Abstract
This study aimed to determine whether a 2-week genistein treatment induced estrogen-like effects in ovariectomized (OVX) Sprague-Dawley rats, after 2 weeks of subcutaneous genistein injections (250 mg/kg of body weight/day). Uterine weight, uterine-to-body weight ratio, femur weight, and femur-to-body weight ratio were all significantly increased with genistein in OVX rats. Body weight was significantly decreased with genistein in OVX rats. Genistein had no effect on the weights of heart, heart-to-body ratio, and fat pad but significantly decreased heart rate and pulse pressure. Genistein had no effect on cardiac GLUT4 protein, oxidative stress, plasma glucose, nonesterified fatty acids, or low-density lipoprotein levels; however, plasma insulin levels were significantly increased. Our results show that a 2-week genistein treatment produced favorable estrogen-like effects on some physical and physiological characteristics in OVX rats. However, based on our experimental conditions, the effects of genistein were not associated with changes in cardiac GLUT4 or oxidative stress.
Key Words: cardiovascular, estrogen alternatives, postmenopausal animal model, soy products
Introduction
Loss of the purported cardioprotective effects of estrogen with onset of menopause is hypothesized to be responsible for the increased incidence of cardiovascular disease and associated elevated morbidity rates in postmenopausal women compared to their premenopausal counterparts.1 Genistein, a phytoestrogen, binds to estrogen receptors, thereby exerting estrogenic effects, and has therefore been proposed as a natural alternative to estrogen replacement.2 Evidence suggests that genistein exhibits a potency similar to that of 17β-estradiol (each administered at 100 μg/kg i.v.) to exert pharmacological postconditioning in a rabbit model of coronary artery occlusion, acting via an estrogen receptor-dependent mechanism, utilizing the phosphatidylinositol 3-kinase/Akt pathway.3 However, unlike the use of estrogen-based hormone therapy, which may increase the risk of coronary artery disease4,5 and uterine cancer,6 genistein therapy has the noted benefit of improving cardiovascular health with contradictory and unequivocal effects on the female reproductive system7 or cancer induction.8
One of the more notable cardiovascular-related effects of genistein is a lowering of blood pressure observed in both hypertensive and normotensive postmenopausal women9,10 or rat models.11 This beneficial effect is purported to be mediated via improvements in endothelial function and reductions in both antioxidant stress in aorta and arterial stiffness.12 Moreover, genistein treatment in postmenopausal women was shown to increase flow-mediated endothelium vasodilation, likely by increasing the nitric oxide to endothelin ratio,11,13–15 and has a beneficial effect on the lipid profile.16 As genistein is pleiotropic in nature, genistein therapy has been associated with improved bone health in postmenopausal women and ovariectomized (OVX) mice.17–20 Furthermore, recent evidence suggests that genistein treatment reduces fat weight and fasting blood glucose and lipid levels in a mouse model of diabetes and has a positive effect on plasma lipid profiles in clinical studies and thus may help prevent obesity-associated diseases.21,22 In addition, we recently demonstrated that genistein exerts an anti-ischemic effect in the hearts of OVX rats following a 2-day treatment period with the same concentration of genistein (250 mg/kg/day) and same route of administration (daily subcutaneous injections) as is used in the current study.23
In view of the aforementioned previously reported protective effects of genistein on cardiovascular functions/systems, the purpose of this study was to better understand genistein's mechanism of action, more specifically in terms of its estrogen-like effects. We examined the effects of a 2-week treatment period of genistein (250 mg/kg/day) on physical and several physiological characteristics of OVX rats, measuring products of oxidative stress, cardiac GLUT4 protein expression, and various cardiovascular plasma markers. We predicted that this study would help explain our previously reported anti-ischemic effects of genistein following a 2-day treatment.
