Abstract
Purpose of review
The humoral immune response to HIV-1 throughout infection is comprised of complex mixtures of antibody isotypes with numerous HIV-1 specificities. However, unlike antibody responses to most infections, protective antibody responses are delayed and do not arise until long after HIV-1 latency is established. We review recent data on HIV-1-specific antibody isotypes induced following HIV-1 transmission: to understand the effects of HIV-1 on B cell and T cell effector responses, to understand the timing of the rise and fall of different anti-HIV-1 antibodies and to understand how antibodies could contribute to protective immunity if they were either pre-existing or elicited immediately after HIV-1 transmission.
Recent findings
Studies of the earliest events following infection by the transmitted/founder virus have recently revealed that early destruction of B cell generative microenvironments may be responsible for delay of potentially protective anti-HIV-1 antibody responses. Unlike the initial CD8+ T cell response to HIV-1, the initial induced antibody response is usually ineffective in controlling virus replication during acute HIV-1 infection.
Summary
The antibody isotypes and specificities elicited during HIV-1 infection can provide a window into deciphering the detrimental effects of HIV-1 on B cell and T cell responses. Additionally, further characterization of the virus inhibitory capabilities of anti-HIV-1 antibody isotypes can define the spectrum of potential protective HIV-1 antibodies that could be readily elicited by experimental vaccines and adjuvants.
Keywords: antibody, humoral responses, isotype, mucosal
Introduction
HIV-1 infection elicits antibody responses of multiple isotypes to proteins encoded by HIV env, gag and pol genes. The isotypes of free antibodies to HIV-1 can be unswitched antibody, IgM, and class-switched antibody isotypes; IgG, IgA, and IgE. In humans, IgG has four subclasses: IgG1, IgG2, IgG3, and IgG4, and IgA has two subclasses: IgA1 and IgA2. Each antibody isotype and subclass may be involved in production of a range of specificities to HIV-1 proteins (i.e. Env, Gag, Tat, Nef, integrase, and reverse transcriptase). The Fab portion of antibody determines the antigen-binding specificity and antibody Fc portion mediates complement component binding and a myriad of Fc receptor-mediated anti-HIV-1 activities of natural killer (NK) cells and monocyte/macrophages (reviewed in [1]). Consequently, antibody isotypes generated during infection determine antibody effector function capabilities (e.g. complement fixation, Fc receptor binding) of the antibodies and represent the specific adaptive humoral response to HIV-1. The functional antiviral capabilities of the humoral response are for the most part limited to antibodies that target envelope. However, levels of antibodies to structural proteins, such as anti-Gag Abs, that do not have known direct antiviral activity, can be indicative of an active T helper cell response [2].
Initial antibody responses to the transmitted/founder HIV-1
Recent studies using single-genome amplification of viral genes coupled with mathematical modeling of the dynamics of HIV-1 evolution have determined that HIV-1 infection by clade B and C viruses is caused by a single quasispecies in approximately 80% of patients [3,4]. The earliest phases of HIV-1 infection during the time following transmission have been defined by stages I–VI by Fiebig et al. [5]. In addition to the detection of p24 protein and viral RNA, the antibody responses to the proteins from the env, gag, and pol genes can mark progression through the early acute phase. The initial free antibodies to HIV-1 are anti-gp41 IgM antibodies, followed by class switching to IgG and IgA antibodies [6]. IgG antibodies to Gag appear at a median time of 18 days (p24, p55) and 33 days (p17) following detectable plasma vRNA. Antibodies to p31 (integrase) are elicited at a median time of 53 days. Antibodies directed to the HIV-1 Env appear in a sequential order (Fig. 1) with anti-gp41 appearing first, predominantly to the immundominant epitope. The initial binding antibody response to gp120 is delayed and appears at 28 days after detectable vRNA compared to the median time to gp41 antibodies of 13 days. For the clade B patients studied, the epitope to which the initial gp120 antibodies target is V3; and these first antibodies (within 40 days from detectable viremia) are non-neutralizing [6] but are closely followed by weakly neutralizing V3 antibodies for heterologous tier 1 HIV-1 isolates [10•]. Mathematical modeling of the early HIV-1-specific IgM and IgG antibody responses indicated that these antibodies generally do not control virus replication in most patients and are not responsible for the initial decline in plasma viral load [6]. Moreover, the antibodies elicited during the first 40 days after detectable plasma viremia did not inhibit virus in standard TZM-bl neutralization assays and did not mediate antibody-dependent cell-mediated virus inhibition (ADCVI) [6]. Among the first neutralizing antibodies to eventually appear during acute infection are predominately variable region-directed antibodies that are detected at approximately 13 weeks’ postinfection in acute clade B-infected patients and at 3–8 weeks’ post-infection for clade C-infected patients [10•]. Most autologous neutralizing antibodies identified thus far have been against variable Env regions [11–14].
