Abstract
This study ascertained whether a proprietary tart cherry juice blend (CherryPharm, Inc., Geneva, NY, USA) associated with anecdotal reports of sleep enhancement improves subjective reports of insomnia compared to a placebo beverage. The pilot study used a randomized, double-blind, crossover design where each participant received both treatment and placebo for 2 weeks with an intervening 2-week washout period. Sleep continuity (sleep onset, wake after sleep onset, total sleep time, and sleep efficiency) was assessed by 2-week mean values from daily sleep diaries and disease severity by the Insomnia Severity Index in a cohort of 15 older adults with chronic insomnia who were otherwise healthy. The tart cherry juice beverage was associated with statistically significant pre- to post-treatment improvements on all sleep variables. When compared to placebo, the study beverage produced significant reductions in insomnia severity (minutes awake after sleep onset); no such improvements were observed for sleep latency, total sleep time, or sleep efficiency compared to placebo. Effect sizes were moderate and in some cases negligible. The results of this pilot study suggest that CherryPharm, a tart cherry juice blend, has modest beneficial effects on sleep in older adults with insomnia with effect sizes equal to or exceeding those observed in studies of valerian and in some, but not all, studies of melatonin, the two most studied natural products for insomnia. These effects, however, were considerably less than those for evidence-based treatments of insomnia: hypnotic agents and cognitive-behavioral therapies for insomnia.
Key Words: insomnia, older adults, sleep enhancement, tart cherry juice
Introduction
Tart cherries are purported to have a number of beneficial health effects, although few data exist to support these claims. One such claim has been for a sleep-enhancing effect of tart cherries, the target of this pilot study.
Available data do support the presence of several phytonutrients in Montmorency tart cherries including the phenolic acids—chlorogenic acid, caffeic acid, and ellagic acid—and the flavonoids—isorhamnetin, kaempferol, quercetin, catechin, epicatechin, procyanidins, and anthocyanins.1 Anthocyanins are the main component of the phenolic compounds found in tart cherries and mainly consist of cyanidin-3-glucosylrutinoside and cyanidin-3-rutinoside. Other anthocyanins like cyanidin-3-sophoroside, cyanidin-3-glucoside, and peonidin-3-glucoside make up less than 10% of the anthocyanin profile.2 One laboratory study has demonstrated a positive linear relationship between the level of anthocyanins in cherries and the degree of protection from oxidative stress in neuronal cells.3 Levels of anthocyanins in tart cherries have been found to exceed those found in sweet cherries and other fruits.4 Tart cherries in particular also have high levels of anti-inflammatory substances3 at a level comparable to a number of nonsteroidal anti-inflammatory products.1 In the few human experiments conducted with tart cherries, a cherry juice blend (CherryPharm, Inc., Geneva, NY, USA) was found to decrease oxidative stress in healthy older adults,5 enhance muscle recovery following a single bout of strength training in a randomized clinical trial,6 and decrease inflammation and oxidative stress following marathon running.7 The food-processing methods used to prepare this particular blend of cherry juice retains levels of phytonutrients approaching those in the whole fruit with one 8 ounce serving containing approximately 60 mg of anthocyanins as measured by high-performance liquid chromatography and 550 mg of total phenolic compounds (unpublished data provided by the manufacturer) as measured by spectrophotometric analysis with the Folin-Ciocalteu reagent, the equivalent of 50 whole tart cherries.1
Interestingly, some participants in the strength training study6 (as well as some college and professional athletes evaluating this tart cherry beverage as a sports recovery drink) made anecdotal reports attributing sleep improvements to the use of the beverage. No systematic studies have been published that test these or other claims with respect to tart cherries and sleep. One putative sleep-promoting pathway for tart cherries is their relatively high content of melatonin,8 a substance with sleep-regulating properties. Another potential pathway is via anti-inflammatory agents in that a number of inflammatory cytokines are intricately related to the modulation of sleep.9
Given these anecdotal reports and a plausible mechanism of action, we designed a pilot study to test the efficacy of a tart cherry juice (TCJ) beverage against placebo in older adults with insomnia. The choice of an elderly cohort was based on the high prevalence rates of insomnia among the elderly10 and the potential benefit of a nonmedication treatment for insomnia for this population, which has a high medication burden. The primary aim was to assess whether there were group differences on prospective self-reports of sleep as assessed by daily sleep diaries and by a retrospective assessment of insomnia severity, while secondary aims were focused on retrospective reports of mood and fatigue.