Materials and Methods
Animals and treatment protocol
All animals used in the experiments were female OVX Sprague-Dawley rats, with an initial weight of 250–300 g (Harlan, Indianapolis, IN, USA). OVX rats were randomly assigned to either the control group (n = 8) or the genistein-treated group (n = 8). The control group consisted of OVX rats injected with dimethyl sulfoxide (200 μL s.c.) for 2 weeks. We chose this group as the control to mimic the clinical setting of postmenopause, confirmed by a significant reduction in uterine weight (0.589 ± 0.074 vs. 0.097 ± 0.009 g [n = 8], P < .001). OVX-treated animals received a single daily subcutaneous genistein injection (250 mg/kg, dissolved in 200 μL of dimethyl sulfoxide [LC Laboratories, Woburn, MA, USA], >99% purity) for a 2-week period. Animals were monitored daily for the presence of sores and signs of stress (i.e., changes in coat appearance, lethargy, failure to thrive as indicated by weight loss). Animals were housed two per cage, with a 12:12-hour light–dark cycle and given food and water ad libitum. Throughout the study rats were fed casein-based powdered diets (prepared by Dr. R.S. MacDonald, Department of Food Science and Human Nutrition, Iowa State University, Ames, IA, USA) and were genistein-free, with an estimated energy content of 16.28 kJ/g.24 All animals used in this study were cared for in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.25 This study was approved by the Midwestern University Institutional Animal Care and Use Committee.
Harvest of tissues
Following treatment, rats were gassed with CO2 in a sealed container, followed by decapitation. Immediately prior to euthanasia, animals were weighed. Immediately after euthanasia, the heart, uterus, femur, and abdominal fat pads were removed, cleaned of extraneous tissue, and weighed. Hearts were stored in liquid nitrogen at −80°C.
Measurement of blood pressure
Systolic and diastolic blood pressure, mean arterial pressure, and heart rate were measured from restrained rats using a pneumatic tail cuff device (NIBP-8, Columbus Instruments, Columbus, OH, USA). Readings were taken before treatment (day 0) and following the 2-week treatment protocol (day 14). Before the cuff was inflated, approximately 1.5 mL of blood was removed from the tail vein using a 25¾-g Vacutainer® (BD, Franklin Lakes, NJ, USA) blood collection set. The blood was placed in heparinized tubes and immediately spun at 3,000 rpm (Eppendorf Mini Spin, Hamburg, Germany), and the plasma was separated and stored at −80°C for later assay.
Measurement of plasma nonesterified fatty acids, low-density lipoproteins, glucose, and insulin
Measurements of plasma nonesterified fatty acids (NEFAs) and glucose were determined by performing the Wako NEFA-HR(2) and Wako Glucose C2 96-well assays, respectively (Wako Diagnostics, Richmond, VA, USA). Plasma insulin was measured using a 96-well assay from Alpco (Salem, NH, USA). Plasma low-density lipoprotein (LDL) was measured using a 96-well assay from BioVision (Mountain View, CA, USA).
Measurement of cardiac GLUT4 protein expression
Standard western blot techniques were used to detect GLUT4 protein (∼40 kDa). Protein concentrations of heart tissues were determined (Pierce, Thermo Scientific, Rockford, IL, USA). In brief, 25 μL of sample was loaded on 10% bis-Tris gels and electrophoresed at 150 V for approximately 120 minutes. Gels were transferred at 100 V for approximately 40 minutes (4°C). Gels were incubated with primary antibody (1:2,500 diluted anti-GLUT4 from rabbit [Calbiochem, Gibbstown, NJ, USA]) overnight at 4°C in 3% milk in phosphate-buffered saline plus 0.1% Tween 20 (PBST). Gels were then incubated with secondary antibody (1:1,500 diluted anti-rabbit immunoglobulin G [Amersham Biosciences, Piscataway, NJ, USA]) in 3% milk in PBST for 1 hour at room temperature. GLUT4 protein was detected with enhanced chemiluminescent substrate (Amersham Biosciences). Membranes were washed with PBST to remove enhanced chemiluminescent substrate and incubated in Restore™ western blot stripping buffer (Pierce, Thermo Scientific) for 5–15 minutes at room temperature. The ECL Plus western blotting detection system (Amersham Biosciences) was used to verify removal of antibodies. Cleaned membranes were incubated with primary antibody (1:2,000 diluted anti-actin [Millipore, Temecula, CA, USA]) at room temperature for 2 hours in 5% milk in PBST. Membranes were incubated with secondary antibody (1:10,000 diluted goat anti-mouse horseradish peroxidase-conjugated immunoglobulin G [Millipore]). The ECL Plus detection system was used to detect actin at 43 kDa, and GLUT4 protein was normalized to the actin.