Figure 1. Sequentially elicited IgG antibodies to HIV-1 envelope epitopes.
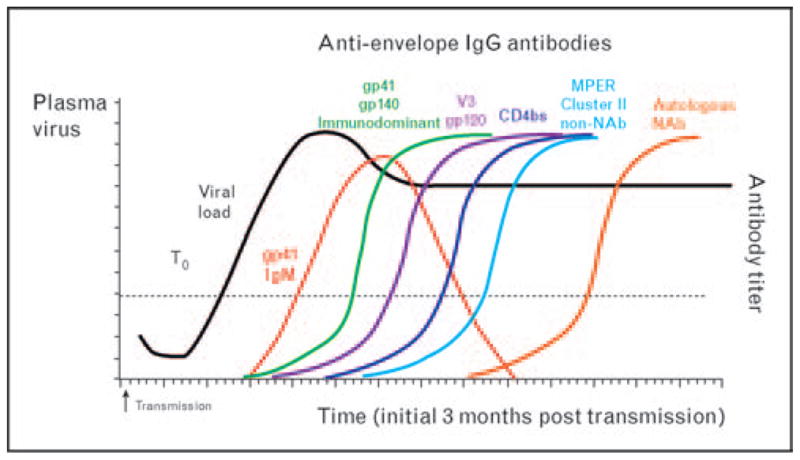
After HIV-1 transmission, antibody isotypes and specificities to the HIV-1 envelope are elicited sequentially [gp41, gp120, CD4bs, MPER (non-neutralizing), autologous neutralizing antibodies]. The first free HIV-1-specific antibody detected in the plasma is anti-gp41 IgM (red line). The immunodominant epitope is one of the known regions in gp41 recognized initially. Anti-gp41 IgM undergoes class switching to IgG and IgA, making gp41 the first protein also recognized by IgG and IgA antibodies. This figure shows the initial IgG response [6] to gp41 (green), gp120 (purple), CD4bs (dark blue), MPER (non-neutralizing) (light blue) through the development of autologous neutralizing antibodies (orange line) [7,8] within the first 3 months from transmission. The dotted line indicates when either plasma viremia or HIV-1-specific antibody is detectable in plasma. T0 is the time at which plasma viremia reaches 100 copies/ml [6,9].
The timing of onset of antibody responses following transmission is delayed in HIV-1 infection compared with other infections. In experimental immunization of uninfected volunteers with ΦX174 bacteriophage, the onset of a measurable neutralizing response is 4 days [15]. Similarly in mice, the onset of an IgM-neutralizing antibody response to vesicular stomatitis virus (VSV) is 4 days [16]. As noted above, the autologous neutralizing antibody response to HIV-1 does not appear until approximately 12 weeks after transmission. Thus, if local and systemic production of autologous neutralizing antibodies could be induced before transmission and/or primed to arise within 4–7 days following transmission, the initial antibody response to HIV-1 may be able to control replication of the transmitted/founder virus. Finally, recent studies of B cells from the gut during early HIV-1 infection suggest induction of class-switched polyclonally activated B cells soon after infection [17••].
Antibody isotypes in broadly neutralizing sera in chronic infection
Recent work has defined antibody specificities and types in plasma/sera and correlated these with the presence of neutralizing antibodies and their breadth. A recent study [18••] examined the distribution of Env-specific IgG1, IgG2, IgG3, IgG4, IgA, and IgM in chronic HIV-1 patients with neutralization breadth. The HIV-1-specific antienvelope response circulating in plasma was predominantly IgG1, supporting the notion that at the chronic stages of HIV-1 infection, the T helper responses were skewed to Th2. IgG2, IgG3, IgG4, and IgM were detected less often, but when IgG2 or IgG3 were detected they were not detected together, indicating that these responses may be differentially regulated [18••]. In addition, HIV-specific IgA responses were also prominent in these HIV-1 patients with broadly neutralizing sera, although among all anti-HIV-1 immunoglobulin isotypes, the IgG1 concentration was the most prevalent.
