Abstract
QT prolongation is the only clinically proven, yet insufficient, electrocardiogram (ECG) biomarker for drug-induced cardiac toxicity. The goal of this study is to evaluate whether JT area, i.e., total area of the T-wave, can serve as an ECG biomarker for drug-induced cardiac toxicity using both signal processing and computational modeling approaches. An ECG dataset that contained recordings from patients under control and sotalol condition was analyzed. In order to relate sotalol-induced ECG changes to its effect on ion channel level, i.e., blockade of the rapid component of the delayed rectifier potassium channel (IKr), varied degrees of IKr blockade were simulated in a slab of ventricular tissue. The mean JT area increased by 36.5% following the administration of sotalol in patients. Simulations in the slab tissue showed that sotalol increased action potential duration preferentially in the midmyocardium, which led to increased transmural dispersion of repolarization and JT area. In conclusion, JT area reflects the transmural dispersion of repolarization and may be a potentially useful surrogate/supplemental ECG biomarker to assess drug safety.
I. Introduction
Cardiac toxicity, particularly proarrhythmic effects, is a major safety concern in the development of both a antiarrhythmic and non-antiarrhythmic drugs. Currently, QT prolongation is the only clinically proven electrocardiogram (ECG) biomarker for drug safety. It is, however, well recognized that QT prolongation alone is inadequate for assessment of drug-induced cardiac toxicity due to the poor correlation between QT prolongation and occurrence of arrhythmias [1]. A large body of research has been dedicated to the exploration of new biomarkers of drug-induced cardiac toxicity (see reviews in [2]). The TRIaD concept has been proposed to emphasize the importance of combined roles of action potential triangulation (T), reverse use dependence of the drug (R), beat-to-beat instability (I) and spatial dispersion of depolarization (D) [3, 4]. It suggests that QT prolongation in the presence of TRIaD preferentially leads to arrhythmias, while QT prolongation without TRIaD may be antiarrhythmic. Thus, new ECG biomarkers obtained by extracting TRIaD from ECG, either alone or combined with QT interval, may provide a better drug safety assessment than QT interval alone.
JT area, i.e., total area of the T-wave, has been shown to be an indicator of temporal and spatial dispersion of repolarization as administration of class III antiarrhythmic drugs (drugs that block potassium channels) increased both JT area and dispersion of repolarization in dogs [5]. The goal of this study is to evaluate the predictive value of JT area as a surrogate/supplemental ECG biomarker for drug-induced toxicity using both signal processing and computational modeling approaches. Specifically, on one hand, ECG signal processing algorithms were developed to analyze changes in JT area induced by sotalol (a class III antiarrhythmic drug) in patients; on the other hand, a multiscale computational modeling study was conducted in order to interpret and evaluate sotalol-induced changes in ECG in terms of the underlying sotalol-induced changes at the ion channel level, i.e., blockade of the rapid component of the delayed rectifier potassium channel (IKr).
II. Methods
A. Signal Processing
The ECG dataset that was employed was obtained from the THEW database (http://thew-project.org/). This dataset contained surface 12-lead ECG recordings from 16 patients (with a history of drug-induced Torsades de pointes) pre and post injection of sotalol. The sampling frequency and resolution were of 1 kHz and 5 μV respectively (see detailed descriptions in [6]). All analyses reported in this study were performed on the lead II ECG. Specifically, ECG baseline wander was eliminated using cubic spline technique. Wave delineation, i.e., identification of fiducial points (peaks and limits for individual P, QRS and T waves), was performed via quadratic spline wavelet transform of the ECG signal, the performance of which has been shown to be accurate and reliable [7].
For each patient, a 30-consecutive beat interval free of ectopic beats was manually selected under control and sotalol condition, respectively. QT interval was measured as the interval between the onset of QRS wave and the end of the T-wave. The heart rate-corrected QT interval, QTc, was calculated using Bazett’s formula. JT area of each beat was computed as the area between the curve and the baseline from J-point to the end point of the T-wave [5]. The baseline referred to the isoelectric segment preceding QRS complex. J-point was defined as 10 ms following the end point of QRS complex. Short-term variability (STV) of JT area was calculated as the mean orthogonal distance from each point in the Poincaré plot to the diagonal (STV = Σ|Dn+1−Dn|/[30×√2], where D is the JT area) [8]. Results were reported as mean±SD. Paired Student’s t-test was used for the statistical comparison of the means between the control and sotalol condition. A P-value less than 0.05 was considered significant.