Determination of nonprotein thiols and thiobarbituric acid-reactive substances
Pulverized samples of frozen heart tissue were homogenized in 10 volumes of cold phosphate buffer (pH 6.8). A portion (0.4 mL) of each homogenate was drawn off and assayed for thiobarbituric acid-reactive substances (TBARS) by the method of Ohkawa et al.26 using 1,1,3,3-tetramethoxypropane as an external standard. Results were reported as nmol of 1,1,3,3-tetramethoxypropane equivalents/g of tissue. For the determination of reduced and oxidized glutathione (GSH and GSSG), the remaining portions of the original homogenates were deproteinized by adding trichloroacetic acid to a final concentration of 5%, incubating at 0°C for 1 hour, and centrifuging at 3,000 g for 10 minutes at 4°C. Aliquots of the deproteinized samples were drawn off, neutralized, and assayed for GSH and GSSG using O-phthalaldehyde according to the method of Senft et al.27 Reduced glutathione (GSH) was used as a standard.
Statistical analysis
Statistical analyses (paired and unpaired t test) performed using Graphpad Prism version 4 (Graphpad Software Inc., San Diego, CA, USA). All results are reported as mean ± SEM values. P < .05 was considered statistically significant.
Results
Effect of genistein on physical characteristics and plasma metabolites
The effect of genistein treatment on the physical characteristics of OVX rats is shown in Table 1. Heart weight, heart-to-body weight ratio, and fat pad weight remained unchanged following the 2-week treatment protocol. Uterine weight and uterine-to-body weight ratio both significantly increased (both P < .0001). Interestingly, femur weight was significantly increased in genistein-treated rats (by approximately 0.08 g, P < .05). Femur-to-body weight ratio was also significantly increased in the genistein-treated group (P < .05). However, the genistein-treated rats weighed significantly less (approximately 14 g, P < .05); thus this increased femur weight is likely not attributed to a concomitant increase in body mass and is therefore likely attributable to the genistein treatment.
Table 1.
Effects of a 2-Week Genistein Treatment Period on Physical Characteristics of Ovariectomized Rats
| Physical characteristic | Control (n = 8) | Genistein (n = 8) |
|---|---|---|
| Body weight (g) | 235.75 ± 3.94 | 221.25 ± 5.30* |
| Uterine weight (mg) | 97.14 ± 8.73 | 347.5 ± 42.37* |
| Uterine-to-body weight ratio | 0.41 ± 0.03 | 1.56 ± 0.02* |
| Heart weight (g) | 0.88 ± 0.02 | 0.95 ± 0.04 |
| Heart-to-body weight ratio | 3.73 ± 0.79 | 4.30 ± 0.12 |
| Femur weight (g) | 0.61 ± 0.03 | 0.69 ± 0.03* |
| Femur-to-body weight ratio | 0.0026 ± 0.00012 | 0.0031 ± 0.00008* |
| Fat pad weight (g) | 1.10 ± 0.21 | 1.11 ± 0.11 |
Data are mean ± SEM values.
P < .05 compared to dimethyl sulfoxide-treated (control) rats.
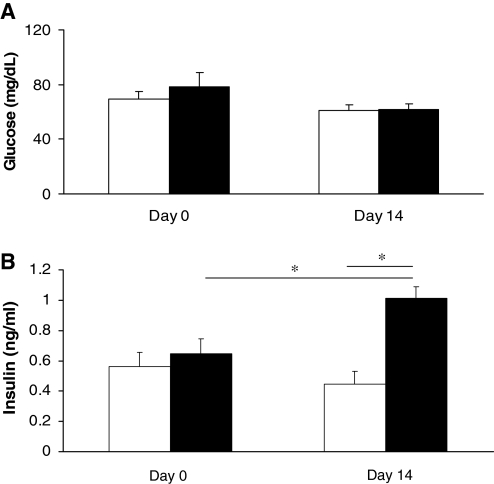
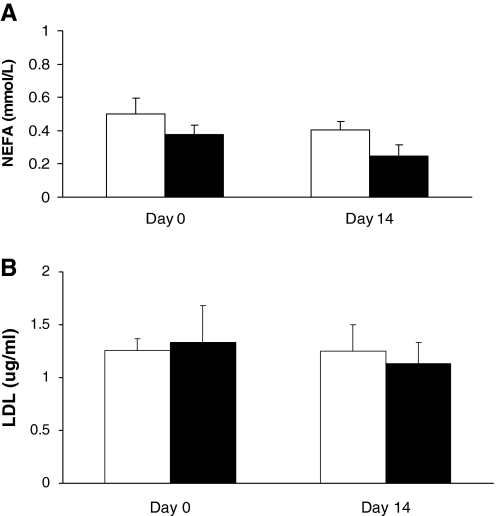
Assessment of plasma glucose levels showed no change in value after the 2-week treatment duration (P = .1226, Fig. 1A). Plasma insulin was significantly increased with the 2-week genistein treatment from 0.645 ± 0.102 to 1.013 ± 0.074 ng/mL (P < .05) and was significantly increased compared to dimethyl sulfoxide controls (1.013 ± 0.074 ng/mL and 0.447 ± 0.086 ng/mL respectively, P < .05) at the end of the 2-week study (Fig. 1B). Measurements of plasma NEFA (Fig. 2A) and LDL (Fig. 2B) levels showed no significant changes after the 2-week genistein treatment compared to controls (P = .0669 and P =.6987, respectively).
