Abstract
Summary
Background and objectives
Insulin has several physiologic actions that include stimulation of cellular glucose and potassium uptake. The ability of insulin to induce glucose uptake by cells is impaired in type 2 diabetes mellitus, but whether potassium uptake is similarly impaired is not known. This study examines whether the cellular uptake of these molecules is regulated in concert or independently.
Design, setting, participants, & measurements
Thirty-two nondiabetic and 13 type 2 diabetic subjects with normal GFR were given a similar, constant metabolic diet for 8 days. On day 9, they were subjected to a hyperinsulinemic euglycemic clamp for 2 hours. Serum and urinary chemistry were obtained before and during the clamp. Glucose disposal rate was calculated from glucose infusion rate during hyperinsulinemic euglycemia. Intracellular potassium and phosphate uptake were calculated by the reduction of extracellular potassium or phosphate content corrected for urinary excretion.
Results
Although glucose disposal rate tended to be lower in type 2 diabetics, cellular potassium uptake was similar between diabetics and nondiabetics. Additionally, although glucose disposal rate was lower with increasing body mass index (R2 = 0.362), cellular potassium (R2 = 0.052), and phosphate (R2 = 0.002), uptake rates did not correlate with body mass index. There was also no correlation between glucose disposal rate and potassium (R2 = 0.016) or phosphate uptake (R2 = 0.053).
Conclusions
Insulin-stimulated intracellular uptake of glucose and potassium are independent of each other. In type 2 diabetes, potassium uptake is preserved despite impaired glucose disposal.
Introduction
Insulin has a multitude of actions on a wide range of cellular processes. In terms of caloric and glucose metabolism, insulin suppresses glycogenolysis, gluconeogenesis, lipolysis and fatty acid release, and protein catabolism and is the principal hormone that stimulates glucose uptake into mainly skeletal muscle and to a certain extent adipocytes (1–6). In subjects with the metabolic syndrome or type 2 diabetes, insulin-stimulated glucose uptake is impaired (7), a condition frequently termed “insulin resistance” in common clinical parlance, although not all insulin actions are necessarily impaired.
Serum potassium concentration ([K+]) reflects total body potassium stores at the steady state, although this relationship can be disturbed in disorders of potassium distribution (8). Plasma [K+] is a major determinant of the resting potential of all cells (9). Hyperkalemia and hypokalemia are silent yet fatal disturbances because of their arrhythmogenic potentials (9). Insulin was shown to be an important regulator of potassium homeostasis shortly after its discovery (10). Basal insulin maintains fasting plasma [K+] within the normal range (11). When insulin levels are suppressed, plasma [K+] rises and pronounced hyperkalemia develops after a potassium load (11). Potassium is a well proven insulin secretagogue in the intact organism and the isolated pancreas (12,13). Insulin is a key defender against exogenous potassium load by using intracellular buffering to minimize hyperkalemia before renal excretion (14).
Hyperkalemia is often encountered in patients with diabetes (8). The insulin-deficient state in type 1 diabetes predisposes to hyperkalemia because of an impaired ability of potassium to enter cells. During hyperglycemic hypertonic states in type 1 and type 2 diabetics, potassium is carried out of cells by convective flux as the most abundant intracellular cation (15,16). Even at the steady state in a significant portion of type 1 and type 2 diabetics, there is an impaired ability of the distal nephron to excrete potassium because of hyporeninemic hypoaldosteronism or tubular insensitivity to aldosterone (17,18). Finally, one wonders whether there is impaired cellular potassium uptake in type 2 diabetes as part of a generalized insulin-resistant state.
One ponders whether there should be any teleogic reasons of coupling glucose to potassium uptake. In the postprandial state of herbivores or carnivores, caloric and potassium influx are concurrent. In a feast-or-famine situation in hunting carnivores, the magnitude of the load is much exaggerated. The simultaneous shift of glucose and potassium into cells makes physiologic sense with the postprandial outpouring of insulin. Similarly, dietary phosphate frequently accompanies caloric intake, and upon entry into cells, glucose is phosphorylated; thus, simultaneous phosphate uptake also makes physiologic sense. However, if potassium, phosphate, and glucose loads are applied discordantly, the simultaneous cellular uptake will clearly present a homeostatic quandary and some means of dissociation is mandatory.
