The centrality of the endothelium in vascular health
The key to prevention of vascular disease is a healthy endothelium. The endothelium lines all blood and lymphatic conduits, and it acts as the Teflon coating for these conduits. This delicate membrane, only a single-cell layer in thickness, exerts tremendous control over vascular tone, structure, and interaction with circulating blood elements (1). To play its central role in vascular homeostasis, the endothelium produces a panoply of paracrine substances. One of the most potent and versatile of these factors is nitric oxide (NO). This simple molecule has diverse effects. It relaxes vascular smooth muscle; inhibits vascular smooth muscle cell migration and proliferation; prevents platelet adherence and aggregation; suppresses adhesion molecules and chemokines mediating inflammation; and promotes endothelial cell survival, proliferation and migration to facilitate restoration of the endothelial lining (2). Therefore it is not surprising that deficiencies of endothelium-derived NO play a role in the initiation and progression of the most common vascular diseases, such as atherosclerosis. Furthermore, biomarkers reflecting the activity of NO synthase (NOS) are independent prognosticators for cardiovascular events and mortality (3-5).
Regulation of NOS activity
Because of its centrality in vascular function, it is important to understand the regulation of the enzyme that produces NO. There are three isoforms of NOS, each of which metabolize the amino acid L-arginine to L-citrulline and NO. The expression and activity of the endothelial isoform (eNOS) is highly regulated by blood flow as well as blood-borne endocrine and paracrine substances. Another endogenous regulator of NOS activity are the methylarginines (6). The mechanism by which the concentration of methylarginines are regulated is the subject of the paper by Torondel and colleagues in this issue of Vascular Medicine (7).
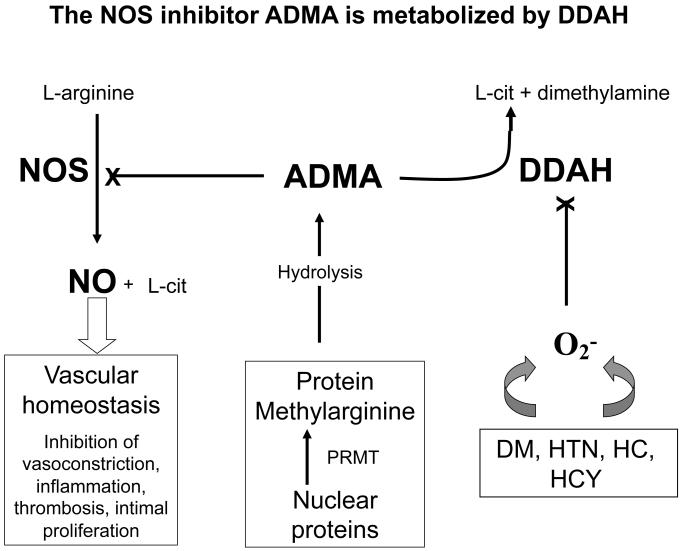
The methylarginines are analogues of arginine in which the terminal guanidino nitrogens of the amino acid are methylated (8). The asymmetric dimethylarginine (ADMA) and the mono-methyl form (NMMA) inhibit the synthesis of NO, by competitively interfering at the L-arginine binding site of NOS (Figure). The symmetric dimethylarginine (SDMA) does not compete at the L-arginine binding site, so does not interfere with NOS activity directly (but could compete for cellular uptake of arginine at the y+ transporter). Each of the methylarginines are derived from proteins (largely histone proteins) containing arginine residues that are methylated. When these proteins are hydrolyzed, the methylarginines are released. Because ADMA is much more prevalent than NMMA, it has received the most study as an endogenous regulator of NOS activity.
Figure.
Regulation of Nitric oxide (NO) by asymmetric dimethylarginine (ADMA). Nitric oxide synthase (NOS) generates the potent vasodilator NO from L-arginine. The synthesis of NO is competitively antagonized by ADMA. This endogenous inhibitor of NOS is derived from the hydrolysis of proteins containing arginine resides that were methylated by protein arginine methyltransferases (PRMT). The intracellular and plasma levels of ADMA are regulated to a great extent by dimethylarginine dimethylaminohydrolase (DDAH) which degrades ADMA to citrulline and dimethylamine. The activity of DDAH is reduced by cellular oxidative stress that is increased in the setting of diabetes mellitus (DM), hypercholesterolemia (HC), hypertension (HC) and hyperhomocysteinemia (HCY). L-cit = L-citrulline.
Whereas SDMA is largely excreted in the urine, in humans about 80% of ADMA and NMMA is cleared by the enzyme dimethylarginine dimethylaminohydrolase (DDAH; 9). DDAH exists as two isoforms, DDAH1 and DDAH2, that have overlapping distributions in all mammalian tissues. Pharmacological antagonism of DDAH activity in vascular rings causes ADMA to accumulate, inducing contraction of the vascular ring (10). Similarly, a partial genetic deficiency of DDAH1 (the complete deficiency of DDAH-1 is embryonically lethal) is associated with elevated plasma ADMA levels, increased blood pressure and systemic vascular resistance (11). Furthermore, animals which overexpress DDAH1 have lower plasma ADMA levels, increased NO synthesis, and lower blood pressure (12). Thus the activity of DDAH appears to be an important determinant of ADMA and NMMA levels, and thus NOS activity.