FIG. 1.
Effect of genistein treatment on plasma (A) glucose (in mg/dL) and (B) insulin (in ng/mL) levels. Data are mean ± SEM values (n = 5 and 6 for glucose and insulin, respectively). *P < .05. The dimethyl sulfoxide-treated control group is represented by open columns, and the genistein-treated group is indicated by the solid columns.
FIG. 2.
Effect of genistein treatment on plasma (A) nonesterified fatty acid (NEFA) (in mmol/L) and (B) low-density lipoprotein (LDL) (in μg/mL) levels. Data are mean ± SEM values (n = 8 for NEFA and 5 for LDL, respectively). The dimethyl sulfoxide-treated control group is represented by open columns, and the genistein-treated group is indicated by the solid columns.
Effect of genistein on in vivo blood pressure
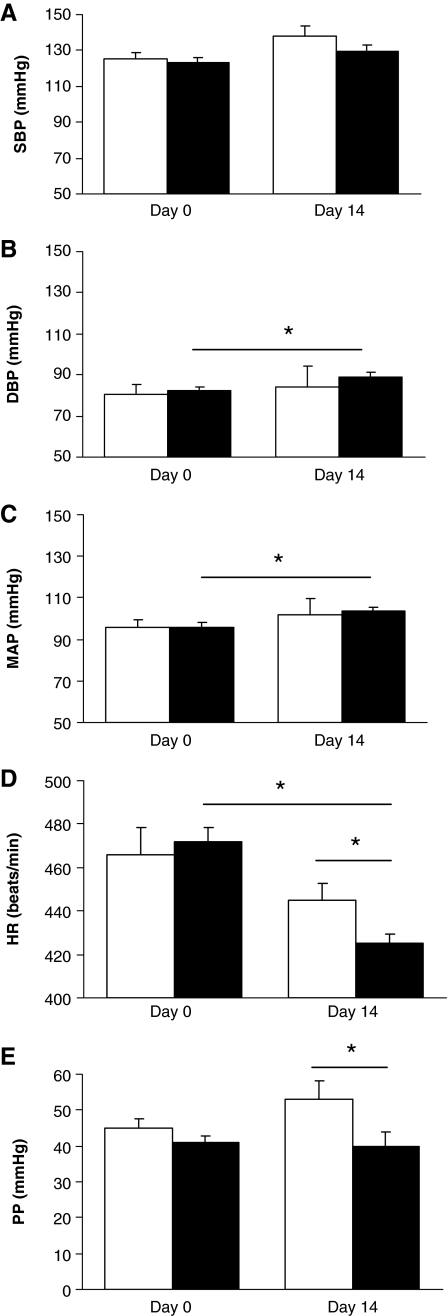
Figure 3 illustrates the effects of genistein on blood pressure. The 2-week genistein-treated group had a significant increase in diastolic blood pressure compared to the start of the study at day 0 (from 82.3 ± 2.1 mm Hg to 89.2 ± 2.1 mm Hg, P = .0421). In addition, the 2-week genistein-treated group had a significant increase in mean arterial pressure compared to the start of the study at day 0 (from 96.0 ± 2.1 mm Hg to 103.7 ± 1.7 mm Hg, P = .0165). However, compared to the control group at 2 weeks, there was no effect of genistein treatment on systolic, diastolic, and mean arterial blood pressure.
FIG. 3.
Effect of genistein treatment on blood pressures and heart rate (HR) in ovariectomized rats: (A) systolic blood pressure (SBP) (in mm Hg), (B) diastolic blood pressure (DBP) (in mm Hg), (C) mean arterial pressure (MAP) (in mm Hg), (D) HR (in beats/minute), and (E) pulse pressure (PP) (in mm Hg). Data are mean ± SEM values (n = 6). *P < .05. The dimethyl sulfoxide-treated control group is represented by open columns, and the genistein-treated group is indicated by the solid columns.
