the definition of the case that is on the margin of clinical dementia and cognitive impairment, mentioned above. Petersen and colleagues have developed criteria for the amnestic type of MCI (aMCI), which is characterized by a memory deficit and subjective complaints of memory impairment [11]. Cases of aMCI identified in the clinic setting have a high probability of converting to AD. It has been argued that these are cases with mild AD, Clinical Dementia Rating (CDR) 0.5, and should be included in the count of AD, although there is disagreement about this [12]. Lopez and colleagues have extended the MCI classification to better reflect the domains of impaired cognition found in the community setting [13] (Fig. 3). Their definition, known as multiple domains MCI, is based on impairment in more than one domain, which does not have to include memory. The concept of MCI is still evolving, and there are data suggesting the conversion rates from MCI to dementia depend on the definition of MCI [14] and whether or not cases are identified in a clinic or a community-based sample [15].
Figure 3.
Prevalence of different MCI sub-types: CHS Pittsburg Cohort [13]
The concept of MCI is binary in the sense that the condition may be present or absent. Even as the debate continues on how to define MCI in this binary sense, there is increased consideration given to cognitive disorders as a continuous phenomenon. Operationalizing this ‘continuous’ concept challenges the use of cut points, which are difficult to establish and somewhat arbitrary (Fig. 4). Changes toward a more linear concept of cognitive impairment would have an important impact on how we measure and evaluate cognitive impairment, and thus on public health activities and clinical practice and therapeutic trials.
Fig 4.
Cognitive disorders are a continuous phenomenon, making cut-points difficult to establish (Launer, unpublished)
4.2 Diagnostic criteria
There are several developments in the area of diagnosis that will have a major impact on the tools and guidelines for case identification. The Alzheimer’s Disease Neuroimaging Initiative (ADNI) is a multi-center study designed to identify and track clinical, physiologic and radiographic biomarkers for preclinical and progressing AD. Recent studies based on ADNI data, for instance, report on the sensitivity and specificity of cerebral spinal fluid markers such as beta amyloid and tau biomarkers. New imaging techniques, such as PET based labeling of the amyloid burden in the brain has, in conjunction with many other studies, provided the basis for the proposal of a cascade of biomarker changes that will eventually lead to a better understanding of the AD disease process and its translation into new clinical biomarkers [16]. At the same time, experts have been reviewing diagnostic guidelines for pre-MCI, MCI and dementia.
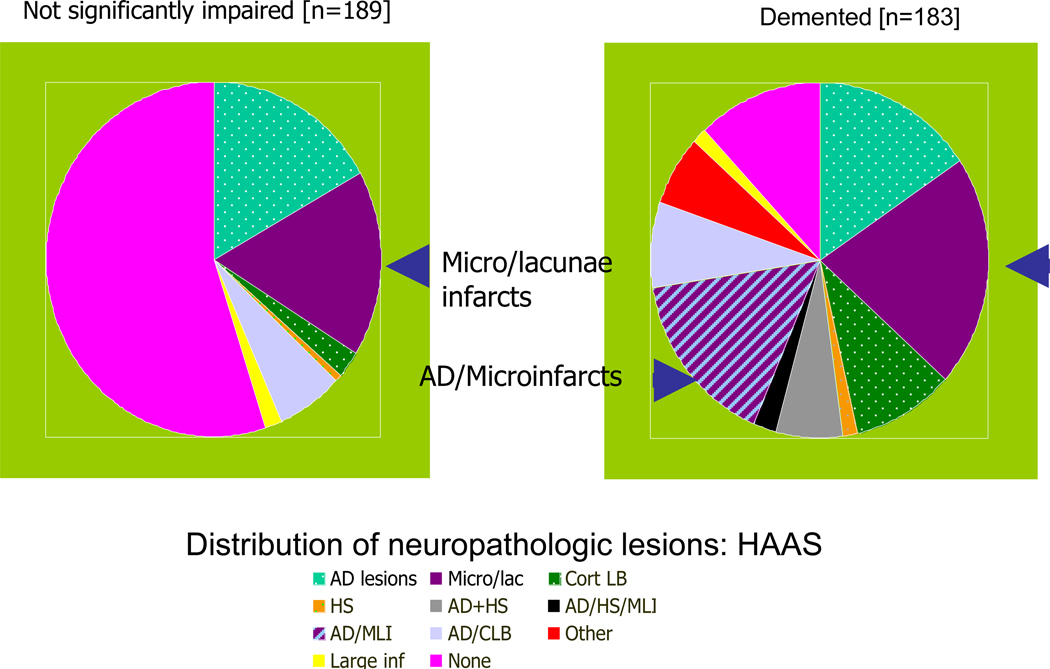
4.4. Neuropathologic lesions
Heterogeneity in the pathology of dementia is another aspect underlying the decisions about who is demented. We know from several community-based autopsy studies that individuals with or without an ante-mortem diagnosis of dementia [17,18] have multiple neuropathologic lesions, including vascular lesions, amyloid-laden plaques, neurofibrillary tangles, hippocampal sclerosis, and Lewy bodies, particularly in the oldest old (Fig. 5). However, for individuals with these lesions, the gross functional impairments are often similar, and, to date, there is little possibility to identify the neuropathologic lesions in living subjects from community-based samples. As a result, the sensitivity of a clinical diagnosis of AD compared to neuropathologic evidence varies considerably and can be lower in community-based samples [19] compared to clinic-based samples [20]. The heterogeneity of disease-causing lesions becomes important when specific treatments or the means to prevent specific pathogenic processes become available.
