Abstract
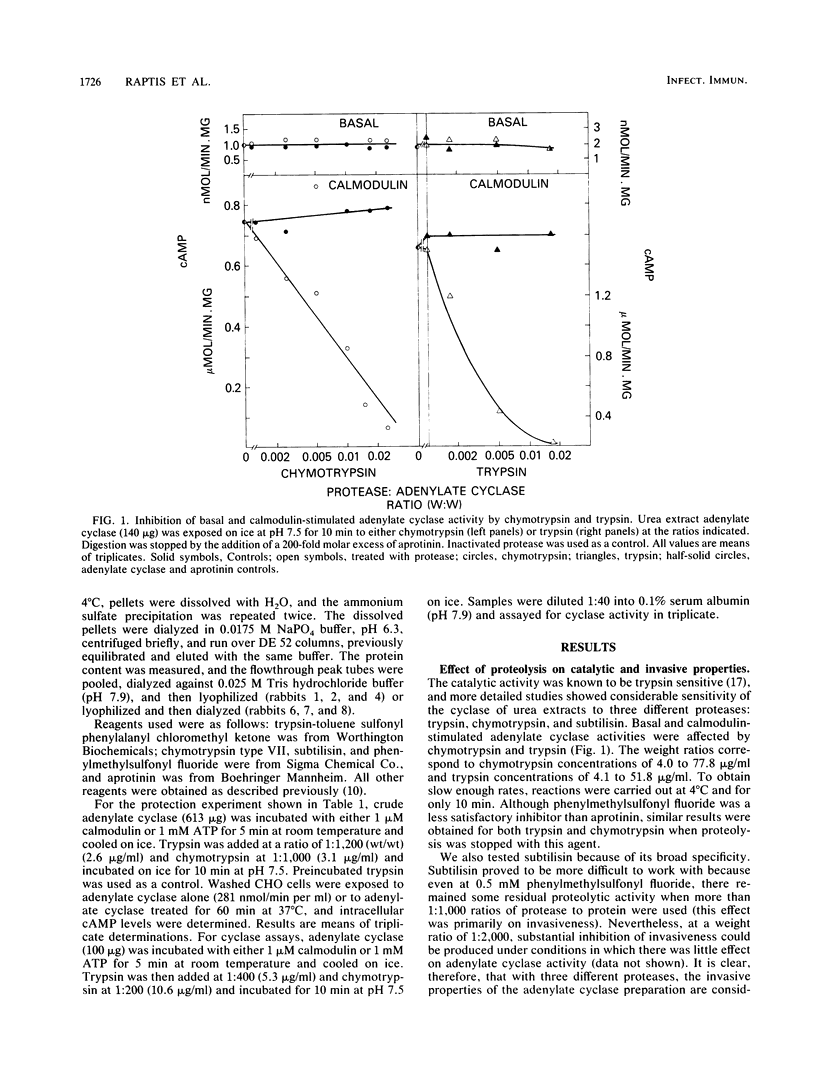
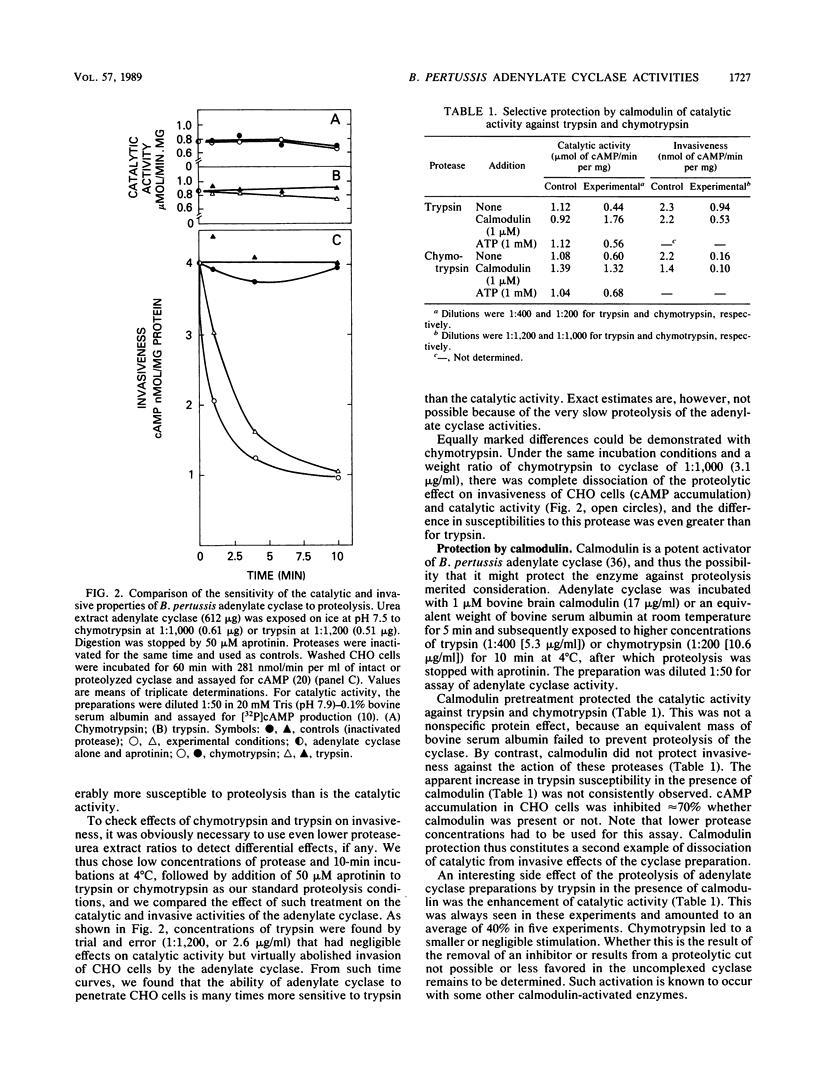
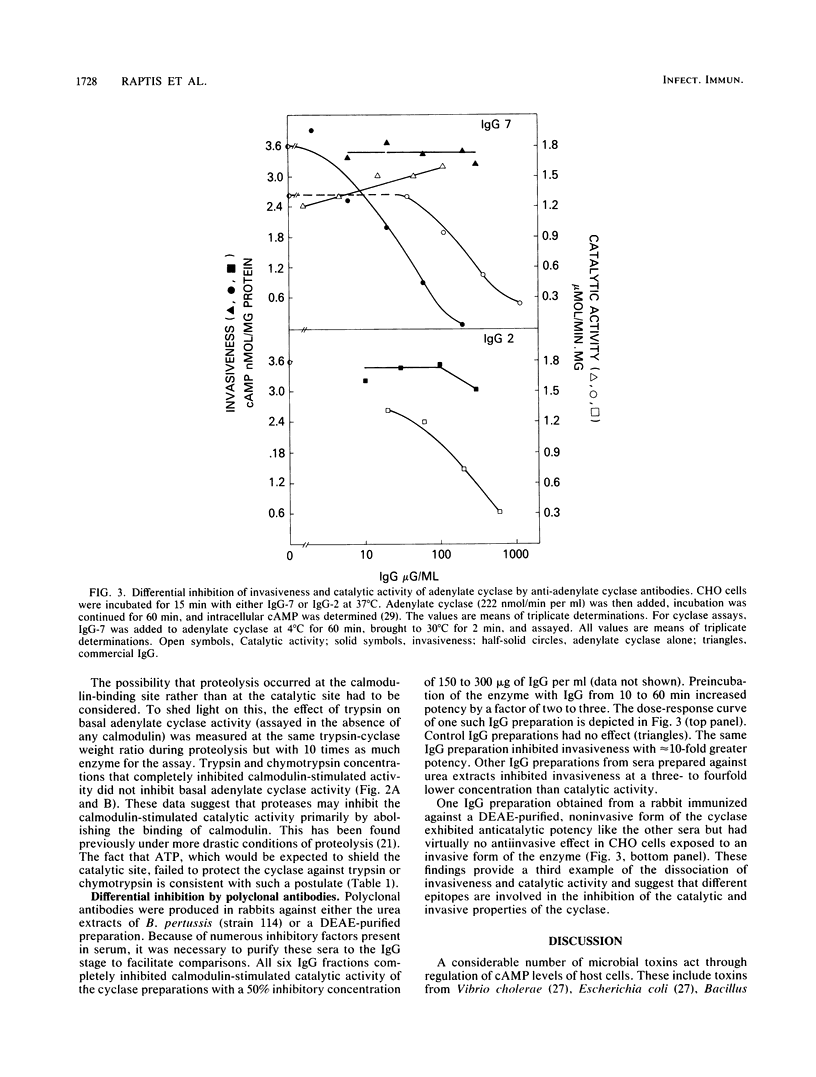
Bordetella pertussis organisms secrete adenylate cyclase, at least one form of which can invade host cells and appears to be a virulence factor. Treatment of urea extracts containing invasive cyclase of B. pertussis with trypsin, chymotrypsin, or subtilisin abolishes the ability to increase intracellular cyclic AMP levels in CHO cells (invasiveness) at concentrations that have minimal or no effects on adenylate cyclase activity. Higher protease concentrations can inhibit catalytic activity, and 1 microM calmodulin protects this catalytic activity, but not invasiveness, against proteolytic inhibition. Rabbit immunoglobulin G (IgG) fractions from antisera prepared against urea extracts inhibited invasiveness at 10-fold-lower concentrations than inhibited catalytic activity. One IgG from a rabbit immunized against a partially purified, noninvasive form of the B. pertussis adenylate cyclase inhibited catalytic activity but was ineffective against invasiveness. We conclude that these two properties of the adenylate cyclase are independent functions that reside on different domains of the same protein or on different proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseeva L. E., Shevchenko L. A., Shimaniuk N. Ia, Rublev B. D., Mishan'kin B. N. Otsenka moduliruiushchego deistviia adenilattsiklazy chumnogo mikroba na peritoneal'nye leikotsity morskoi svinki s pomoshch'iu khemiliuminestsentsii. Zh Mikrobiol Epidemiol Immunobiol. 1987 Jul;(7):59–63. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R. Adenylate cyclase activity during phenotypic variation of Bordetella pertussis. J Gen Microbiol. 