Abstract
Objective. Treatment algorithms in RA include factors associated with poor prognosis; however, many patients remain erosion free despite years of disease. Our objective was to characterize the group of RA patients without erosions and identify its clinical predictors.
Methods. Our study was conducted within a prospective observational cohort of RA patients recruited from the outpatient practice of an academic medical centre. We studied patients with bilateral hand radiographs at cohort baseline and 2-year follow-up assessed with Sharp/van der Heijde scores (SHS). The primary outcome was erosion-free status at baseline and 2-year follow-up. We assessed baseline values of the following as potential correlates: age at RA onset, gender, RA duration, BMI, 28-joint DAS (DAS-28), CRP, anti-CCP status, tender and swollen joint counts, functional status [multidimensional HAQ (MDHAQ)], tobacco use and RA treatments. Variables with P ≤ 0.25 in the univariate analyses were assessed using backward selection in multivariable logistic regression models.
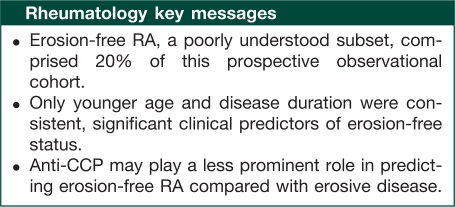
Results. Of the 271 subjects included, 21% (n = 56) were considered erosion free. Forty-six per cent (n = 26) of this group was anti-CCP positive compared with 56% (n = 121) in subjects with erosions present. Mean RA duration for erosion-free subjects was 3.9 years compared with 4.6 years in erosive subjects. Treatments for RA did not differ between the two groups. In the multivariable-adjusted analysis, significant predictors of erosion-free status were younger age at onset and shorter RA duration.
Conclusion. In our cohort, 21% of subjects were erosion free at baseline and 2 years. Few baseline clinical characteristics significantly predicted erosion-free status.
Keywords: Rheumatoid arthritis, Disease progression, Prognosis
Introduction
RA is a chronic inflammatory joint disease that, if left untreated, leads to damage of articular cartilage and development of bone erosions. In some patients, the disease process ultimately destroys affected joints. Three subgroups of RA patients can be delineated with respect to bone erosions: Group 1—patients who develop or have worsening bone erosions over time despite treatment; Group 2—patients who present with erosions, but the erosions remain unchanged over time; and Group 3—patients who present without erosions and remain erosion free. In prospective cohort studies and clinical trials, RA subjects with progressive erosive disease (Group 1) are compared with those who do not progress, which includes those with erosions and do not progress (Group 2) and those who never develop erosions (Group 3) [1–4]. Therefore, understanding predictors for who will remain erosion free is not a simple converse of the predictors for progressors. Few studies focus on the third group of RA patients who remain erosion free during follow-up. In one 10-year prospective cohort study, ∼17% of RA subjects remained erosion free [5]. A review of clinical trial data found that subjects with RA duration >1–2 years without bone erosions at baseline were unlikely to develop bone erosions during treatment [6].
Most clinical studies and trials concentrate on Group 1 RA patients who have progressive erosive disease, because they stand to benefit the most from early aggressive treatment [4, 7–9]. However, an improved understanding of Group 3 RA patients who remain erosion free, will inform practitioners regarding patients who would likely have minimal joint damage regardless of therapy. In addition, identifying this group and its clinical characteristics will facilitate genetic and biologic studies that can further elucidate the pathogenesis of RA and its subtypes. Thus, rather than focusing on who will develop erosions or has stable erosions, we characterized patients who have not developed erosions nor did so during a 2-year follow-up and attempted to identify predictors.
Methods
Study population
We conducted this study within the Brigham Rheumatoid Arthritis Sequential Study (BRASS), a prospective, observational cohort of 1105 RA patients seen at Brigham and Women’s Hospital, Boston, MA, USA. Subjects were aged ≥18 years and had a diagnosis of RA from their rheumatologist.
Data collection
Baseline evaluation, conducted at the time of recruitment and not disease onset, included demographic and clinical information, evaluation for the 1987 ACR classification criteria for RA [10], the multidimensional HAQ (MDHAQ) [11], 28-joint DAS (DAS-28 calculated with CRP) [12], tobacco use (ever/never), laboratory testing and bilateral posterior–anterior (PA) hand radiographs. A physical examination with 28-joint count, assessment of pain and disease activity by a physician, and similar evaluations by the patient were collected at baseline evaluation. Bilateral hand and wrist radiographs were performed at baseline and 2 years (see below). For further details on BRASS and data collection variables, please refer to Iannaccone et al. [13].