Materials and Methods
Participants
Forty-three potential participants who responded to local newspaper advertisements or brochures left in local physician offices, health centers, and senior centers were screened by telephone for preliminary eligibility. Nineteen participants ≥65 years of age who complained of insomnia but were otherwise healthy, active, and medically stable and taking no hypnotic or sedating medications were scheduled for an intake visit. This consisted of the informed consent process, a physical examination, a 12-lead electrocardiogram, clinical laboratory evaluations, structured diagnostic interviews, and a self-report instrument battery. Inclusionary criteria also included a typical bedtime of between 9:00 p.m. and 12:00 a.m., a sleep problem frequency >3 nights/week with a duration ≥6 months, meeting research diagnostic criteria for primary insomnia,11 an Insomnia Severity Index (ISI) ≥10, and a minimum of 30 minutes of either sleep-onset latency (SL) or wake after sleep onset (WASO). Additional exclusionary criteria included any unstable medical or psychiatric illness, a positive screen for substances of abuse, the use of any sedating or hypnotic medications, symptoms suggestive of sleep disorders other than primary insomnia, and any diagnosis of diabetes or elevated glucose levels on baseline clinical chemistries (to exclude patients who may not monitor their sugar intake adequately given the potential addition of a fruit juice to their diet).
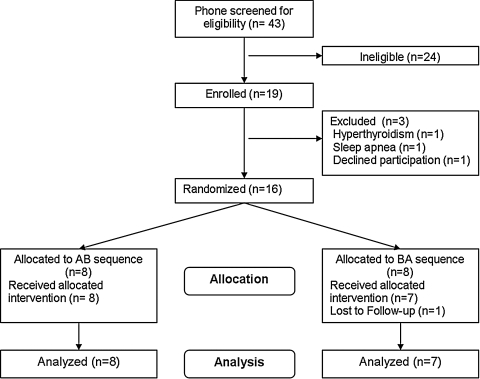
The analyzed sample (Fig. 1 shows the participant flow diagram) consisted of 15 participants with a mean (SD) age of 71.6 (5.4) years and a body mass index of 25.8 (4.6) kg/m2, and seven were women. Baseline level of sleep disturbances include group means of approximately 25 minutes SL, 80 minutes WASO, 6.5 hours of total sleep time (TST), 80% sleep efficiency (SE), and an ISI score of 15. These suggest a moderate to severe level of insomnia that is more related to sleep maintenance than to sleep onset problems.
FIG. 1.
Participant flow diagram.
Study design
This was a randomized, placebo-controlled, double-blind, crossover trial conducted at the University of Rochester Medical Center (Rochester, NY, USA) and approved by its Institutional Review Board. Following written informed consent and intake assessment, participants who were not excluded and received written permission to participate from their primary care physician were block randomized (in blocks of two with additional stratification by gender) to an AB/BA order of beverage. The study consisted of four 2-week periods (a total of 8 weeks) following enrollment: (1) baseline, (2) beverage A or B (based on randomization), (3) washout with no study beverages, and (4) beverage B or A. Assessments were made at baseline, following the first beverage period, and following the second beverage period. During each of the 14-day periods on beverage each participant was instructed to drink two 8-ounce servings of the assigned beverage with one serving in the morning between 8 and 10 a.m. and one serving in the evening 1–2 hours before bedtime to avoid excess fluid intake immediately before bed. Participants recorded on the daily sleep diary whether any dose was missed or taken outside of the prescribed dosing times; they also returned both empty and unused containers at the end of each 2-week treatment period, which were counted.