Previous studies have addressed whether potassium and glucose uptake are coupled. DeFronzo et al. observed a relationship between the decline in plasma [K+] and insulin level as well the total amount of glucose taken up by cells (19). Arslanian et al. concluded that insulin-dependent diabetics have impaired potassium uptake (20). However, Cohen et al. found independent actions of insulin on glucose and potassium uptake (21). Our study contains a larger number of human subjects and intends to encompass a wider range of “insulin sensitivity” comparing nondiabetic to diabetic subjects. We conclude that glucose and potassium uptake are differentially regulated and that impaired glucose disposal does not affect potassium uptake.
Materials and Methods
Subjects
There were 45 study subjects. The diagnosis of type 2 diabetes was made by the participants' personal physicians before enrollment on the basis of elevated fasting or random serum glucose (≥126 mg/dl or ≥200 mg/dl, respectively) on two separate occasions. Nondiabetics included subjects with a broad range of body mass indices (BMIs) without a known history of type 2 diabetes that was confirmed by a fasting glucose of <126 mg/dl (highest value was 106 mg/dl). Diabetic subjects were excluded if they were treated with insulin and/or thiazolidinediones. Treatment included metformin alone (n = 6), a sulfonyl urea alone (n = 2), metformin and sulfonyl urea (n = 3), or diet alone (n = 2). The Institutional Review Board at the University of Texas Southwestern Medical Center approved the study, and all participants provided informed consent. All subjects consumed a fixed metabolic diet for 8 days as outpatients for the first 5 days, and then as inpatients for 3 days at the General Clinical Research Center at University of Texas Southwestern Medical Center starting on the evening of the 5th day. On the evening of day 8, subjects fasted overnight except for 300 ml of water at bedtime.
Hyperinsulinemic Euglycemic Insulin Clamp Technique
On day 9, breakfast was withheld and subjects underwent hyperinsulinemic euglycemic clamp (22) starting at 8:00 a.m. with insulin infusion at 80 mU/m2 of body surface area for 2 hours. A 20-g/dl glucose solution was started after 4 minutes of insulin infusion to maintain plasma glucose concentration at the fasting levels throughout the clamp procedure. Plasma insulin was determined by a modification of the method of Yallow and Berson (23,24). Blood for plasma glucose levels was drawn every 5 minutes from an arterialized dorsal hand vein kept in a hotbox at 70°C (25). A glucose analyzer (YSI, Yellow Springs, OH) was used to measure plasma glucose, and the rate of the glucose infusion was adjusted to maintain euglycemia. Peripheral venous blood was drawn without stasis from an antecubital vein for electrolytes and lipid profile at 8:00 and 10:00 a.m. Two timed urine specimens were collected between 6:00 and 8:00 a.m. (preclamp) and 8:00 and 10:00 a.m. (hyperinsulinemia). To ensure adequate urinary output, 250 ml of water was given orally at 6:00 and 8:00 a.m. Urine was collected under mineral oil and kept refrigerated until analysis. Plasma and urinary electrolytes and plasma lipid profiles were measured by an autoanalyzer (Beckman, Synchron CX9ALX, Brea, CA).
Calculations and Analytical Methods
Glucose disposal rate during the hyperinsulinemic phase with complete suppression of gluconeogenesis and glycogenolysis, and absence of glycosuria was calculated as equivalent to the infusion rate of glucose from time 80 to 120 minutes. A fall in plasma [K+] or phosphate concentration was defined as the level obtained at 8:00 a.m. minus that obtained at 10:00 a.m. Instead of using the “potassium clamp” described by Choi and co-workers, (26) we used a balance approach because it is technically difficult to do simultaneous glucose and potassium clamps. Cellular [K+] or phosphate uptake was calculated by subtracting the urinary excretion rate from the fall in extracellular potassium or phosphate content during the clamp. Extracellular fluid volume was calculated as 0.2 L/kg × body weight in kilograms. This method is more accurate than the decrement in plasma [K+] but technically simpler than the potassium clamp. Phosphorus uptake was similarly calculated as the difference between the fall in extracellular phosphorus uptake and urinary phosphorus excretion.