Laboratory assessment
Blood was collected at the baseline evaluation and measured using the following protocols. We measured RF using immunoturbidimetric technique on the Cobas Integra 700 analyser (Roche Diagnostics, Indianapolis, IN, USA), using reagents and calibrators from Roche. Anti-CCP was measured using a second-generation ELISA assay (INOVA Diagnostics, Inc., San Diego, CA, USA) with a titre of >20 U/ml considered as positive. High-sensitivity CRP was measured using reagents from Diasorin (Saluggia, Italy). Titres of <5 mg/l were considered normal.
Radiological assessment
Radiographs of both hands were obtained at baseline and 2 years. Four Brigham and Women’s Hospital radiologists scored the radiographs according to the Sharp/van der Heijde method in the hands only [14, 15], in random order without knowledge of the clinical data. Sixteen joints on each side of the body were scored for erosions (score range 0–5): 0 = no erosions; 1 = discrete erosion, with increasing points dependent on the amount of surface area affected; 3 = extends over imaginary middle of bone; and 5 = complete collapse of bone (total erosion score range: 0–160). Fifteen joints on each side of the body were scored for joint space narrowing (JSN) (score range 0–4) in each hand and wrist with: 0 = normal; 1 = focal or doubtful; 2 = generalized, <50% of original joint space; 3 = generalized, >50% of original joint space or subluxation; and 4 = bony ankylosis (total JSN score range 0–120) [15, 16]. The total erosion score was calculated by adding the erosion scores in both hands and wrists. Similarly, the total JSN score was calculated by adding the JSN scores in both hands and wrists. The Sharp/van der Heijde score (SHS) = total erosion score + total JSN score; the SHS can range from 0 to 280. The inter-rater reliability for the SHS in our study was 0.93 (calculated from the scores of two radiologists who read 90% of the radiographs).
The study was limited to subjects who had SHS for both baseline and 2 years and with RA duration ≤10 years (patients diagnosed between 1999 and 2009). We limited RA duration to allow for TNF inhibitors (TNFi) as a treatment option for all subjects in this study. The Partners Institutional Review Board approved all aspects of this study.
Statistical analysis
The primary outcome was sustained erosion-free status over 2 years. A subject was considered erosion free if they had a total erosion score of zero at baseline and 2 years. For the sensitivity analysis, a subject with a total erosion score of ≤1 at baseline and 2 years was considered erosion free.
The clinical characteristics of erosion-free subjects was compared with the erosive subjects using Student’s t-test for normally distributed variables and the Wilcoxon’s rank sum for those with non-normal distributions. Chi-square tests were used for categorical variables. Clinical variables with a P ≤ 0.25 were considered in a multivariable model along with age, gender and disease duration. Significant clinical predictors for erosion-free status were analysed using logistic regression with backward selection.
To examine the possible effects of treatment, baseline MTX and TNFi use were included into the final model as dichotomous variables (yes/no). TNFi’s included infliximab, etanercept and adalimumab. These treatment variables were considered confounders if they changed the point estimates of the existing predictors by >10%. The final model with the addition of the medication variables was also assessed for improvements in model fit using the Bayesian information criterion (BIC), discrimination using the area under the receiver operating characteristic curve (c-statistic) and calibration of risk using the Hosmer–Lemeshow test. Subjects were also stratified by RA duration of ≤2, >2–5 and >5–10 years to assess for significant differences in the percentage of subjects who were erosion free in each group. Furthermore, we assessed the concordance between anti-CCP positivity and RF positivity in erosion-free subjects using the kappa statistic. All analyses were conducted using SAS v9.2 (SAS Institute, Cary, NC, USA).