Study beverages
The treatment juice was a proprietary juice blended from whole Montmorency tart cherries and apple juice processed to shelf stable conditions and provided by the manufacturer CherryPharm, Inc. The placebo was a beverage intended to have similar sugar, acid, and visual properties but without the phytonutrient content found in the TCJ blend treatment juice. The placebo was prepared by mixing unsweetened black cherry Kool-Aid® soft drink mix (Kraft North America, Ryebrook, NY, USA) with water, coloring, a clouding agent, and sucrose to match the color, sugar content, and final soluble solids content by weight of the proprietary whole TCJ. Both the TCJ and the placebo were packed in the same clear, 8 ounce, polyethylene terephthalate containers, labeled with the identical CherryPharm product label, and packaged in cases marked “A” or “B.” Both investigators and participants were blind to which cases (or which individual containers) contained TCJ or placebo.
Measures
Instruments administered only at the intake interview included the MINI International Psychiatric Interview, Mini Mental Status Exam, and a structured clinical interview developed and Institution Review Board-approved for this study. Instruments administered at intake as well as at the two post-treatment study visits included the ISI, which is a validated seven-item instrument that assesses difficulty initiating and maintaining sleep, daytime consequences, worry about sleep, and satisfaction with sleep quality,12 a medical history inventory, a medical symptoms checklist, the Multidimensional Fatigue Inventory,13 the Beck Depression Inventory,14 and the Beck Anxiety Inventory.15 In addition, participants maintained daily sleep diaries throughout the study period, which include items from which the sleep continuity variables may be calculated (SL, WASO, TST, and SE).
Primary outcome measures and statistical considerations
We hypothesized that participants would report greater improvements over baseline in insomnia severity, as assessed by the ISI, and sleep continuity, as assessed by 2-week mean sleep diary values of SL, WASO, TST, and SE, from drinking the TCJ compared to the placebo. Tests of period and carryover effects for these variables were conducted using analysis of variance tests, and no significant order or period effects were observed in this crossover trial. Accordingly, participants receiving TCJ, regardless of sequence, were combined as a treatment group (n = 15) and served as their own controls. For each outcome variable, a general linear model for repeated measures was used to test the group (placebo vs. treatment juice) by time (baseline vs. post-treatment) effect. In addition, paired-samples t tests were conducted to assess within-group effects of TCJ and of placebo separately. Three variables (SL, TST, and the Multidimensional Fatigue Inventory) were not normally distributed; these data were transformed using the square root method to allow the same parametric tests to be applied to all data. Secondary outcomes were not tested for period or carryover effects but were subjected to the same within and between-group comparisons.
There were no missing data for any of the primary or secondary outcome measures because of, in part, all instruments being reviewed for completeness at each study visit. One participant missed the last three bottles while taking TCJ and returned these bottles. We did not adjust the data for this one subject. Significance testing was two-tailed with α set at 0.05. Although corrections for multiple comparisons are not typically applied to this set of outcome variables in insomnia trials, for readers wishing to compare results adjusted for correlations between these variables, the Bonferroni-adjusted α is.023. All analyses were conducted with SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Effect sizes were calculated with Cohen's d.16
Results
There were no main effects for time or group. Detailed within- and between-group results are presented in Table 1. Within-group, pre–post-treatment changes for TCJ were significant for ISI (P < .05), SL (P < .05), WASO (P < .01), TST (P < .01), and SE (P < .05) with moderate effect sizes. There was a within-group effect of placebo on TST only (P < .05). In the between-group analyses, compared to placebo, TCJ was associated with a significant reduction on the ISI (P < .05) and in WASO (P < .01), with no such findings for SL, TST, or SE. Effect sizes are moderate. There were no significant improvements on measures of fatigue, depression, or anxiety.
Table 1.