Comparison between groups was evaluated using the t test with a two-tailed P value, and a paired t test was used for paired analyses. A χ2 test was used to compare percentages between groups. The Pearson correlation coefficient was used to assess correlation between different parameters. Statistical analyses were performed with SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Patient Characteristics
The diabetic and nondiabetic groups were similar in age, gender, gender race, cholesterol, and basal creatinine levels (Table 1). Mean BMI was in the obese range for the diabetic group and in the overweight range for the nondiabetic group. There was also a higher percentage of Hispanics in the diabetic than the nondiabetic group. In addition, the diabetic group had a lower LDL, lower HDL, higher triglyceride, and higher fasting glucose than the nondiabetic group.
Table 1.
Patient characteristics
| Characteristics | Nondiabetic Group | Diabetic Group | P |
|---|---|---|---|
| Number of patients | 32 | 13 | |
| Age (years) | 51 ± 12 | 53 ± 9 | 0.72 |
| Percent male | 59.4% | 53.8% | 0.73 |
| Height (cm) | 168 ± 11 | 173 ± 12 | 0.17 |
| Weight (kg) | 84 ± 25 | 96 ± 16 | 0.12 |
| BMI (kg/m2) | 29.5 ± 6.6 | 32.1 ± 3.8 | 0.10 |
| Race (%) | |||
| Caucasian | 78.1% | 76.9% | 0.76 |
| African American | 18.8% | 23.1% | 0.93 |
| Asian | 3.1% | 0.0% | |
| Hispanic | 6.3% | 23.1% | 0.27 |
| non-Hispanic | 93.8% | 76.9% | 0.27 |
| Serum creatinine (mg/dl) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.95 |
| Creatinine clearance (ml/min) | 116.7 ± 27.9 | 121.0 ± 22.8 | 0.62 |
| LDL cholesterol (mg/dl) | 132 ± 36 | 106 ± 38 | 0.04 |
| Total cholesterol (mg/dl) | 200 ± 38 | 187 ± 52 | 0.36 |
| HDL cholesterol (mg/dl) | 40 ± 11 | 36 ± 6 | 0.07 |
| Triglyceride (mg/dl) | 136 ± 65 | 225 ± 174 | 0.02 |
| Glucose (mg/dl) | 92 ± 7 | 118 ± 34 | 0.02 |
| Hemoglobin A1c (%) | 5.2 ± 0.3 | 6.1 ± 0.7 | <0.01 |
Values are mean ± standard deviation. BMI, body mass index.
Plasma and Urinary Chemistry
The diabetic group had a numerically higher preclamp plasma insulin level than the nondiabetic group, but the difference was not statistically significant (12.0 ± 8.4 versus 8.6 ± 3.6 μU/ml, P = 0.25) (Table 2). The diabetic group had higher preclamp (118 ± 34 versus 92 ± 7 mg/dl, P = 0.02) and postclamp (100 ± 15 mg/dl versus 91 ± 7 mg/dl, P = 0.05) glucose concentrations than the nondiabetic group. There were no differences in serum creatinine, potassium, and phosphate levels between the two groups before and after insulin.
Table 2.