Role of DDAH in regulating NOS activity
Torondel and co-workers have extended this concept by using adenoviral vectors to enhance the endothelial expression of DDAH (7). They used adenoviral constructs encoding DDAH1 or DDAH2 to overexpress the enzymes in human endothelial cells. The adenoviral transfection with either the DDAH 1 or 2 construct resulted in a modest increase in DDAH activity of about 50%. This modest increase in DDAH activity was associated with a modest decline (about 25%) of ADMA in the conditioned medium, consistent with the effect of DDAH to reduce ADMA levels. Surprisingly, this modest effect on ADMA was associated with a striking (3-fold) increase in nitrate levels in the conditioned medium. Similar results were obtained with murine carotid arteries in which the endothelium was exposed to the DDAH adenoviral construct. Nitrate is a stable breakdown product of NO, and in these studies (where there are no other significant sources of nitrate) reflects the activity of endothelial NOS. It is possible that the slight reduction in ADMA in the conditioned medium reflected a larger reduction in intracellular ADMA. However, small changes in plasma and tissue ADMA concentrations have been reported to be associated with significant changes in NOS activity in previous studies. For example, in the DDAH-1 transgenic mouse, a decline in plasma ADMA levels from 1.6 to 0.7 uM is associated with a doubling of urinary nitrogen oxide (12). In these same animals there is a striking enhancement of NO-modulated endothelial processes such as angiogenesis in response to hindlimb ischemia. Tissue ADMA levels in the ischemic calf muscles of transgenic DDAH animals are reduced by comparison to controls (18.3 vs 9.2 nmol/g protein), and tissue NOS activity is increased 60% (13). In the current study, endothelium-dependent vasodilation was improved by the DDAH transfection of the carotid artery intima of wildtype mice. In mice that are partially deficient in DDAH1 (DDAH1+/−), there is an impairment of endothelium dependent vasodilation that was reversed in this study by transfection with either DDAH isoform. To summarize, by increasing the endothelial expression of DDAH using adenoviral constructs encoding DDAH-1 or DDAH-2, the investigators increased the metabolism of ADMA. The effect on ADMA levels and NOS activity of either construct was similar (as opposed to data recently provided by Pope AJ and colleagues which suggest that DDAH-2 may regulate NO synthesis through an ADMA-independent mechanism). The subsequent reduction in ADMA concentration permitted an increase in NOS activity, generating more NO and improving endothelium-dependent vasorelaxation.
Clinical relevance of DDAH modulation
When the endothelium of a human artery is exposed to elevated levels of LDL-cholesterol, glucose, or homocysteine, it begins to manifest signs of dysfunction, eg. Impaired ability to induce vasodilation. The mechanisms of endothelial vasodilator dysfunction are multifactorial, but it is clear that elevated levels of ADMA play a role (15-17). In some cases, the elevation in plasma ADMA may be due to impaired renal function (6). This is in part due to the fact that ADMA is excreted in the urine, but also because the kidney is a rich source of DDAH and degrades ADMA. In patients with renal insufficiency, plasma ADMA levels are an independent risk factor for progression of renal disease and mortality (18).
However, the most common cause of elevated plasma ADMA levels may be impaired DDAH activity. Because DDAH contains a reactive sulfhydryl group in its active catalytic site, which is vulnerable to oxidative or nitrosative stress (19). Exposure of endothelial cells to high levels of LDL-cholesterol, glucose, or homocysteine cause an endothelial oxidative stress that attenuates DDAH activity and increases levels of ADMA in the conditioned medium, an effect that can be reversed by thiol antioxidants (20-22). Similarly, in individuals with hypercholesterolemia, diabetes or insulin resistance, hypertension or hyperhomocysteinemia, plasma ADMA levels are elevated, and endothelial vasodilator function is impaired (8). The adverse effects of elevated plasma ADMA on endothelial vasodilator function can be acutely reversed by administration of the NOS substrate L-arginine, as one might expect in the case where there is an elevated concentration of the competitive inhibitor of NOS(23). However, long-term administration of L-arginine may not be a viable option for maintaining normal levels of NO generation (24).
Is DDAH a therapeutic target in the treatment of vascular disease?
Torondel and colleagues suggest that gene therapy to increase local vascular expression of DDAH may be used to treat or prevent some vascular diseases, such as restenosis in the setting of balloon angioplasty or stenting. Although there is strong pre-clinical evidence that enhancing local vascular synthesis of NO can prevent restenosis (25), the application of gene therapy in humans faces technical and regulatory obstacles. Pharmacological approaches to enhance DDAH activity may be a more tractable. Indeed, agents which increase DDAH activity (angiotensin converting enzyme inhibitors, angiotensin receptor antagonists, metformin, PPAR agonists) or expression (such as estrogen, retinoic acid, and FXR agonists) are known (8). Novel agents designed to specifically increase the expression of DDAH would likely have other transcriptional effects, which could increase the risk of off-target adverse effects. Another approach would be to directly enhance the activity of DDAH, as with an allosteric modulator of enzyme activity. This is not a typical pharmaceutical approach, although recent success with allosteric modulators (26) may increase interest in this approach to drug development.