Within- and Between-Group Effects of Placebo and Cherry Juice on Sleep and Other Outcomes
| |
|
Effect size (d) |
Time × group |
||
|---|---|---|---|---|---|
| Mean (SD) | Within | Between | F | P | |
| Primary outcome | |||||
| ISI | |||||
| Baseline | 15.5 (2.7) | ||||
| Placebo | 14.9 (3.6) | 0.19 | |||
| Cherry juice | 13.2 (2.8)* | 0.86 | 0.55 | 5.65 | .025 |
| SL (minutes)a | |||||
| Baseline | 24.7 (15.1) | ||||
| Placebo | 23.7 (14.8) | 0.07 | |||
| Cherry juice | 21.1 (17.1)** | 0.23 | 0.17 | 1.25 | .274 |
| WASO (minutes) | |||||
| Baseline | 78.9 (32.7) | ||||
| Placebo | 79.1 (38.6) | -0.01 | |||
| Cherry juice | 62.1 (37.4)** | 0.49 | 0.46 | 8.56 | .007 |
| TST (minutes)a | |||||
| Baseline | 388.3 (48.8) | ||||
| Placebo | 409.2 (43.9)* | 0.47 | |||
| Cherry juice | 417.6 (54.2)** | 0.59 | 0.18 | 0.47 | .528 |
| SE | |||||
| Baseline | 79.2 (7.0) | ||||
| Placebo | 81.4 (8.2) | 0.30 | |||
| Cherry juice | 82.9 (8.6)* | 0.49 | 0.18 | 0.88 | .355 |
| Secondary outcomes | |||||
| MFIa | |||||
| Baseline | 49.1 (12.5) | ||||
| Placebo | 44.1 (9.4) | 0.47 | |||
| Cherry juice | 46.2 (11.5) | 0.25 | 0.21 | NS | |
| BDI | |||||
| Baseline | 8.5 (4.3) | ||||
| Placebo | 6.4 (4.3) | 0.50 | |||
| Cherry juice | 7.3 (4.0) | 0.26 | 0.14 | NS | |
| BAI | |||||
| Baseline | 6.8 (4.4) | ||||
| Placebo | 5.0 (4.3) | 0.42 | |||
| Cherry juice | 5.8 (4.0) | 0.24 | 0.20 | NS | |
d, the measure of effect size (Cohen's d); BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; MFI, Multidimensional Fatigue Inventory; NS, not significant.
These raw data were transformed to normalize data for significance testing.
Within-group pre–post-treatment improvement of *P < .05. **P < .01.
Discussion
The findings from this preliminary study suggest that a TCJ can modestly improve sleep in older adults with insomnia. At the within-group level, TCJ was associated with significant improvements on each of the sleep continuity variables as well as the ISI. Compared to placebo, the ISI score and WASO were reduced. Possibly because of a floor effect (this sample did not have high levels of sleep onset problems) there was no significant improvement on SL. The amount of improvements and their related effect sizes are smaller than those achieved in pharmacotherapy or behavioral trials (effect sizes of ∼1.0 for WASO, TWT, and SE and ∼0.5 for TST). Further, the magnitude of the improvements at the group mean level was such that participants continued to have a significant amount of sleep disturbance. In fact, using clinical benchmarks, the sample, as a whole, continued to evidence insomnia. The magnitude of improvements do exceed those reported for valerian17 and equal some, but not all, reported outcomes for exogenous melatonin.18 Neither of these other natural products, however, is considered effective treatment for insomnia.
There are a number of limitations in this pilot study. Although we powered the study to detect moderate effects on the primary outcome variables, the sample size is small. In addition, there was no polysomnographic assessment of sleep as either a screening tool or as an outcome. Participants who were not screened out by subjective report and structured interviews, but still had apnea, may have gone unidentified in this study. Between-group differences would have been mitigated by the crossover design as such participants would have served as their own controls. Polysomnography is also considered the gold standard with respect to the objective measurement of sleep. Although polysomnography is a standard assessment method in most pharmaceutical trials, it is rarely achieved in pre–post-treatment insomnia intervention trials outside of industry support. In fact, the vast majority of studies used as rational by a National Institutes of Health State of the Science Conference to recommend cognitive-behavioral therapy for insomnia as a first-line treatment for insomnia are based on subjective data.19 This is also the case in trials of depression and anxiety, where the norm is for primary outcomes to be based on subjective reports using validated instruments.
It is also true that given the selected sample of healthy elderly individuals with insomnia, these findings may not generalize to other populations. Moreover, this cohort suffered primarily from sleep maintenance insomnia and had modest sleep onset difficulties, so it is most prudent to consider the effect of tart cherries on SL to be undetermined as there was a floor effect in this sample. In addition, the cherry juice used in this study was a proprietary blend (CherryPharm, Inc.) made from fresh tart cherries so that findings may not generalize to cherry juice made from concentrate or to eating 1–2 servings of tart cherries per day (as participants ingested the equivalent of approximately 100 cherries per day).