Results
Five hundred and sixty subjects in BRASS had baseline and 2-year follow-up radiographs formally assessed with SHS, with 271 subjects having ≤10 years of disease at baseline. Of these 271, 56 (21%) subjects had sustained erosion-free status at baseline and 2 years. Age at onset, disease duration and MDHAQ scores significantly differed between the erosion-free and remaining subjects (Table 1). Erosion-free subjects were younger, with a mean (s.d.) age of 45.0 (14.6) years compared with subjects with erosions with mean (s.d.) age of 51.3 (13.2) years. Erosion-free subjects also had shorter RA duration and lower MDHAQ scores. We found no significant differences in medication use at baseline including MTX, TNFi and HCQ. Among subjects not on a TNFi at baseline, 22% of erosion-free subjects were on a TNFi at Year 2 compared with 20% for those with erosions (P = 0.70). Thirty-four per cent of erosion-free subjects were in DAS-28 remission (DAS-28 < 2.6) at baseline compared with 26% of other subjects (P = 0.24). We found that 46% of erosion-free subjects and 56% of other subjects were anti-CCP positive (P = 0.19). Among the erosion-free subjects, those who were anti-CCP positive also tended to be RF positive (κ = 0.71) (Table 2). The percentage of subjects remaining erosion free at 2-year follow-up was 26% for those with ≤2 years of disease duration at baseline, 20% for those with >2–5 years and 15% for >5–10 years (P = 0.18). JSN scores were highly correlated with erosions and were significantly different between the erosion-free group and subjects with erosions (P ≤ 0.0001).
Table 1.
Characteristics of RA subjects who remained erosion free over 2 years compared with subjects with erosions (n = 271)
| Characteristic | Erosion free, n (%) = 56 (20.7) | Erosions present, n (%) = 215 (79.3) | P-value |
|---|---|---|---|
| Age at onset, mean (s.d.), years | 45.0 (14.6) | 51.3 (13.2) | 0.0026 |
| Gender: female, n (%) | 44 (78.6) | 175 (81.4) | 0.63 |
| Disease duration, mean (s.d.), years, | 3.4 (3.0) | 4.5 (3.1) | 0.03 |
| Tobacco use (ever/never), n (%) | 25 (44.6) | 103 (47.9) | 0.46 |
| BMI, mean (s.d.) | 27.4 (5.9) | 26.5 (5.3) | 0.34 |
| Fulfils 1987 ACR criteria, n (%) | 55 (98.2) | 209 (97.2) | 0.68 |
| Functional assessment | |||
| MDHAQ, mean (s.d.) | 1.4 (1.5) | 1.9 (1.4) | 0.018 |
| DAS | |||
| DAS, mean (s.d.) | 3.7 (1.7) | 3.8 (1.5) | 0.65 |
| Swollen joint count, mean (s.d.) | 6.1 (7.2) | 6.4 (6.8) | 0.72 |
| Tender joint count, mean (s.d.) | 7.8 (8.5) | 7.0 (7.2) | 0.49 |
| Baseline JSN score, median (IQR) | 0 (0) | 2 (0,10) | <0.0001 |
| Serological studies | |||
| RF positive, n (%) | 24 (42.9) | 112 (52.1) | 0.22 |
| Anti-CCP positive, n (%) | 26 (46.4) | 121 (56.3) | 0.19 |
| CRP titre, median (IQR), mg/dl | 3.6 (1.3, 8.7) | 2.8 (0.95, 6.3) | 0.53 |
| Medications at baseline, n (%) | |||
| MTX | 24 (42.9) | 97 (45.1) | 0.76 |
| TNFi | 15 (26.8) | 61 (28.4) | 0.81 |
| HCQ | 10 (17.9) | 54 (25.1) | 0.26 |
Table 2.
Anti-CCP and RF status of erosion-free subjects
| Anti-CCP positive, n | Anti-CCP negative, n | Total, n (%) | |
|---|---|---|---|
| RF positive, n | 21 | 3 | 24 (42.9%) |
| RF negative, n | 5 | 27 | 32 (57.1%) |
| Total, n (%) | 26 (46.4%) | 30 (53.5%) | 56 (100%) |
In the multivariable model, two baseline variables remained significant predictors of erosion-free status—younger age and shorter disease duration (Table 3). Each 5-year increase in age at RA onset resulted in a 20% decrease in odds for sustained erosion-free status [odds ratio (OR) 0.80; 95% CI 0.71, 0.91; P = 0.0003]. Every 1-year increase in disease duration resulted in ∼14% decrease in odds for remaining erosion free in 2 years (OR 0.86; 95% CI 0.77, 0.96; P = 0.006).
Table 3.