Serum and urine data before and after clamp
| Data | Preclamp/Postclamp | Nondiabetic Group | Diabetic Group | P |
|---|---|---|---|---|
| Plasma [K+] (mM) | Preclamp | 4.0 ± 0.4 | 4.0 ± 0.2 | 0.58 |
| Postclamp | 3.5 ± 0.2a | 3.6 ± 0.3a | 0.41 | |
| Plasma phosphorus concentration (mM) | Preclamp | 0.93 ± 0.11 | 1.00 ± 0.16 | 0.11 |
| Postclamp | 0.57 ± 0.11b | 0.69 ± 0.19b | 0.05 | |
| Plasma creatinine (mg/dl) | Preclamp | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.95 |
| Postclamp | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.70 | |
| Plasma insulin (μU/ml) | Preclamp | 8.6 ± 3.6 | 12.0 ± 8.4 | 0.25 |
| Postclamp | 149.3 ± 34.1b | 151.6 ± 36.2b | 0.85 | |
| Plasma glucose (mg/dl) | Preclamp | 92 ± 7 | 118 ± 34 | 0.02 |
| Postclamp | 91 ± 7 | 100 ± 15 | 0.05 | |
| UKV (mmol/2 h) | Preclamp | 1.11 ± 0.90 | 0.70 ± 0.86 | 0.17 |
| Postclamp | 1.24 ± 0.71 | 0.67 ± 0.56 | 0.02 | |
| UPV (mmol/2 h) | Preclamp | 3.17 ± 3.18 | 3.53 ± 5.54 | 0.83 |
| Postclamp | 1.67 ± 1.12b | 1.41 ± 0.97 | 0.48 |
Values are mean +/− standard deviation. [K+], potassium concentration; UKV, urine potassium excretion rate; UPV, urine phosphorus excretion rate.
P value compares nondiabetic group to diabetic group. Comparison of pre- versus postclamp values:
P < 0.05,
P < 0.01.
Glucose, Potassium, and Phosphate Uptake
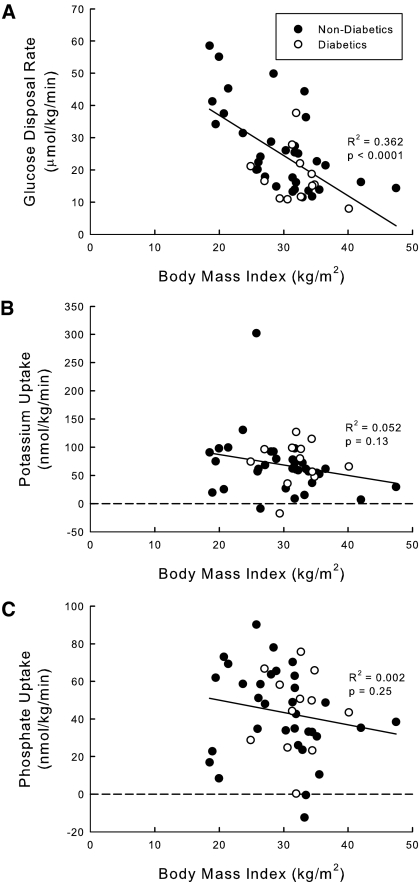
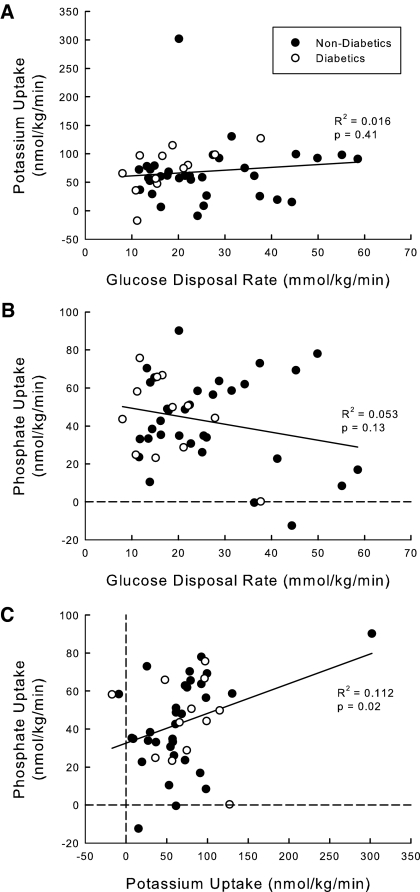
There was a great deal of overlap of glucose disposal rate between the two groups of subjects (Table 3). Type 2 diabetics have numerically lower glucose disposal rates than nondiabetics (19.4 ± 9.5 versus 26.1 ± 13.2 μmol/kg per min, P = 0.10). This is not surprising because the absolute segregation of subjects into two distinct groups is artificial. It is more meaningful to use a continuous independent variable such as BMI as a surrogate. As expected, glucose disposal rate falls as BMI rises when the two groups are analyzed together (Figure 1A). In contrast, potassium (Figure 1B) and phosphate (Figure 1C) uptake do not correlate at all with BMI. The fall in plasma [K+] was similar between the diabetic and nondiabetic groups (0.5 ± 0.2 versus 0.5 ± 0.3 mM, P = 0.90). There were also no differences in potassium uptake (72.2 ± 37.8 versus 66.8 ± 53.5 nmol/kg per min, P = 0.74) or phosphate uptake (40.9 ± 24.3 versus 44.3 ± 22.7 nmol/kg per min, P = 0.65) rate between the diabetic and nondiabetic groups. Despite the fall in glucose disposal rate with increasing BMI, there was no relationship between rate of potassium uptake (Figure 2A) or phosphate uptake (Figure 2B) to glucose disposal rate, and there was no correlation between potassium and phosphate uptake.