B. Computational Modeling
The Chaste simulator (http://web.comlab.ox.ac.uk/chaste/cardiac_index.html) was used to investigate the effect of sotalol, i.e., IKr blockade, on pseudo-ECG in a slab of ventricular tissue. The cubic slab was of 0.45 cm edge length and consisted of three transmural layers, i.e., epicardial (0.11 cm), midmyocardial (0.17 cm) and endocardial (0.17 cm) layers [9]. The electrical activity of the slab was simulated using the bidomain model [10]. The bidomain model represents the cardiac tissue with both intracellular and extracellular continua, separated by the membrane. The intracellular potential (Φi, mV) and extracellular potential (Φe, mV) satisfy the following equations:
| (1) |
| (2) |
where Im and Istim are the volume density of the transmembrane current and stimulus respectively (μA/cm3); Ĝi and Ĝe are the intracellular and extracellular conductivity tensor respectively (mS/cm).
Membrane kinetics was represented by the Mahajan-Shiferaw rabbit ventricular model [11]. Transmural heterogeneities in the slow component of the delayed rectifier potassium channel (IKs) and the transient outward current (Ito) were simulated as in previous studies [12]. Homogeneous IKr blockade was simulated by decreasing the maximum conductance of IKr from its control value to 0% in steps of 20% throughout the slab.
To ensure steady-state propagation, the slab was paced from the entire endocardial surface at a basic cycle length of 300 ms. Action potentials (APs) and pseudo-ECG during the last pacing beat were analyzed. Transmural dispersion of repolarization (TDR) was measured as the maximum difference in AP duration (APD) from any two ventricular sites. Extracellular unipolar potential (pseudo-ECG) was recorded from the center of the outmost epicardial layer. QT interval was measured as the interval between the peaks of the Q-wave and the T-wave. JT area was calculated by integrating pseudo-ECG above the isoelectric line over the JT interval.
The computational mesh contained 162,000 tetrahedral elements. Simulations were run with 5-μs time steps on a 4-processor computer (AMD Phenom(tm) 9600B Quad-core Processor 1.15 GHz 3.9 GB RAM).
III. Results
A. Signal Processing
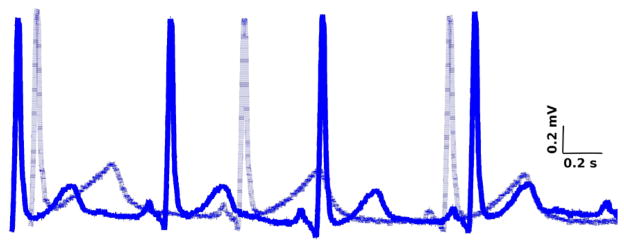
Fig. 1 illustrates representative traces of lead II-ECG recorded in a patient before (solid line) and after (dashed line) administration of sotalol. It is clear that sotalol increased JT area dramatically.
Fig. 1.
Superimposition of representative traces of lead II-ECG before (solid line) and after (dashed line) sotalol injection.
Statistical analysis (Table 1) showed that sotalol led to significant increase in RR interval, QT interval, QTc interval, JT area and STV of JT area. Their mean values increased by 24.5%, 26.6%, 13.2%, 36.5% and 54.8%, respectively following the administration of sotalol.
TABLE I.
Ecg Analysis for patients under control and sotalol condition
| Control | Sotalol | P-value | |
|---|---|---|---|
| RR Interval (ms) | 856.6±155.4 | 1066.9±143.4 | 1.6e-5 |
| QT Interval (ms) | 404.7±39.1 | 512.2±35.0 | 1.6e-7 |
| QTc Interval (ms)* | 432.6±28.3 | 489.7±32.0 | 2.9e-7 |
| JT Area (mV•ms) | 31.5±18.8 | 43.0±30.7 | 0.04 |
| STVJT (mV•ms)* | 3.1±2.3 | 4.8±2.6 | 0.008 |
QTc = heart rate-corrected QT interval; STVJT = short-term variability of JT area.