The 2-week genistein-treated group had a significant decrease in heart rate compared to the control group (444.7 ± 8.2 beats/minute and 425.0 ± 4.6 beats/minute, respectively, P = .0491). The 2-week genistein-treated group had a significantly decreased pulse pressure compared to the control group (53.0 ± 5.0 mm Hg and 40.0 ± 3.8 mm Hg, respectively, P = .0494).
Effect of genistein on cardiac GLUT4 expression, glutathione, and TBARS
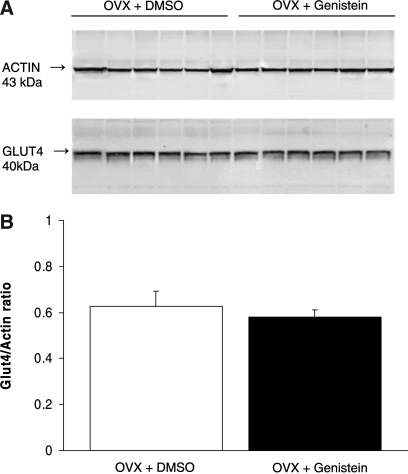
Figure 4 shows no significant change (P = .4883) in cardiac GLUT4/actin ratio measured using western blot analysis in rats treated with genistein for 2 weeks compared to dimethyl sulfoxide controls (n = 6 for either dimethyl sulfoxide only- or genistein-treated groups, Fig. 4). Table 2 shows there was also no effect of genistein on the cardiac oxidative stress as measured by the nonprotein thiols and TBARS levels.
FIG. 4.
(A) Western blots obtained from each rat heart in each group. (B) Effect of genistein treatment on cardiac GLUT4 expression in ovariectomized (OVX) rats. Data are mean ± SEM values (n = 6). DMSO, dimethyl sulfoxide.
Table 2.
Effects of a 2-Week Genistein Treatment Period on Cardiac Nonprotein Thiols and Thiobarbituric Acid-Reactive Substances Content
| Parameter | DMSO | Genistein |
|---|---|---|
| Nonprotein thiols | ||
| nmol of GSH equivalents/g of tissue | 2,023 ± 28 | 2,118 ± 108 |
| nmol of GSSG equivalents/g of tissue | 500 ± 57 | 499 ± 36 |
| GSH/GSSG (reduced/ oxidized) ratio | 4.24 ± 0.42 | 4.07 ± 0.28 |
| TBARS (nmol of TMP equivalents/g of tissue) | 250 ± 46 | 258 ± 32 |
Data are mean ± SEM values (n = 6–8).
GSH, reduced glutathione; GSSG, oxidized glutathione; TBARS, thiobarbituric acid-reactive substances; TMP, 1,1,3,3-tetramethoxypropane.
Discussion
Genistein, a naturally occurring phytoestrogen, has repeatedly been described as an alternative to estrogen, as exemplified in a recent study demonstrating that genistein exerted a similar potency to 17β-estradiol via an estrogen receptor-dependent mechanism, utilizing the phosphatidylinositol 3-kinase/Akt pathway.3 This study aimed to follow up on our previous work demonstrating that in OVX rats, 2 days of subcutaneous injections with genistein improved ischemic tolerance (i.e., contractility and cardiac output improved to ∼75% and ∼40% of the measured preischemic function).23 In this study, the use of a more chronic application of genistein (2 weeks vs. 2 days) would better represent physiologically the responses that would likely ensue from taking such daily supplements.