Table 3.
Cellular uptakes and urinary excretion rates during hyperinsulinemia for three solutes in nondiabetic versus diabetic group
| Nondiabetic Group | Diabetic Group | P | |
|---|---|---|---|
| Glucose disposal rate (μmol/kg per min) | 26.1 ± 13.2 | 19.4 ± 9.5 | 0.10 |
| Change in plasma [K+] (mM) | −0.5 ± 0.3 | −0.5 ± 0.2 | 0.90 |
| Potassium uptake (nmol/kg per min) | 66.8 ± 53.5 | 72.2 ± 37.8 | 0.74 |
| Change in UKV (mmol/2 h) | 0.13 ± 1.05 | −0.025 ± 0.94 | 0.65 |
| Change in plasma phosphorus concentration (mM) | −0.37 ± 0.12 | −0.32 ± 0.12 | 0.21 |
| Phosphate uptake (nmol/kg per min) | 44.3 ± 22.7 | 40.9 ± 24.3 | 0.65 |
| Change in UPV (mmol/2 h) | −1.50 ± 3.00 | −2.12 ± 5.80 | 0.72 |
Values are mean +/− standard deviation. [K], potassium concentration; UKV, urine potassium excretion rate; UPV, urine phosphorus excretion rate.
Figure 1.
Relationship of (A) glucose, (B) potassium, and (C) phosphate uptake as a function of body mass index (BMI). Forty-five subjects with a wide range of BMIs underwent hyperinsulinemic euglycemic clamp. Glucose disposal rate was calculated from the glucose infusion required to maintain euglycemia. Potassium and phosphate uptake were calculated as the reduction in extracellular potassium or phosphate corrected for urinary excretion. The correlation coefficient was calculated for the relationship between the x and y values as well as the P value for the R2.
Figure 2.
Relationship between uptake of three solutes: (A) potassium versus glucose, (B) phosphate versus glucose, and (C) phosphate versus potassium. Forty-five subjects with a wide range of BMIs underwent hyperinsulinemic euglycemic clamp. Glucose disposal rate was calculated from the glucose infusion required to maintain euglycemia. Potassium and phosphate uptake were calculated as the reduction in extracellular potassium or phosphate corrected for urinary excretion. The correlation coefficient was calculated for the relationship between the x and y values as well as the P value for the R2.
Discussion
Insulin shifts glucose and potassium from the extracellular to the intracellular compartment. The primary goal of this study was to examine whether these are coupled actions using subjects with a wide range of glucose disposal rates. There was no correlation between potassium uptake and BMI and between glucose disposal rate and the fall in plasma [K+] or potassium uptake. A similar lack of relationship is observed with phosphate. This indicates that the actions of insulin on glucose and potassium or phosphate uptake are independently regulated.