Fig. 5.
The pathology underlying dementia is heterogeneous [16]
4.5. Secular change in risk factors
A final issue to consider when counting dementia cases is the secular change in putative risk factors for dementia, and how the changes may alter who is in a high risk group. For instance, risk factors such as smoking and hypertension have decreased [21], but obesity [22] and diabetes [23] are projected to increase over time, and in younger individuals. These trends may have the potential of shifting the age-related trajectory of prevalent and incident disease. At the other end, secular changes in longevity or treatment possibilities will also affect the numbers of prevalent and incident cases of dementia.
5. Conclusions
In the population setting, the purpose of identifying a dementia case will strongly dictate the approach to assessment. These purposes can include: (1) etiologic research to identify new risk factors or to understand how known risk factors contribute to dementia and its sub-types; (2) prediction of who will become demented, which is based on subject characteristics; (3) monitoring and documenting secular trends in dementia; (4) planning for resources needed to clinically diagnose and care for people with dementia; (5) estimating the size of the commercial market for a new drug; or (6) characterizing cases with the aim of identifying who might benefit from particular intervention at what stage of the disorder.
The different goals can be achieved with different levels of phenotype data. Studies with research objectives to examine the etiology of dementia will continue to require deep phenotyping, and the complexity of this effort will increase as more is understood about the syndrome. For studies aimed to acquire general prevalence figures for health-resource planning and related activities, a broad approach that identifies functionally impaired individuals may be sufficient, at least at our current level of understanding.
In summary, given one’s goals, the following questions should be addressed to arrive at the best approach to count cases of dementia. Why we are counting will determine the cascade of decisions about study design including: What should we count: function, specific symptoms, or lesions? When should we start counting: in middle age, when the disease process likely begins; at age 65 years, the average age when people typically retire (although this is likely to change), or should we start at a later age, when the incidence of dementia is higher? Who should we count: total populations, including those individuals residing in nursing homes, only community-dwelling individuals, or specific sub-groups that differ demographically or in risk factor burden? How much coverage: a nationally representative sample, or smaller defined populations? Answering these questions can guide the formulation of standardized or harmonized protocols to assess dementia in a way that is appropriate for the purpose. There is no one best way.
Footnotes
Disclosure statement for author
The author has no conflicts to disclose.
References
- 1.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer's disease in a community population of older persons: Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 3.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–S9. [PubMed] [Google Scholar]
- 4.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: Results from the 90+ Study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 5.Beard CM, Kokmen E, O'Brien PC, Kurland LT. The prevalence of dementia is changing over time in Rochester, Minnesota. Neurology. 1995;45:75–79. doi: 10.1212/wnl.45.1.75. [DOI] [PubMed] [Google Scholar]
- 6.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 7.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 8.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 9.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The Aging, Demographics, and Memory Study: Study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 10.Henderson AS, Huppert FA. The problem of mild dementia. Psychol Med. 1984;14:5–11. doi: 10.1017/s0033291700003020. [DOI] [PubMed] [Google Scholar]
- 11.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: Time to revise diagnostic criteria. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 14.Saxton J, Snitz BE, Lopez OL, Ives DG, Dunn LO, Fitzpatrick A, et al. Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry. 2009;80:737–743. doi: 10.1136/jnnp.2008.160705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, et al. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211. e7. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White L. Brain lesions and dementia: the HAAS. Journal of Alzheimer's Disease. 2009:18713–18725. [Google Scholar]
- 18.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 19.Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 20.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 21.Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, Mensah G, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med. 2006;166:2348–2355. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 23.Mainous AG, 3rd, Baker R, Koopman RJ, Saxena S, Diaz VA, Everett CJ, et al. Impact of the population at risk of diabetes on projections of diabetes burden in the United States: An epidemic on the way. Diabetologia. 2007;50:934–940. doi: 10.1007/s00125-006-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]