We predicted that genistein, administered via subcutaneous injections over a 2-week period, would have estrogen-like effects on the OVX rats. A major strength of this study is the ability to eliminate common cardiovascular risk factors as contenders for genistein's mechanism of action, in addition to monitoring genistein's effect over a longer time period. Of the physical characteristics we measured, only uterine weight, uterine-to-body weight ratio, femur weight, and femur-to-body weight ratio were significantly increased following the 2-week treatment with genistein. Our observed effect of genistein on uterine weight is consistent with previous reports in the literature. For example, Rimoldi et al.28 showed in OVX rats that a 3-month oral regimen of genistein (54 mg/kg/day) significantly increased uterine weight. Our observed increase in femur weight was also in accord with previous studies demonstrating that genistein (administered for either 3 or 7 weeks) increased bone formation in OVX rats.29
On the other hand, some of our data were in stark contradiction with previously published evidence. A 3-week exposure to dietary genistein (1,500 mg/kg of food) has been shown to successfully decrease body fat in older OVX mice.30 In contrast, we observed no effect of genistein on fat pad weight after a 2-week duration. This could perhaps be explained by the following differences in our studies: the dose of genistein that we used was lower (250 mg/kg vs. 1,500 mg/kg), the route of administration was different (subcutaneous injection vs. oral), our duration of exposure to genistein was shorter (2 weeks vs. 3 weeks), and, lastly, we used rats versus mice. A reduction in fat pad weight by genistein has been attributed to an increase in lipolysis and adipocyte apoptosis.30
Osteoporosis is a widely known phenomenon occurring in the estrogen-depleted state, i.e., following ovariectomy or postmenopause. The effects of bone loss in OVX mice have been shown to be reversed by a 4-week treatment of either 0.7 mg/day genistein or 0.03 μg/day 17β-estradiol administered via daily subcutaneous injections.17 In addition, genistein (54 mg/day in the form of two oral tablets for 24 months) has been shown to increase bone mineral density in osteopenic postmenopausal women.20 These results are consistent with our observed increase (∼0.08 g) in femur weight with the 2-week genistein treatment; however, we report this effect with a shorter treatment duration.
Evidence in the literature suggests that genistein typically decreases blood pressure.31 Mahn et al.11 showed that male rats fed a soy-enriched diet for up to 10 months had significantly lowered systolic and diastolic blood pressures. Similarly, Li et al.32 showed that genistein treatment (0.4 mg/kg/day for 21 days) decreased mean arterial pressure in OVX rats. Furthermore, a 6-week dietary genistein regimen (2.0 g/kg of diet) has been shown to decrease systolic blood pressure in male spontaneously hypertensive rats.33 Interestingly, there is also evidence in the literature to suggest that soy can exert hypertensive actions; a clinical study using hypertensive male and female subjects concluded that a 3-month dietary soy supplementation did not lower blood pressure, but rather marginally increased blood pressure.10 We observed no change in systolic, diastolic, and mean arterial pressure, comparing genistein-treated and control groups at day 14. The 2-week genistein treatment significantly decreased heart rate and pulse pressure compared to controls at day 14.
Several studies have documented the effects of genistein on glucose uptake and metabolism in tissues. For instance, in the adipocyte, genistein treatment has been associated with a decrease in glucose uptake and oxidation.34–36 In contrast, in skeletal muscle and L6 myotubes, increased glucose uptake was reported.37 Genistein may increase glucose uptake in muscle cells through an up-regulation of the insulin-dependent translocation of the glucose transporter, GLUT4, into the plasma membrane, as well as by increased insulin-independent mechanisms.37 The precise role of genistein on cardiac GLUT4 expression remains unclear, although we have demonstrated that an acute 2-day genistein treatment in OVX rats reduced cardiac GLUT4 levels.23 Those data suggested that decreased GLUT4 levels could be related to the genistein-mediated ischemic tolerance.23 In the present study, cardiac GLUT4 levels remained unchanged at the end of the 2-week genistein treatment. The reasons for these differences are not clear but may reflect the duration of the treatment period and consequently the amount of genistein metabolized. It is also possible that the decrease in GLUT4 seen following 2 days of treatment is a transient effect that is later restored. Regardless, the observation that genistein treatment is anti-ischemic also suggests that changes in glucose metabolism involving GLUT4 may not necessarily be a mechanism of action of genistein even if this is a concept widely linked to improved left ventricular function during reperfusion.38,39
A role for genistein's effects in regulating oxidative stress in cardiac tissue has not been explored adequately to date. Most studies have used mixed phytoestrogen supplements given to rats or mice or added phytoestrogens acutely to cell lines or other tissues. Given that these studies have used inconsistent phytoestrogen dosages and treatment regimens (or routes), therefore the results of this study need to be analyzed in the light of the limited available literature. Nonetheless, most previous studies indicate that treatment with genistein improves the antioxidant status in tissues, as reflected by increases or prevention of loss of intracellular GSH levels, by decreased oxidized glutathione to GSH ratios, or by restoring levels of TBARS. This role of genistein is also confirmed in soy-deficient diets, which cause mitochondrial levels of glutathione to decrease as a result of increased production of reactive oxygen species.40–44 In contrast with these studies, our data failed to show an increase in cardiac GSH content with genistein treatment. However, this does not suggest that the oxidative stress capacity of genistein-treated hearts is limited because we recently reported that a 2-day treatment period increases tolerance of hearts to severe ischemia.23 Cellular defenses against oxidative stress also include superoxide dismutase, catalase, and glutathione peroxidase, all of which are increased with either estrogen or genistein.11,45,46
In conclusion, the results of this study demonstrate favorable effects of genistein on physical characteristics in the OVX rat, used here as a model for the postmenopausal condition. These favorable effects are evidenced by increased uterine weight and femur weight, along with reduced heart rate and pulse pressure. However, these effects appear not to be associated with cardiac GLUT4 expression and cardiac tissue oxidative stress. Further studies are required to better elucidate genistein's mechanism of action.