B. Computational Modeling
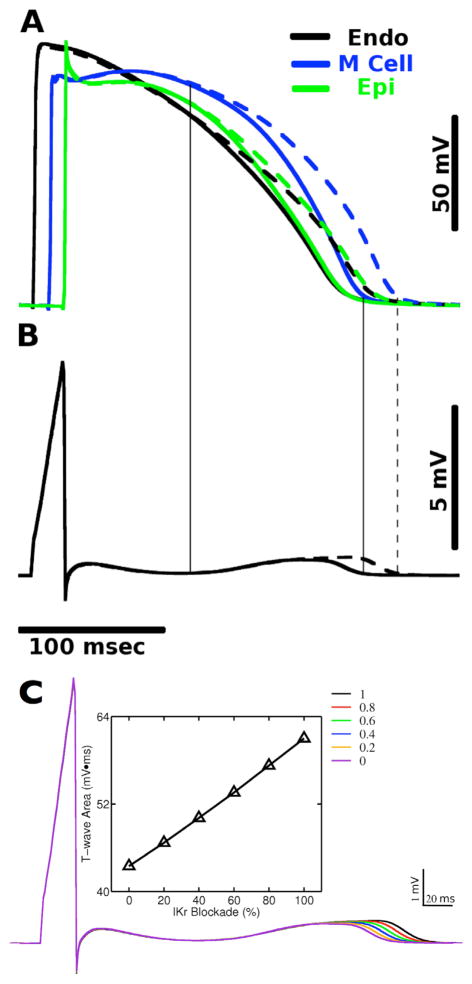
Fig. 2 shows the time course of the AP from a representative site in each transmural layer (Fig. 2A) and also the pseudo-ECG (Fig. 2B) for control (solid lines) and 100% IKr blockade (dashed lines) condition, respectively. Complete IKr blockade led to APD prolongation by 19, 23, and 19 ms in epicardial, midmyocardial and endocardial layers, respectively. Vertical lines indicate that for both conditions, the onset of the T-wave corresponds to the time when AP in the midmyocardial layer starts to deviate from that in the epicardial and endocardial layer, and the end of the T-wave corresponds to the time when APs in all three layers returns to the rest state. These findings are consistent with experiments on dog ventricular wedge preparations [13].
Fig. 2.
Action potentials (AP) from a representative node of each transmural layer (A) and pseudo-ECG (B) for control (solid lines) and 100% IKr blockade (dashed lines) condition, respectively. (C) Pseudo-ECG and total t-wave area for 0%, 20%, 40%, 60% and 100% IKr blockade respectively.
Fig. 2C presents changes in JT area with varying degrees of IKr blockade. As the degree of IKr blockade increased from 0% to 100%, JT area increased almost linearly by 38.0%, accompanied with a gradual increase in TDR by 17.0% and a gradual increase in QT interval by 20.0%.
IV. DISCUSSION
This combined signal processing and computational modeling study demonstrated that sotalol increased APD preferentially in the midmyocardium, and consequently led to increased transmural dispersion of repolarization and JT area. JT area appeared to be a potential ECG biomarker for better predicting and understanding drug-induced cardiac toxicity.
Torsades de pointes (TdP) is a polymorphic ventricular tachycardia that can potentially degenerate into life-threatening ventricular fibrillation. QT prolongation is one major characteristics of TdP. But drug-induced QT prolongation, even to a significant extent, might not lead to occurrence of TdP [3, 8]. As a matter of fact, in our study, although the mean QTc interval was 489.7 ms following sotalol injection, which is above the established cut-off values (450 ms for men and 460 ms for women [14]), no episodes of TdP were observed among all patients. This, together with existing studies, suggests the necessity to identify a new biomarker for drug toxicity instead of using QT prolongation alone.
Our ECG signal analysis showed that sotalol resulted in significant increase in JT area, the mean value of which increased by 36.5% when compared with control. This is consistent with the experimental study in dog ventricles where the mean JT area increased by 90.0% following administration of class III antiarrhythmic drugs [5]. Furthermore, in our results, the percentage increase in the mean JT area was nearly three times greater than that in mean QTc interval, suggesting a better sensitivity to drug-induced changes in JT area. It is noted that our analysis was performed on a single lead. Studies have shown that the accuracy and sensitivity to drug-induced QT prolongation varied when using different ECG leads [15]. Thus the optimal lead for detecting changes in JT area may be determined by repeating the same analysis on other leads.
Our computational modeling study demonstrated the preferential prolongation of APD in the midmyocardium following IKr blockade, consistent with the findings in dog ventricular wedge preparations [13]. Such heterogeneous changes in transmural distribution of APD led to dispersion of repolarization and thus increased JT area. Specifically, with 100% IKr blockade, the prolongation of APD in midmyocardium was 4 ms more than that in both epicardium and endocardium. The corresponding increase in JT area was 38.0% comparing to the control condition. This confirmed the high sensitivity in JT area as discussed above.