Classic physiologic studies in animals and humans support dissociative regulation. Potassium and sodium movement in the isolated diaphragm in response to insulin occurs in the absence of glucose (27). There is temporal separation of potassium and glucose uptake (28,29), and insulin stimulates potassium uptake in doses at which there is no appreciable glucose flux (30). Rats fed a high-fat diet developed slower uptake of glucose and potassium, creating an apparent view of concordant regulation (31). However, the high-fat diet resulted in a dramatically lower potassium ingestion, which is likely the reason for the reduction of cellular potassium buffering. When the high-fat diet was supplemented with higher potassium to neutralize the reduction in dietary potassium, cellular potassium uptake was not different than control. This is strong evidence to support discordant regulation.
Limited human studies have been performed. Hyperinsulinemic euglycemic clamps performed on 20 adolescents with insulin-dependent diabetes and 10 age-matched controls disclosed a slightly lower insulin-induced fall in plasma [K+] in diabetic subjects and a correlation between glucose disposal rate and fall in serum [K+] in control but not in diabetic subjects (20). It is unclear why this relationship is not maintained in diabetics if cell entry of glucose and potassium are coupled. Patients with acanthosis nigricans and insulin resistance have reduced glucose disposal rate, but the fall in plasma [K+] was not affected (21). DeFronzo et al. studied 29 normal nondiabetic subjects and analyzed the potassium and glucose balance in the splanchnic bed and the periphery (19). This study is cited (20) to support concordant glucose and potassium uptake by cells because of the positive correlation of the fall in plasma [K+] (which is a composite of splanchnic and peripheral potassium transport) with glucose utilization. Note that the DeFronzo study separately examined splanchnic and peripheral potassium handling, which are both very complex; in fact, the authors concluded that there was no evidence in either system that potassium uptake is coupled to glucose uptake (19).
We studied a larger number of human subjects with a wide range of glucose disposal rates, including subjects with high BMIs and some features of the metabolic syndrome to frank type 2 diabetics, and showed clear dissociation of glucose disposal from potassium uptake. We also examined phosphate, which is another electrolyte for which the shift is regulated by insulin but the cellular mechanisms are not defined (32,33). Because the utilization of glucose mandates its conversion to glucose-6-phosphate, concomitant stoichiometrically equivalent coupled phosphate uptake into the same cells seems logical. The interpretation of phosphate flux is more complex because the fall in plasma phosphate triggers a rapid secondary renal retention (34). This can be due to the effect of falling plasma phosphate concentration or a direct effect of insulin on the tubule (35). But even correcting for this, we still did not observe a correlation between glucose and phosphate uptake, further suggesting that transport of different solutes are differentially regulated by insulin.
Insulin receptor binding initiates a complicated cascade eventuating in translocation of the facilitative glucose transporter GLUT4 to the plasma membrane to affect glucose uptake (36). Insulin stimulates potassium cellular uptake by elevation and increased sensitivity to intracellular sodium, translocation and activation Na+/K+-ATPase, and inhibition of potassium efflux (30,37–43). The mechanism of insulin-stimulated phosphate uptake is unknown. It is clear that insulin receptor activation leads to divergent glucose and potassium regulatory pathways, allowing them to be independently regulated at a postreceptor level. The postreceptor defect described in patients with the metabolic syndrome or type 2 diabetes mellitus affects the arm, leading to glucose uptake but not potassium uptake. An alternative and rather different view is that the reduction of glucose uptake is a physiologic adaptive mechanism in which the cells protect themselves against caloric overload and appropriately and specifically downregulate the glucose uptake pathway (44). In this model, there will be no reason to restrict entry of other solutes that do not bear calories. Finally, insulin resistance may affect glucose and potassium because serum [K+] per se may exert an effect on insulin-induced potassium uptake (43), and any disturbance in serum [K+] may self-rectify the defect.