We identified one patient with broadly neutralizing antibodies targeting the gp41 envelope membrane proximal external region (MPER) and the antibody isotype of the affinity-purified antibody with neutralization breadth was IgG1 [19]. Immunoglobulin isotype data of the nature of broadly neutralizing antibodies will be required to determine if the different antienvelope IgG subclasses correlate with particular specificities of broad neutralizing antibodies (i.e. MPER, CD4bs).
Regulation of class switching
The classical route for exogenous antigens to stimulate B cells to undergo immunoglobulin class switching occurs in the lymph nodes. The properties of the infectious agent antigen, such as size, ability to activate complement, ability to bind to antigen-presenting cell receptors and/or form immune complexes can all influence B cell immunoglobulin class switching. Epithelial cells in mucosal tissue reacting to inflammatory signals produce B-cell activating factor, BAFF, and IL-10, which can also activate naïve B cells to undergo class switch recombination (reviewed in [20••]). Moreover, gp120 carbohydrates can bind to C-type mannose receptors on B cells and induce activation-induced cytidine deaminase (AID) and immunoglobulin class switching [21]. This type of HIV-1 envelope interaction may be responsible for a component of the polyclonal B cell activation and class switching seen in early HIV-1 infection [17••]. Moreover, carbohydrate on Env can interact with mannose receptors on dendritic cells resulting in immunosuppressive responses [22]. It remains to be determined whether induction of mucosal production of locally class switched and protective anti-HIV-1-specific IgG and IgA can be induced by various vaccine strategies.
HIV-1-specific IgG subclass responses
IgG1
In both acute and chronic HIV infection, anti-Env antibodies are predominantly IgG1 [23–28] and of the IgG subclasses, IgG1 has the broadest response to Env, Gag, and Pol proteins [29]. Importantly, HIV-1 Env-specific IgG1 antibodies can mediate antiviral functions. HIV-1-specific IgG1 can bind to FcR and mediate antibody-dependent cellular cytotoxicity (ADCC) of HIV-1-infected cells [30]. Additionally, a recent report using TZM-bl cells expressing four major human Fc receptors showed that the strongest effect of gp41 MPER-specific antibodies was in the context of the IgG1 subclass [31•].
A recent study that characterized the IgG subclasses and IgA antibodies in HIV-2 heterosexual transmission found an inverse association between anti-Env C2C3 IgG and CD4+ T cells [32]. They hypothesized that because the C2V3C3 region of the HIV-2 envelope has immunosuppressive properties, the antibodies that target this region decrease its immunosuppressive properties leading to immune activation and CD4 loss. Similar to HIV-1, the predominant IgG subclass response to HIV-2 is IgG1; however, IgG3 was also found in significant concentrations.
IgG2
Anti-Env IgG2 can be detected at various stages throughout HIV-1 infection [33,34•]; however, the level of anti-Env IgG2 is low compared with the other subclasses [23–25]. Classically, IgG2 antibodies tend to be elicited to carbohydrate moieties. Although a rare human broadly neutralizing antibody, 2G12 mAb, binds to high mannose residues on HIV-1 Env [35], the heavily glycosylated HIV-1 envelope fails to routinely elicit anti-carbohydrate neutralizing antibodies [36]. Interestingly the lack of anti-gp41 Env IgG2 antibodies was associated with progression to AIDS [37]. In addition, the presence of anti-Env IgG2 antibodies in long-term nonprogressors was concluded to be a correlate of virus control and T cell help [38] suggesting a salutary effect of a vaccine if anti-HIV-1 Env IgG2 antibodies could be induced.
IgG3
Although anti-Env plasma antibodies have been described to be predominantly IgG1, anti-Env IgG3 is the second most predominant IgG subclass [39]. IgG3 can have greater in-vitro neutralizing ability compared with IgG1 potentially due to an enhanced flexibility of the immunoglobulin hinge region [40]. In accordance with finding different subclass predominance for Env and Gag, differential regulation of anti-Env and anti-Gag antibodies has been described [2]. Anti-Gag IgG3 antibodies (in particular anti-p17) are found more frequently in early infection [23–25,30]. Furthermore, anti-Gag IgG3 appears early in acute infection and then declines [29] (Tomaras GD, Yates NL, Haynes BF, unpublished data). Several studies found a decrease in anti-HIV IgG3 during disease progression [24,30]. Additionally, in one study [41], more total IgG3 was found in HIV patients with high viral loads and major B cell dysfunction, than HIV-1-negative patients, suggesting that at different stages of HIV-1 infection, the level of antibody isotypes may reflect immune perturbations.