1985 Jan;131(1):27–38. doi: 10.1099/00221287-131-1-27. [DOI] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R., Schultz J. E., Rogel A., Hanski E. Cloning of the adenylate cyclase genetic determinant of Bordetella pertussis and its expression in Escherichia coli and B. pertussis. Microb Pathog. 1988 May;4(5):335–344. doi: 10.1016/0882-4010(88)90061-7. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Endoh M., Takezawa T., Nakase Y. Adenylate cyclase activity of Bordetella organisms. I. Its production in liquid medium. Microbiol Immunol. 1980;24(2):95–104. doi: 10.1111/j.1348-0421.1980.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile F., Raptis A., Knipling L. G., Wolff J. Bordetella pertussis adenylate cyclase. Penetration into host cells. Eur J Biochem. 1988 Aug 15;175(3):447–453. doi: 10.1111/j.1432-1033.1988.tb14215.x. [DOI] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Gordon V. M., Leppla S. H., Hewlett E. L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56(5):1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hewlett E. L., Roberts C. O., Wolff J., Manclark C. R. Biphasic effect of pertussis vaccine on serum insulin in mice. Infect Immun. 1983 Jul;41(1):137–144. doi: 10.1128/iai.41.1.137-144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono K., Suzuki H. Morphological transformation of Chinese hamster cells by acylpeptides, inhibitors of cAMP phosphodiesterase, produced by Bacillus subtilis. J Biol Chem. 1985 Sep 15;260(20):11252–11255. [PubMed] [Google Scholar]
- Kessin R. H., Franke J. Secreted adenylate cyclase of Bordetella pertussis: calmodulin requirements and partial purification of two forms. J Bacteriol. 1986 Apr;166(1):290–296. doi: 10.1128/jb.166.1.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J Biol Chem. 1988 Feb 25;263(6):2612–2618. [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Masure H. R., Oldenburg D. J., Donovan M. G., Shattuck R. L., Storm D. R. The interaction of Ca2+ with the calmodulin-sensitive adenylate cyclase from Bordetella pertussis. J Biol Chem. 1988 May 15;263(14):6933–6940. [PubMed] [Google Scholar]