Finally, this study was not designed to address mechanism of action. If the mechanism for sleep enhancement is melatonin, perhaps anecdotal reports of sleep improvements by college and professional athletes is related to circadian regulation of sleep afforded by melatonin. This is a testable hypothesis. Similarly, a study undertaken with the study beverage in a population with diagnosed circadian rhythm disturbances would be informative. Alternatively, given that several pro-inflammatory cytokines are involved in the regulation of sleep,9 the anti-inflammatory properties of tart cherries may be a mechanism of action.
In conclusion, the results of this study suggest that the particular TCJ blend used in this study has modest beneficial effects on sleep in older adults with insomnia. Given that these results were achieved following only a brief treatment period (2 weeks), that mechanism of action is unknown, and that optimal dosing strategies (amount, time, frequency, and duration) are equally uncertain, further study of the sleep-promoting effects of tart cherries is warranted.
Acknowledgments
W.R.P. receives salary support from grant K23 NR010408 from the National Institutes of Health and research support from the American Academy of Sleep Medicine and the VA Center of Excellence at Canandaigua.
Author Disclosure Statement
CherryPharm, Inc., the maker of the tart cherry juice, funded this research study in its entirety. No other competing financial interests exist.
References
- 1.Kim DO. Heo HJ. Kim YJ. Yang HS. Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005;53:9921–9927. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- 2.Kirakosyan A. Seymour EM. Urcuyo-Llanes DE. Kaufman PB. Bolling SF. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009;115:20–25. [Google Scholar]
- 3.Wang HB. Nair MG. Strasburg GM. Chang YC. Booren AM. Gray JI. Dewitt D. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod. 1999;62:294–296. doi: 10.1021/np980501m. Erratum in: J Nat Prod 1999;62:802. [DOI] [PubMed] [Google Scholar]
- 4.Wang HB. Nair MG. Iezzoni AF. Strasburg GM. Booren AM. Gray JI. Quantification and characterization of anthocyanins in Balaton tart cherries. J Agric Food Chem. 1997;45:2556–2560. [Google Scholar]
- 5.Traustadottir T. Davies SS. Stock AA. Su Y. Heward CB. Roberts LJ. Harman SM. Proprietary tart cherry juice blend decreases oxidative stress in healthy older adults. J Nutr Dis. 2009;139:1896–1900. doi: 10.3945/jn.109.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly DAJ. Mchugh MP. Padilla-Zakour OI. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. 2006;40:679–683. doi: 10.1136/bjsm.2005.025429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howatson G. Mchugh MP. Hill JA. Brouner J. Jewell AP. van Someren KA. Shave RE. Howatson SA. Efficacy of tart cherry juice in reducing muscle damage, inflammation and oxidative stress following marathon running. Med Sci Sports Exerc. 2009;5:507–508. [Google Scholar]
- 8.Burkhardt S. Tan DX. Manchester LC. Hardeland R. Reiter RJ. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus) J Agric Food Chem. 2001;49:4898–4902. doi: 10.1021/jf010321+. [DOI] [PubMed] [Google Scholar]
- 9.Opp MR. Cytokines and sleep: the first hundred years. Brain Behav Immun. 2004;18:295–297. doi: 10.1016/j.bbi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19(3 Suppl):S7–S15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 11.Edinger JD. Bonnet MH. Bootzin RR. Doghramji K. Dorsey CM. Espie CA. Jamieson AO. McCall WV. Morin CM. Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM. Insomnia: psychological assessment and management. Sleep. 1993;15:302–305. [Google Scholar]
- 13.Smets EM. Garssen B. Bonke B. De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT. Ward CH. Mendelson M. Mock J. Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT. Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corp.; San Antonio, TX: 1990. [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 17.Taibi DM. Landis CA. Petry H. Vitiello MV. A systematic review of valerian as a sleep aid: safe but not effective. Sleep Med Rev. 2007;11:209–230. doi: 10.1016/j.smrv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Buscemi N. Vandermeer B. Hooton N. Pandya R. Tjosvold L. Hartling L. Baker G. Klassen TP. Vohra S. The efficacy and safety of exogenous melatonin for primary sleep disorders—a meta-analysis. J Gen Intern Med. 2005;20:1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep. 2005;28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]