IgG4
IgG4 antibody responses are typically a predominant response to chronic antigenic stimulation, such as in the setting of chronic parasite exposure. HIV-1-specific IgG4 responses have been found in a few studies either in patients in a parasite endemic area, [42] or in haemophiliac patients [43] and plasma donors (Tomaras GD, Haynes BF, unpublished data) in whom repeated antigen exposure was likely. Furthermore, HIV-1-specific IgG4 was found more readily in chronically HIV-1-infected patients [26,30].
IgA
HIV-1 is transmitted predominately through mucosal surfaces; thus understanding mucosal antibody responses is important for the development of effective preventive strategies. HIV-1 can cross the epithelial cell barriers through movement of virions through intracellular junctions (Thomas Hope, personal communication). HIV-1-specific antibodies that can bind virions may be able to prevent HIV-1 transmission via traditional neutralization, virion aggregation, or inhibition of viral movement across epithelial barriers [44]. Mucosal HIV-1-specific antibodies have been shown to inhibit the transcytosis route of HIV-1 migration through epithelial cells [45,46]. Moreover, functional antibodies that inhibit HIV-1 via Fc receptor-mediated functions such as ADCC can be found in cervicovaginal fluids [47], raising the notion that these binding antibodies may be a component of a protective anti-HIV-1 antibody response.
Evidence for the potential protective role of mucosal antibodies comes from studies in which the presence of HIV-1-specific IgA potentially correlated with protection [48]. Neutralizing IgA of unknown specificity has been found in genital secretions from high-risk HIV-1-uninfected sex workers and correlated with subsequent protection from HIV-1 acquisition [49,50•]. Another recent study examined HIV-1-specific cervical IgA in commercial sex workers and found that these antibodies were not neutralizing but did correlate with the number of HIV-1 exposures [51]. HIV-specific IgA can also be detected in saliva and a recent study has found specificities for the V1/V2 region of the HIV-1 envelope that could neutralize primary virus isolates from clades A, B, and C [52]. It remains an open question as to whether mucosal vaccination will be required to induce protective antibody responses at mucosal surfaces.
Binding antibodies and virus clearance
In addition to Fab binding, effector functions of antibodies can depend on the interaction of antibodies with Fc receptors on B cells, NK cells, dendritic cells, neutrophils, and monocyte macrophages. Part of the functional role of antibodies depends on the engagement of Fc receptors [FcγRI (CD64), FcγRIIa/b (CD32), and FcγRIIIa (CD16)] on the surface of effector cells [53]. ADCC occurs when HIV-1-specific antibodies, predominately IgG1 and IgG3, bind to their antigens presented on the surface of infected cells. FcR on NK cells can then target these antibodies and direct the cytotoxic activities of the effector NK cells. Binding antibodies can also act directly on the HIV-1 virion through opsonization – a classical antibody effector function. FcR on the surface of monocyte macrophages and neutrophils can bind to the constant region of immunoglobulin molecules, and this binding can be modulated by immunoglobulin Fc glycosylation [54]. If anti-HIV-1 immunoglobulin molecules are bound to virions and multiple FcR are engaged, then immunoglobulin cross-linking occurs resulting in phagocytosis of HIV-1. In addition to phagocytosis, antibody Fc-mediated activities leading to complement deposition on the virion can lead to direct virolysis [55], although the virion can bind complement components directly in the absence of antibody [56]. Moreover, complement coated virions can be more efficiently transferred to follicular dendritic cells and CD21+ B cells (reviewed in [57••]). A recent study has demonstrated disruption of FDC networks in the generative microenvironments in early HIV-1 infection with loss of approximately 50% of Peyer’s patch germinal centers within the first 80 days of HIV-1 infection [17••]. Many questions remain regarding the role of binding antibodies (i.e. opsonization, ADCC, aggregation, and inhibition of cell-to-cell transmission) in controlling versus facilitating HIV-1 replication and infection in vivo. However, FcR genotype was reported to predict progression perhaps due to the roles of FcR in clearance of immune complexes [58]. An ultimate test of protective function is the passive transfer of binding antibodies (with specific antiviral activities) to naïve animals to determine if the animals are protected from virus challenge or SIV/SHIV-induced disease. Antibody-dependent cell-mediated virus inhibition has already been shown to be an important effector mechanism in a passive protection study in nonhuman primates [59]. Further analyses on potential correlations between binding antibodies with diverse antiviral functions and protection from HIV-1 in humans are warranted.