In addition to providing further foundation to search for the underlying mechanisms and reasons for this divergent regulation, there are some clinical implications. When treating hyperkalemia, insulin remains efficacious in diabetics and nondiabetics and one does not need to resort to b-agonists, and diabetics do not require different doses of insulin to shift potassium. Because the commonly encountered “insulin-resistant” patients actually have preserved insulin-induced potassium disposal, one wonders why their high insulin levels are not causing hypokalemia. This remains to be explored, but it is conceivable that any disturbance in serum [K+] is capable of regulating insulin-stimulated potassium uptake (43). Our study confirms that insulin independently regulates glucose and potassium uptake into cells and this independence explains why in noninsulin-dependent diabetic insulin resistance leads to impaired insulin uptake into cells but has no effect on the cell's potassium disposal.
Disclosures
None.
Acknowledgments
This work was supported by the National Institutes of Health (M01-RR00633, P01-DK20543, R01-DK081423, and K23-RR21710), the Charles Pak and Donald Seldin Endowment for Metabolic Research, and the Simmons Family Foundation. We are grateful to the expertise of Mr. John Poindexter for biostatistical support, the nursing staff of the University of Texas Southwestern General Clinical Research Center, and the technical staff at the Mineral Metabolism Clinical Laboratory.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “A Critically Swift Response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle,” on pages 1513–1516.
References
- 1. Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR: Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest 76: 2306–2311, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lafontan M, Langin D: Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Lewis GF, Zinman B, Groenewoud Y, Vranic M, Giacca A: Hepatic glucose production is regulated both by direct hepatic and extrahepatic effects of insulin in humans. Diabetes 45: 454–462, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ: Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest 90: 2348–2354, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magkos F, Wang X, Mittendorfer B: Metabolic actions of insulin in men and women. Nutrition 26: 686–693, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shepherd PR, Kahn BB: Glucose transporters and insulin action—Implications for insulin resistance and diabetes mellitus. N Engl J Med 341: 248–257, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Graham TE, Kahn BB: Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res 39: 717–721, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Kamel KS, Lin SH, Halperin ML: Clinical disorders of hyperkalemia. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York, Elsevier, 2008, pp 1387–1406 [Google Scholar]
- 9. Levy DL, Goldstein SAN. Effect of electrolyte disorders on excitable membranes. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York, Elsevier, 2008, pp 1407–1428 [Google Scholar]
- 10. Briggs AP, Koechig I, Doisy EA, Weber CJ: Some changes in the composition of blood due to the injection of insulin. J Biochem 58: 1924 [Google Scholar]
- 11. DeFronzo RA, Sherwin RS, Dillingham M, Hendler R, Tamborlane WV, Felig P: Influence of basal insulin and glucagon secretion on potassium and sodium metabolism. Studies with somatostatin in normal dogs and in normal and diabetic human beings. J Clin Invest 61: 472–479, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dluhy RG, Axelrod L, Williams GH: Serum immunoreactive insulin and growth hormone response to potassium infusion in normal man. J Appl Physiol 33: 22–26, 1972 [DOI] [PubMed] [Google Scholar]
- 13. Gomez M, Curry DL: Potassium stimulation of insulin release by the perfused rat pancreas. Endocrinology 92: 1126–1134, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Fenn WO: The deposition of potassium and phosphate with glycogen in rat liver. J Biol Chem 128: 297–307, 1939 [Google Scholar]
- 15. Ammon RA, May WS, Nightingale SD: Glucose-induced hyperkalemia with normal aldosterone levels. Studies in a patient with diabetes mellitus. Ann Intern Med 89: 349–351, 1978 [DOI] [PubMed] [Google Scholar]
- 16. Goldfarb S, Cox M, Singer I, Goldberg M: Acute hyperkalemia induced by hyperglycemia: Hormonal mechanisms. Ann Intern Med 84: 426–432, 1976 [DOI] [PubMed] [Google Scholar]