It should be mentioned that for certain vascular diseases, inhibition of DDAH activity to increase ADMA accumulation may be useful (27). For example, the cardiovascular collapse caused by sepsis is due to excessive upregulation of the inducible form of NO synthase (iNOS). The excessive amount of NO produced in sepsis suppresses contractile activity of vascular and myocardial smooth muscle, and can result in septic shock. In this case, ADMA might act as a brake on iNOS, reducing the excessive production of NO. Accordingly, in this scenario, an antagonist of DDAH could increase endogenous ADMA levels in a therapeutic manner. However, the enthusiasm for this approach has been tempered by the unsuccessful trials of pharmacological NOS antagonists.
To conclude, the enzyme DDAH regulates the intracellular and plasma levels of the endogenous NOS inhibitor ADMA. Because NO is a key regulator of vascular homeostasis; and because NO deficiency seems to be a major factor in the initiation and progression of vascular diseases; approaches to enhance NO synthesis could provide a powerful approach toward vascular health. Therapeutic modulation of DDAH activity (so as to reduce ADMA and increase NO synthesis) holds promise as a novel therapeutic avenue.
Acknowledgements and Disclosures
This work was supported in part by grants from the National Institutes of Health (K12 HL087746, RC2HL103400, 1U01HL100397), the California Tobacco Related Disease Research Program of the University of California (18XT-0098), the American Heart Association (#0970036N), The Wallace H. Coulter Translational Research Grant Program, and the California Institute for Regenerative Medicine (RS1-00183), and the Stanford Cardiovascular Institute. Dr. Cooke is the inventor of patents owned by Stanford University for diagnostic and therapeutic applications of the NOS pathway from which he receives royalties. Dr. Cooke is a consultant for NiCox.
References
- 1.Aird WC. Endothelial biomedicine. Cambridge University Press; New York, NY: 2007. [Google Scholar]
- 2.Cooke JP. Flow, NO, and atherogenesis. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000 Apr 25;101(16):1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 4.Valkonen VP, Paiva H, Salonen JT, et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–2128. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 5.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009 Mar 31;119(12):1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 7.Torondel, et al. Vasc Med. THIS ISSUE. [Google Scholar]
- 8.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004 Apr 20;109(15):1813–8. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 9.Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005 Jul;10(Suppl 1):S73–81. doi: 10.1191/1358863x05vm597oa. [DOI] [PubMed] [Google Scholar]
- 10.MacAllister RJ, Parry H, Kimoto M, et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007 Feb;13(2):198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 12.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger M, Wang B, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. DDAH Regulates NO Synthesis: Genetic and physiological evidence. Circulation. 2003;108:1043–1048. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 13.Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP. Overexpression of Dimethylarginine Dimethylaminohydrolase (DDAH) Reduces Tissue ADMA Levels and Enhances Angiogenesis. Circulation. 2005 Mar 22;111(11):1431–8. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 14.Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. J Biol Chem. 2009 Dec 18;284(51):35338–47. doi: 10.1074/jbc.M109.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi F, Asagmi T, Cooke JP, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 17.Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, Szuba A, Malinow MR, Wascher TC, Pachinger O, Cooke JP. Endothelial dysfunction induced by hyperhomocysteinemia: Role of ADMA. Circulation. 2003 Aug 26;108(8):933–8. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 18.Kielstein JT, Zoccali C. Asymmetric dimethylarginine: a novel marker of risk and a potential target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens. 2008 Nov;17(6):609–15. doi: 10.1097/MNH.0b013e328314b6ca. [DOI] [PubMed] [Google Scholar]
- 19.Murray-Rust J, Leiper J, McAlister M, et al. Structural insights into the hydrolysis of cellular nitric oxide synthase inhibitors by dimethylarginine dimethylaminohydrolase. Nat Struct Biol. 2001;8:679–683. doi: 10.1038/90387. [DOI] [PubMed] [Google Scholar]
- 20.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel Mechanism for Endothelial Dysfunction: Dysregulation of Dimethylarginine Dimethylaminohydrolase. Circulation. 1999 Jun 22;99(24):3092–5. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 21.Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 22.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven G, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002 Aug 20;106(8):987–92. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 23.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992 Oct;90(4):1248–53. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson A, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: No benefit and possible harm. Circulation. 2007 Jul 10;116(2):188–95. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 25.von der Leyen HE, Gibbons GH, Morishita R, Lewis NP, Zhang L, Nakajima M, Kaneda Y, Cooke JP, Dzau VJ. Gene therapy inhibiting neointimal vascular lesion: In vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C-H, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Monzingo AF, Hu S, Schaller TH, Robertus JD, Fast W. Developing dual and specific inhibitors of dimethylarginine dimethylaminohydrolase-1 and nitric oxide synthase: toward a targeted polypharmacology to control nitric oxide. Biochemistry. 2009 Sep 15;48(36):8624–35. doi: 10.1021/bi9007098. [DOI] [PMC free article] [PubMed] [Google Scholar]