Clinical relevance of isotype distribution
Several studies have found correlations with particular HIV-1-specific antibody isotype responses and control of infection or long-term nonprogressor status. The predominant antibody types and specificities that stand out from these analyses are anti-Env IgG2 and anti-Gag antibodies. Despite total IgG2 antibodies being an abundant isotype after IgG1 in serum from uninfected individuals, anti-Env IgG2 antibodies are sporadically detected in chronic HIV-1-positive patient sera at low levels [18••,23–25,33,34•]. Due to studies that found a correlation between HIV-1-specific (Env or Gag) IgG2 antibodies with virus control and T cell help [38,60], it is thought that elicitation of a more robust IgG2 response in HIV-1 infection might be of some benefit. However, the function of these IgG2 antibodies is not clear as IgG2 does not bind complement well and only weakly mediates ADCC. Of note, a potentially protective IgG2 subclass response has been demonstrated in age-related anti-measles responses [61]. Studies like the one showing an association between IFN-γ producing HIV-1-specific CD4 Th1 cells and HIV-1-specific IgG2 antibodies [60] suggest that knowledge of the antibody isotypes present may provide some insight into the cellular responses to HIV-1 as well.
Many studies have examined the correlation of anti-Gag antibodies with clinical disease and have found that these antibodies, although they are non-neutralizing, were associated with a more delayed disease progression and in some cases decline during AIDS defining illnesses [62–68], likely due to their presence or affinity being associated with a strong CD4 T helper response [2,69].
Frequency and timing of induction of IgG1 broad neutralizing antibody responses
Broadly neutralizing antibodies are not routinely made in HIV-1 infection, and when they are made, they arise late as highlighted by four recent studies. Scheid et al. [70••] found that mixtures of anti-gp140 antibodies (albeit at supraphysiologic amounts of antibody concentrations) in part reconstituted the breadth of neutralizing antibodies found in plasma. Simek et al. [71] studied over 1200 chronically infected patients and found 1% with extreme breadth. Shen et al. [19] screened over 300 patients for MPER-neutralizing antibody and found one patient with a 2F5-mAb-like plasma antibody with neutralization breadth that arose after the first year of infection. This study and that by Sather et al. [72] demonstrated that the natural development of broad neutralizing antibodies can take several years to develop [19,72]. Although elite neutralizers are rare (~1%), a larger proportion (~20–25%) of HIV-1-infected patients does develop cross-reactive neutralizing antibodies (reviewed in [73]).
Profound effects on the antibody repertoire by HIV-1 is evident by alterations of the kappa/lambda (κ/λ) antibody light chain ratios, which were skewed to kappa in the lamina propria of terminal ileum gut-associated lymphoid tissues (GALTs) in acute and early HIV-1 infection [17••]. This skewing toward an increased use of the kappa (κ) antibody light chain was also founding p140-specificantibodiesfrom memory B cells in chronic HIV-1 (compared to non-gp140 antibodies and historical controls) [70••,74].
Thus, rapid maturation of an effective antibody response is probably circumvented by the follicular damage and germinal cell loss occurring soon after HIV-1 infection [17••]. In addition, there is a profound and rapid cytokine storm [9] in acute HIV-1 infection that likely contributes to the immunopathology of early infection and the lack of an appropriate maturing antibody response.
Conclusion
HIV-1 transmission severely affects the overall B cell response, resulting in an ineffective antibody response. Although potentially protective antibody types are elicited at much later times throughout the course of infection, they are ineffective in controlling viral replication at the time that they develop. Thus, for a vaccine to prevent HIV infection, it will be critical for some level of the antibody with specificity to the transmitted/founder HIV-1 to be present prior to transmission and, optimally, with a rapid secondary response arising soon thereafter. An understanding of all of the potential antibody-mediated effector functions (e.g. FcR anti-HIV-1 functions) other than classical neutralizing antibodies will probably be critical in designing an effective HIV-1 vaccine.
Acknowledgments
G.D.T. is supported by the National Institutes of Health (NIH/NIAID) grants: RO1AI052779, U19AI067854 (Center for HIV/AIDS Vaccine Immunology), AI068618 (HIV Vaccine Trials Network) (Duke Center for AIDS Research), and the Bill and Melinda Gates Foundation (38619). B.F.H. is supported by the NIH/NIAIDS grants; U19AI067854 (Center for HIV/AIDS Vaccine Immunology), PO1AI061734, PO1AI052816, and the Bill and Melinda Gates Foundation (38643, 38617).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 454).