- 17. Schambelan M, Sebastian A, Rector FC, Jr: Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): Role of increased renal chloride reabsorption. Kidney Int 19: 716–727 1981 [DOI] [PubMed] [Google Scholar]
- 18. Weidmann P: Hyporeninemic hypoaldosteronism and the differential diagnosis of hyperkalemia. Schweiz Med Wochenschr 112: 1764–1774, 1982 [PubMed] [Google Scholar]
- 19. DeFronzo RA, Felig P, Ferrannini E, Wahren J: Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol 238: E421–E427, 1980 [DOI] [PubMed] [Google Scholar]
- 20. Arslanian S, Austin A: Impaired insulin mediated potassium uptake in adolescents with IDDM. Biochem Med Metab Biol 46: 364–372, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Cohen P, Barzilai N, Lerman A, Harel H, Szylman P, Karnieli E: Insulin effects on glucose and potassium metabolism in vivo: Evidence for selective insulin resistance in humans. J Clin Endocrinol Metab 73: 564–568, 1991 [DOI] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Herbert V, Lau KS, Gottlieb CW, Bleicher SJ: Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25: 1375–1384, 1965 [DOI] [PubMed] [Google Scholar]
- 24. Yalow RS, Berson SA: Immunoassay of endogenous plasma insulin in man. J Clin Invest 39: 1157–1175, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M: Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976 [DOI] [PubMed] [Google Scholar]
- 26. Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH: Short-term K(+) deprivation provokes insulin resistance of cellular K(+) uptake revealed with the K(+) clamp. Am J Physiol Renal Physiol 280: F95–F102, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Creese R: Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol 197: 255–278, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andres R, Baltzan MA, Cader G, Zierler KL: Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest 41: 108–115, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kestens PJ, Haxhe JJ, Lambotte L, Lambotte C: The effect of insulin on the uptake of potassium and phosphate by the isolated perfused canine liver. Metabolism 12: 941–950, 1963 [PubMed] [Google Scholar]
- 30. Zierler KL, Rabinowitz D: Effect of very small concentrations of insulin on forearm metabolism. Persistence of its action on potassium and free fatty acids without its effect on glucose. J Clin Invest 43: 950–962, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi CS, Lee FN, McDonough AA, Youn JH: Independent regulation of in vivo insulin action on glucose versus K(+) uptake by dietary fat and K(+) content. Diabetes 51: 915–920, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Harrop GA, Jr, Benedict EM: The participation of inorganic substance in carbohydrate metabolism. Proc Soc Exp Biol Med 20: 683–697, 1923 [Google Scholar]
- 33. DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ: The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55: 845–855, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hammerman MR, Rogers S, Hansen VA, Gavin JR, III: Insulin stimulates Pi transport in brush border vesicles from proximal tubular segments. Am J Physiol 247: E616–E624 1984 [DOI] [PubMed] [Google Scholar]
- 35. Klip A: The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab 34: 481–487, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Ewart HS, Klip A: Hormonal regulation of the Na(+)-K(+)-ATPase: Mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol 269: C295–311, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Fehlmann M, Freychet P: Insulin and glucagon stimulation of (Na+-K+)-ATPase transport activity in isolated rat hepatocytes. J Biol Chem 256: 7449–7453, 1981 [PubMed] [Google Scholar]
- 38. Gourley DR: Effect of insulin on potassium exchange in normal and ouabain-treated skeletal muscle. J Pharmacol Exp Ther 148: 339–347, 1965 [PubMed] [Google Scholar]
- 39. Grinstein S, Erlij D: Insulin unmasks latent sodium pump sites in frog muscle. Nature 251: 57–58, 1974 [DOI] [PubMed] [Google Scholar]
- 40. McGill DL, Guidotti G: Insulin stimulates both the alpha 1 and the alpha 2 isoforms of the rat adipocyte (Na+,K+) ATPase. Two mechanisms of stimulation. J Biol Chem 266: 15824–15831, 1991 [PubMed] [Google Scholar]
- 41. Rosic NK, Standaert ML, Pollet RJ: The mechanism of insulin stimulation of (Na+,K+)-ATPase transport activity in muscle. J Biol Chem 260: 6206–6212, 1985 [PubMed] [Google Scholar]
- 42. Youn JH, McDonough AA: Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Unger RH: Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab 14: 398–403, 2003 [DOI] [PubMed] [Google Scholar]