Acknowledgments
This work was supported by Midwestern University intramural funding awarded to L.A.-N. and T.L.B. and the National Institutes of Health (R15 DK071625-01A2, awarded to L.A.-N.). B.M. was the recipient of a Midwestern University DO Summer Fellowship. The authors would like to thank Dr. R. MacDonald (Department of Food Science and Human Nutrition, Iowa State University, Ames, IA) for providing the casein-based genistein-free diet.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Subbiah MT. Estrogen replacement therapy and cardioprotection: mechanisms and controversies. Braz J Med Biol Res. 2002;35:271–276. doi: 10.1590/s0100-879x2002000300001. [DOI] [PubMed] [Google Scholar]
- 2.Suetsugi M. Su L. Karlsberg K. Yuan YC. Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- 3.Tissier R. Waintraub X. Couvreur N, et al. Pharmacological postconditioning with the phytoestrogen genistein. J Mol Cell Cardiol. 2007;42:79–87. doi: 10.1016/j.yjmcc.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Manson J. Hsia J. Johnson K, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 5.Hulley S. Grady D. Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 6.Furness S. Roberts H. Marjoribanks J. Lethaby A. Hickey M. Farquhar C. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev. 2009;2:CD000402. doi: 10.1002/14651858.CD000402.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Anthony MS. Clarkson TB. Hughes CL. Morgan TM. Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system or peripubertal rhesus monkeys. J Nutr. 1996;126:43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Sliva D. Suppression of cancer invasiveness by dietary compounds. Mini Rev Med Chem. 2008;8:677–688. doi: 10.2174/138955708784567412. [DOI] [PubMed] [Google Scholar]
- 9.Teede HJ. Dalais FS. Kotsopoulos D. Liang Y-L. Davis S. McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 10.Teede HJ. Giannopoulos D. Dalais FS. Hodgson J. McGrath BP. Randomized, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am College Nutr. 2006;25:533–540. doi: 10.1080/07315724.2006.10719569. [DOI] [PubMed] [Google Scholar]
- 11.Mahn K. Borras C. Knock GA, et al. Dietary soy isoflavone-induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 12.Teede H. McGrath B. DeSilva L. Cehun M. Fassoulakis A. Nestel P. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- 13.Squadrito F. Altavilla D. Squadrito G, et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45:454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 14.Squadrito F. Altavillo D. Morabito N, et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 15.Hall W. Formanuik N. Harnpanich D, et al. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J Nutr. 2008;138:1288–1292. doi: 10.1093/jn/138.7.1288. [DOI] [PubMed] [Google Scholar]
- 16.Goodman-Gruen D. Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131:1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 17.Ishimi Y. Arai N. Wang X, et al. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem Biophys Res Commun. 2000;274:697–701. doi: 10.1006/bbrc.2000.3175. [DOI] [PubMed] [Google Scholar]
- 18.Wu J. Wang X. Chiba H, et al. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004;53:942–948. doi: 10.1016/j.metabol.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Atteritano M. Marini H. Minutoli L, et al. Effects of phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 20.Marini H. Minutoli L. Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women. Ann Intern Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 21.Park SA. Choi M-S. Cho S-Y, et al. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006;79:1207–1233. doi: 10.1016/j.lfs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Ørgaard A. Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- 23.Al-Nakkash L. Markus B. Bowden K. Batia L. Prozialeck WC. Broderick TL. Effects of acute, 2-day genistein treatment on cardiac function, ischemic tolerance in ovariectomized rats. Gender Med. 2009;6:488–497. doi: 10.1016/j.genm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Al-Nakkash L. Clarke LL. Rottinghaus GE. Chen YJ. Cooper K. Rubin LJ. Dietary genistein stimulates anion secretion across female murine intestine. J Nutr. 2006;136:2785–2790. doi: 10.1093/jn/136.11.2785. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- 26.Ohkawa H. Ohishi N. Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Senft AP. Dalton TP. Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe O-phthalaldehyde. Analyt. Biochem. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- 28.Rimoldi G. Christoffel J. Seidlova-Wuttke D. Jarry H. Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect. 2007;115:62–68. doi: 10.1289/ehp.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chanawirat A. Khemapech S. Patumraj S. Siriviriyakul P. Genistein replacement therapy on endothelial dysfunction and bone loss in bilateral ovariectomized rats. Clin Hemorheol Microcirc. 2006;34:309–314. [PubMed] [Google Scholar]