- 1.Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 2.Binley JM, Klasse PJ, Cao Y, et al. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma antigp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 8.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Davis KL, Gray ES, Moore PL, et al. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. This elegant study details the early development of V3-specific neutralizing antibody responses in acute infection (clades B and C) and in vaccines (clade B) using HIV-2/HIV-1 V3 Env scaffolds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore PL, Gray ES, Choge IA, et al. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J Virol. 2008;82:1860–1869. doi: 10.1128/JVI.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honnen WJ, Krachmarov C, Kayman SC, et al. Type-specific epitopes targeted by monoclonal antibodies with exceptionally potent neutralizing activities for selected strains of human immunodeficiency virus type 1 map to a common region of the V2 domain of gp120 and differ only at single positions from the clade B consensus sequence. J Virol. 2007;81:1424–1432. doi: 10.1128/JVI.02054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rong R, Bibollet-Ruche F, Mulenga J, et al. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J Virol. 2007;81:1350–1359. doi: 10.1128/JVI.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinter A. Roles of HIV-1 Env variable regions in viral neutralization and vaccine development. Curr HIV Res. 2007;5:542–553. doi: 10.2174/157016207782418470. [DOI] [PubMed] [Google Scholar]
- 15.Peacock DB, Jones JV, Gough M. The immune response to thetaX 174 in man. I. Primary and secondary antibody production in normal adults. Clin Exp Immunol. 1973;13:497–513. [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr T, Naim HY, Bachmann MF, et al. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat Med. 1998;4:945–948. doi: 10.1038/nm0898-945. [DOI] [PubMed] [Google Scholar]
- 17••.Levesque MC, Moody MA, Hwang K, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000107. in press. This study addresses the effect of HIV-1 transmission on the B cell subsets in the blood and terminal ileum GALTs in acute and early HIV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. A comprehensive analysis of neutralizing antibody specificities and antibody isotypes in plasma from patients with HIV-1 neutralization breadth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen X, Parks RJ, Montefiori DC, et al. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J Virol. 2009;83:3617–3625. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. An excellent review of recent studies on innate immune recognition of pathogens and the critical role this plays in mucosal IgA class switching. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Qiao X, Klasse PJ, et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 22.Shan M, Klasse PJ, Banerjee K, et al. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klasse J, Blomberg J. Patterns of antibodies to human immunodeficiency virus proteins in different subclasses of IgG. J Infect Dis. 1987;156:1026–1030. doi: 10.1093/infdis/156.6.1026. [DOI] [PubMed] [Google Scholar]
- 24.McDougal JS, Kennedy MS, Nicholson JK, et al. Antibody response to human immunodeficiency virus in homosexual men. Relation of antibody specificity, titer, and isotype to clinical status, severity of immunodeficiency, and disease progression. J Clin Invest. 1987;80:316–324. doi: 10.1172/JCI113075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalife J, Guy B, Capron M, et al. Isotypic restriction of the antibody response to human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988;4:3–9. doi: 10.1089/aid.1988.4.3. [DOI] [PubMed] [Google Scholar]
- 26.Mathiesen T, Chiodi F, Broliden PA, et al. Analysis of a subclass-restricted HIV-1 gp41 epitope by omission peptides. Immunology. 1989;67:1–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Mergener K, Enzensberger W, Rubsamen-Waigmann H, et al. Immunoglobulin class- and subclass-specific HIV antibody detection in serum and CSF specimens by ELISA and Western blot. Infection. 1987;15:317–322. doi: 10.1007/BF01647729. [DOI] [PubMed] [Google Scholar]
- 28.Sundqvist VA, Linde A, Kurth R, et al. Restricted IgG subclass responses to HTLV-III/LAV and to cytomegalovirus in patients with AIDS and lymphadenopathy syndrome. J Infect Dis. 1986;153:970–973. doi: 10.1093/infdis/153.5.970. [DOI] [PubMed] [Google Scholar]