In summary, JT area has the potential to serve as a surrogate or supplemental ECG biomarker other than QT prolongation to assess drug safety. Further evaluation is needed in order to establish the predictive value of JT area. A combined signal processing and computational modeling study as conducted here provides a unique way in the search for new biomarker of proarrhythmic risk. Successful identification of such biomarkers can help prevent abandonment of potentially useful drug compounds.
Limitations
The ECG dataset examined in this study was collected from a limited number of patients and only involved one type of drugs. Future studies with a larger sample size and more varieties of drug compounds, such as classes I and IV antiarrhythmic drugs that block sodium and calcium channels respectively, are feasible and will help establish and define the predictive value of JT area. Additionally, in this study, sotalol-induced increase in QTc was more important (i.e., has a smaller P-value) than that in JT area. Further investigations of the predictive value of JT area for drugs that do not have a significant QT prolongation effect are currently undertaken in our group. As to computational modeling, the ionic model used in this study was the rabbit model, while the most appropriate membrane model to use in our study would have been a human model for obvious reasons. Variations in JT area due to species-related differences could quantitatively change the results. However, the mechanisms uncovered in this study would remain the same.
Acknowledgments
The authors would like to thank the Chaste team for the technical help in using Chaste.
This work was supported by the preDiCT project, which is funded by the European Commission under Grant FP7-2008-IST. Blanca Rodriguez holds a MRC Career Development Award.
Contributor Information
Xiao Jie, Email: xiao.jie@comlab.ox.ac.uk, Computing Laboratory, Oxford University, Oxford OX1 3QD, UK (phone: +44 (0)1865-610807; fax: +44 (0)1865-280300.
Blanca Rodriguez, Email: blanca@comlab.ox.ac.uk, Computing Laboratory, Oxford University, Oxford OX1 3QD, UK (phone: +44 (0)1865-610807; fax: +44 (0)1865-280300.
Esther Pueyo, Email: epueyo@unizar.es, Department of Electronic Engineering and Communications, University of Zaragoza, Zaragoza 50118, Spain.
References
- 1.Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Thomsen MB, Matz J, Volders PG, Vos MA. Assessing the proarrhythmic potential of drugs: current status of models and surrogate parameters of torsades de pointes arrhythmias. Pharmacology and Therapeutics. 2006 Oct;112(1):150–170. doi: 10.1016/j.pharmthera.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103(15):2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 4.Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. Journal of Cardiovascular Electrophysiology. 2006 Mar;17(3):337–340. doi: 10.1111/j.1540-8167.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 5.van Opstal JM, Verduyn SC, Winckels SK, Leerssen HM, Leunissen JD, Wellens HJ, et al. The JT-area indicates dispersion of repolarization in dogs with atrioventricular block. Journal of Interventional Cardiac Electrophysiology. 2002 Jun;6(2):113–120. doi: 10.1023/a:1015302415323. [DOI] [PubMed] [Google Scholar]
- 6.Kaab S, Hinterseer M, Nabauer M, Steinbeck G. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome--a case-control pilot study using i.v. sotalol. Eur Heart J. 2003;24(7):649–657. doi: 10.1016/s0195-668x(02)00806-0. [DOI] [PubMed] [Google Scholar]
- 7.Martínez JP, Almeida R, Olmos S, Rocha AP, Laguna P. A wavelet-based ECG delineator: evaluation on standard databases. IEEE Trans Biomed Eng. 2004;51(4):570–581. doi: 10.1109/TBME.2003.821031. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004 Oct 19;110(16):2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- 9.Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch AD. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circulation Research. 2004;95(12):1216–1224. doi: 10.1161/01.RES.0000150055.06226.4e. [DOI] [PubMed] [Google Scholar]
- 10.Henriquez CS. Simulating the electrical behavior of cardiac tissue using the bidomain model. Critical Review in Biomedical Engineering. 1993;21:1–77. [PubMed] [Google Scholar]
- 11.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, et al. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophysical Journal. 2008;94(2):392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh MA, Cobbe SM, Smith GL. Heterogeneous changes in action potential and intracellular Ca2+ in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovascular Research. 2000;45(2):397–409. doi: 10.1016/s0008-6363(99)00360-0. [DOI] [PubMed] [Google Scholar]
- 13.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998 Nov 3;98(18):1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 14.Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80:1301–1308. doi: 10.1161/01.cir.80.5.1301. [DOI] [PubMed] [Google Scholar]
- 15.Sadanaga T, Sadanaga F, Yao H, Fujishima M. An evaluation of ECG leads used to assess QT prolongation. Cardiology. 2006;105(3):149–154. doi: 10.1159/000091227. [DOI] [PubMed] [Google Scholar]