- 30.Kim H-K. Nelson-Dooley C. Della-Fera MA, et al. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136:409–414. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 31.Carlson S. Peng N. Prasain J. Wyss J. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gender Med. 2008;5(Suppl A):S76–S85. doi: 10.1016/j.genm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H-F. Wang L-D. Qu S-Y. Phytoestrogen genistein decreases contractile response to aortic artery in vitro and arterial blood pressure in vivo. Acta Pharmacol Sin. 2004;25:313–318. [PubMed] [Google Scholar]
- 33.Si H. Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr. 2008;138:297–304. doi: 10.1093/jn/138.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith R. Tiesinga J. Shah N. Smith J. Jarett L. Genistein inhibits insulin-stimulated glucose transport and decreases immunocytochemical labeling of GLUT4 carboxyl-terminus without affecting translocation of GLUT4 in isolated rat adipocytes: additional evidence of GLUT4 activation by insulin. Arch Biochem Biophys. 1993;300:238–246. doi: 10.1006/abbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- 35.Bazuine M. van den Broek P. Maassen A. Genistein directly inhibits GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;326:511–514. doi: 10.1016/j.bbrc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 36.Nomura M. Takahashi T. Nagata N, et al. Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol Pharm Bull. 2008;31:1403–1409. doi: 10.1248/bpb.31.1403. [DOI] [PubMed] [Google Scholar]
- 37.Lee M. Kim C. Hoang D, et al. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biol Pharm Bull. 2009;32:504–508. doi: 10.1248/bpb.32.504. [DOI] [PubMed] [Google Scholar]
- 38.McVeigh JJ. Lopaschuk GD. Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol. 1990;259:H1070–H1085. doi: 10.1152/ajpheart.1990.259.4.H1079. [DOI] [PubMed] [Google Scholar]
- 39.Broderick TL. Quinney HA. Barker CC. Lopaschuk GD. Beneficial effect of carnitine on mechanical function of rat hearts reperfused after a transient period of global ischemia is accompanied by a stimulation of glucose oxidation. Circulation. 1993;87:972–981. doi: 10.1161/01.cir.87.3.972. [DOI] [PubMed] [Google Scholar]
- 40.Mizutani K. Ikeda K. Nishikata T. Yamori Y. Phytoestrogens attenuate oxidative DNA damage in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Hypertens. 2000;18:1833–1840. doi: 10.1097/00004872-200018120-00018. [DOI] [PubMed] [Google Scholar]
- 41.Boadi W. Iyere P. Adunyah S. In vitro exposure to quercetin and genistein alters lipid peroxides and prevents the loss of glutathione in human progenitor mononuclear (U937) cells. J Appl Toxicol. 2005;25:82–88. doi: 10.1002/jat.1049. [DOI] [PubMed] [Google Scholar]
- 42.Aneja R. Upadhyaya G. Prakesh S. Dass S. Chandra R. Ameliorating effect of phytoestrogens on CCl4-induced oxidative stress in the livers of male Wistar rats. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:201–213. doi: 10.1081/bio-200055908. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim W. Habib H. Chow C. Bruckner G. Isoflavone-rich soy isolate reduces lipid peroxidation in mouse liver. Int J Vitam Nutr Res. 2008;78:217–222. doi: 10.1024/0300-9831.78.45.217. [DOI] [PubMed] [Google Scholar]
- 44.Palanisamy N. Viswanathan P. Anuradha C. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren Fail. 2008;30:645–654. doi: 10.1080/08860220802134532. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki K. Koike H. Matsui H, et al. Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCAP and PC-3. Int J Cancer. 2002;99:846–852. doi: 10.1002/ijc.10428. [DOI] [PubMed] [Google Scholar]
- 46.Strehlow K. Rotter S. Wassmann S, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]