- 29.Wilson KM, Johnson EI, Croom HA, et al. Incidence immunoassay for distinguishing recent from established HIV-1 infection in therapy-naive populations. AIDS. 2004;18:2253–2259. doi: 10.1097/00002030-200411190-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ljunggren K, Broliden PA, Morfeldt-Manson L, et al. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin Exp Immunol. 1988;73:343–347. [PMC free article] [PubMed] [Google Scholar]
- 31•.Perez LG, Costa MR, Todd CA, et al. Utilization of IgG Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane proximal external region of gp41. J Virol. 2009;83:7397–7410. doi: 10.1128/JVI.00656-09. This study details the role of FcγRI and FcγRIIb with IgG subclass and antibody specificity in antibody-mediated neutralization of HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcelino JM, Nilsson C, Barroso H, et al. Envelope-specific antibody response in HIV-2 infection: C2V3C3-specific IgG response is associated with disease progression. AIDS. 2008;22:2257–2265. doi: 10.1097/QAD.0b013e3283155546. [DOI] [PubMed] [Google Scholar]
- 33.Chiodi F, Mathiesen T, Albert J, et al. IgG subclass responses to a transmembrane protein (gp41) peptide in HIV infection. J Immunol. 1989;142:3809–3814. [PubMed] [Google Scholar]
- 34•.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. A comprehensive analysis of the binding and neutralizing antibody responses in a virus controller cohort that found that ADCC was higher in the virus controllers than in chronics. Among the binding antibody responses studied, anti-immunodominant and anti-MPER IgG1 and IgG2 were detected in this study, with IgG1-immunodominant antibodies found in lower concentrations in the virus controller group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trkola A, Purtscher M, Muster T, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines. 2006;5:347–363. doi: 10.1586/14760584.5.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lal RB, Heiba IM, Dhawan RR, et al. IgG subclass responses to human immunodeficiency virus-1 antigens: lack of IgG2 response to gp41 correlates with clinical manifestation of disease. Clin Immunol Immunopathol. 1991;58:267–277. doi: 10.1016/0090-1229(91)90141-v. [DOI] [PubMed] [Google Scholar]
- 38.Ngo-Giang-Huong N, Candotti D, Goubar A, et al. HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses. 2001;17:1435–1446. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- 39.Broliden PA, Morfeldt-Mansson L, Rosen J, et al. Fine specificity of IgG subclass response to group antigens in HIV-1-infected patients. Clin Exp Immunol. 1989;76:216–221. [PMC free article] [PubMed] [Google Scholar]
- 40.Scharf O, Golding H, King LR, et al. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol. 2001;75:6558–6565. doi: 10.1128/JVI.75.14.6558-6565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beniguel L, Begaud E, Peruchon S, et al. Isotype profiles of antigp160 antibodies from HIV-infected patients in plasma and culture supernatants. Immunol Lett. 2004;93:57–62. doi: 10.1016/j.imlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Kovacsovics-Bankowski M, Carini C, Kashala O, Essex M. Isotypic distribution of HIV-1-specific antibodies in individuals from central Africa. Viral Immunol. 1992;5:243–248. doi: 10.1089/vim.1992.5.243. [DOI] [PubMed] [Google Scholar]
- 43.Klasse PJ, Berntorp E, Hansson BG. An aberrant subclass pattern of HIV-specific immunoglobulin G in sera from haemophiliacs. AIDS. 1988;2:311–313. [PubMed] [Google Scholar]
- 44.Haynes BF, Shattock RJ. Critical issues in mucosal immunity for HIV-1 vaccine development. J Allergy Clin Immunol. 2008;122:3–9. doi: 10.1016/j.jaci.2008.03.036. quiz 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomsel M, Heyman M, Hocini H, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 46.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166:6257–6265. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 47.Battle-Miller K, Eby CA, Landay AL, et al. Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1-infected women. J Infect Dis. 2002;185:439–447. doi: 10.1086/338828. [DOI] [PubMed] [Google Scholar]
- 48.Rowland-Jones S. Dimers are a girl’s best friend. Nat Med. 1997;3:1199–1200. doi: 10.1038/nm1197-1199. [DOI] [PubMed] [Google Scholar]
- 49.Hirbod T, Broliden K, Kaul R. Genital immunoglobulin A and HIV-1 protection: virus neutralization versus specificity. AIDS. 2008;22:2401–2402. doi: 10.1097/QAD.0b013e328314e3a6. [DOI] [PubMed] [Google Scholar]
- 50•.Hirbod T, Kaul R, Reichard C, et al. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS. 2008;22:727–735. doi: 10.1097/QAD.0b013e3282f56b64. Several studies have reported potentially protective immune responses in highly exposed, persistently HIV-1-seronegative (HEPS) individuals. This recent study found neutralizing IgA antibodies in the lower genital tract that was associated with reduced rates of HIV-1 acquisition. Of note, these IgA antibodies were not HIV-1-specific and the specificity of these neutralizing IgA antibodies remains unknown. [DOI] [PubMed] [Google Scholar]