- Monneron A., Ladant D., d'Alayer J., Bellalou J., Bârzu O., Ullmann A. Immunological relatedness between Bordetella pertussis and rat brain adenylyl cyclases. Biochemistry. 1988 Jan 26;27(2):536–539. doi: 10.1021/bi00402a005. [DOI] [PubMed] [Google Scholar]
- Moss J., Burns D. L., Hsia J. A., Hewlett E. L., Guerrant R. L., Vaughan M. NIH conference. Cyclic nucleotides: mediators of bacterial toxin action in disease. Ann Intern Med. 1984 Nov;101(5):653–666. doi: 10.7326/0003-4819-101-5-653. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Symes P., Conboy M., Weiss A. A., Hewlett E. L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987 Oct 15;139(8):2749–2754. [PubMed] [Google Scholar]
- Raptis A., Knipling L. G., Gentile F., Wolff J. Modulation of invasiveness and catalytic activity of Bordetella pertussis adenylate cyclase by polycations. Infect Immun. 1989 Apr;57(4):1066–1071. doi: 10.1128/iai.57.4.1066-1071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Shattuck R. L., Storm D. R. Calmodulin inhibits entry of Bordetella pertussis adenylate cyclase into animal cells. Biochemistry. 1985 Nov 5;24(23):6323–6328. doi: 10.1021/bi00344a001. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sugisaki Y., Gunge N., Sakaguchi K., Yamasaki M., Tamura G. Kluyveromyces lactis killer toxin inhibits adenylate cyclase of sensitive yeast cells. Nature. 1983 Aug 4;304(5925):464–466. doi: 10.1038/304464a0. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Londos C., Hewlett E. L. Bordetella pertussis: multiple attacks on host cell cyclic AMP regulation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:161–172. [PubMed] [Google Scholar]