- 51.Horton RE, Ball TB, Wachichi C, et al. Cervical HIV-specific IgA in a population of commercial sex workers correlates with repeated exposure but not resistance to HIV. AIDS Res Hum Retroviruses. 2009;25:83–92. doi: 10.1089/aid.2008.0207. [DOI] [PubMed] [Google Scholar]
- 52.Granados-Gonzalez V, Piedrahita LD, Martinez M, et al. Neutralizing interclade cross-reactivity of HIV-1 V1/V2-specific immunoglobulin A in Colombian and French cohorts. AIDS. 2009:23. doi: 10.1097/qad.0b013e328329d134. in press. [DOI] [PubMed] [Google Scholar]
- 53.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 54.Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989;259:347–353. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 56.Stoiber H, Speth C, Dierich MP. Role of complement in the control of HIV dynamics and pathogenesis. Vaccine. 2003;21 (Suppl 2):S77–S82. doi: 10.1016/s0264-410x(03)00203-2. [DOI] [PubMed] [Google Scholar]
- 57••.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. A recent expert review on the mechanisms of B cell dysfunction in HIV-1 with accompanying time line of research highlights since 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forthal DN, Landucci G, Bream J, et al. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 59.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 60.Martinez V, Costagliola D, Bonduelle O, et al. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J Infect Dis. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 61.Toptygina AP, Pukhalsky AL, Alioshkin VA. Immunoglobulin G subclass profile of antimeasles response in vaccinated children and in adults with measles history. Clin Diagn Lab Immunol. 2005;12:845–847. doi: 10.1128/CDLI.12.7.845-847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lange JM, Coutinho RA, Krone WJ, et al. Distinct IgG recognition patterns during progression of subclinical and clinical infection with lymphadenopathy associated virus/human T lymphotropic virus. Br Med J (Clin Res Ed) 1986;292:228–230. doi: 10.1136/bmj.292.6515.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lange JM, de Wolf F, Goudsmit J. Markers for progression in HIV infection. AIDS. 1989;3 (Suppl 1):S153–160. doi: 10.1097/00002030-198901001-00023. [DOI] [PubMed] [Google Scholar]
- 64.Lange JM, Paul DA, Huisman HG, et al. Persistent HIV antigenaemia and decline of HIV core antibodies associated with transition to AIDS. Br Med J (Clin Res Ed) 1986;293:1459–1462. doi: 10.1136/bmj.293.6560.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheingsong-Popov R, Panagiotidi C, Bowcock S, et al. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infection. Br Med J. 1991;302:23–26. doi: 10.1136/bmj.302.6767.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber JN, Clapham PR, Weiss RA, et al. Human immunodeficiency virus infection in two cohorts of homosexual men: neutralising sera and association of antigag antibody with prognosis. Lancet. 1987;1:119–122. doi: 10.1016/s0140-6736(87)91964-7. [DOI] [PubMed] [Google Scholar]
- 67.Chargelegue D, Stanley CM, O’Toole CM, et al. The affinity of IgG antibodies to gag p24 and p17 in HIV-1-infected patients correlates with disease progression. Clin Exp Immunol. 1995;99:175–181. doi: 10.1111/j.1365-2249.1995.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forster SM, Osborne LM, Cheingsong-Popov R, et al. Decline of antip24 antibody precedes antigenaemia as correlate of prognosis in HIV-1 infection. AIDS. 1987;1:235–240. [PubMed] [Google Scholar]
- 69.Spira TJ, Kaplan JE, Holman RC, et al. Deterioration in immunologic status of human immunodeficiency virus (HIV)-infected homosexual men with lymphadenopathy: prognostic implications. J Clin Immunol. 1989;9:132–138. doi: 10.1007/BF00916941. [DOI] [PubMed] [Google Scholar]
- 70••.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. Using HIV-1 gp140 protein as bait, this study isolated HIV-1-neutralizing antibodies from HIV-1-specific memory B cells from the blood of HIV-1 elite controllers. Of interest to the work covered in this review, upon examination of the antibody repertoire, they found skewing toward kappa (κ) antibody light chain. [DOI] [PubMed] [Google Scholar]
- 71.Simek MD, Rida W, Priddy FH, et al. HIV-1 elite neutralizers: individuals with broad and potent neutralizing activity identified using a high throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009 doi: 10.1038/nm.1949.. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Scheid JF, Mouquet H, Feldhahn N, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