Clinical model for predicting erosion-free status at 2-year follow-up
| Initial modela | Partial model | Final modelb | |
|---|---|---|---|
| Variables | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age of RA onset (q5 years) | 0.82 (0.72, 0.93) | 0.80 (0.71, 0.90) | 0.80 (0.71, 0.91) |
| Gender: male | 1.7 (0.76, 3.7) | 1.6 (0.74, 3.5) | 1.6 (0.74, 3.5) |
| RA duration, years | 0.86 (0.77, 0.97) | 0.90 (0.78, 0.97) | 0.86 (0.77, 0.96) |
| Anti-CCP negative | 1.3 (0.70, 2.5) | 1.4 (0.74, 2.6) | – |
| MDHAQ | 0.81 (0.65, 1.0) | – | – |
aInitial model: age, gender, disease duration + all variables from univariate analysis with P < 0.25. bFinal model: significant variables only + gender.
In the sensitivity analysis where erosion-free status was defined as a total erosion score of ≤1 at baseline and 2 years, more subjects met the criteria for erosion-free status because the threshold for erosion-free status was lowered. Using this endpoint, 83 (31%) subjects were erosion free vs 56 (21%) subjects using the primary outcome definition. In the analysis using the secondary outcome definition, we found that younger age (OR 0.82; 95% CI 0.73, 0.91; P = 0.0002) and shorter disease duration (OR 0.90; 95% CI 0.82, 0.99; P = 0.045) remained significant predictors for erosion-free status. As well, the absence of anti-CCP (OR 1.8; 95% CI 1.1, 3.1; P = 0.048) became a significant predictor.
The addition of baseline use of MTX or TNFi into the final model did not change the point estimates of the existing variables nor were they significant predictors (see supplementary table, available as supplementary data at Rheumatology Online). The addition of variables for medications resulted in a decrease in the goodness of fit for the model (increased the BIC) with a minimal change in the c-statistic and Hosmer–Lemeshow P-value (see supplementary table, available as supplementary data at Rheumatology Online). The findings were similar with the addition of both variables in the same model and those on combination therapy with MTX and TNFi (data not shown). Medications added to the model in the sensitivity analysis where erosion free was defined as a total erosion score ≤1, also did not reach statistical significance and did not alter the point estimates of the existing variables, age, gender, disease duration and anti-CCP status.
Discussion
We believe this is one of the first studies from a large prospective RA cohort focused on characterizing erosion-free status and its predictors. With increasing knowledge regarding the pathogenesis of RA and the multitude of treatment options, it is important to understand not only which RA patients are likely to develop erosions and are at risk for progressive joint destruction, but also those who may never develop joint damage. Patients who are not at risk for bone erosions may theoretically do well with less potent therapies. While new biologic RA treatments have proven relatively safe in short- to medium-term studies, their high cost and unknown long-term safety make it imperative for clinicians to not over-treat patients who will do well without increasingly potent therapies. The focus of this study, erosion-free status, is one aspect of a good prognosis.
Erosion-free RA subjects comprised 21% of our cohort with RA disease duration of ≤10 years, which is consistent with findings from a previous study [5]. Since we found no published studies focused on erosion-free status, we included factors found to be significant for erosive disease from the literature in our univariate analysis. These factors included gender, RF status, elevated acute-phase reactants, level of disability and presence of arthritis in ≥3 joints [3, 5, 8, 17–20]. Across the majority of studies, the presence of anti-citrullinated peptide antibodies (ACPAs) is a significant risk factor if not the most important factor for erosive disease in RA [3, 5, 8, 17–20]. However, we found that many of these factors were not useful in distinguishing erosion-free subjects from stable and progressive erosive patients. Only younger age at onset and shorter disease duration were significant factors for predicting erosion-free RA status after 2 years.
Notably, anti-CCP status was not as important in predicting erosion-free status compared with its importance in predicting erosive disease. In our analysis, the absence of anti-CCP was not significant in the primary analysis where the strictest definition for erosion-free status was used (total erosion score = 0). Anti-CCP status was significant in our sensitivity analysis where erosion free was defined as a total erosion score of ≤1 at recruitment and at 2 years. These findings are likely due to lack of power stemming from the relatively small number of subjects who remained erosion free in our study. Alternatively, it is possible that although anti-CCP plays an important role in determining individuals at risk for worsening erosive disease, it has less influence in differentiating those who will remain erosion free from individuals who have stable erosive disease and progressive erosive disease.
Our findings demonstrate that simply taking the converse from studies of worsening erosive disease is not the optimal approach to understanding erosion-free status in RA. In this study, we compared subjects with erosion-free RA with those with erosions regardless of whether they progressed. This is in contrast to studies focused on understanding erosions that compare progressive erosive disease with those who have stable disease, which includes stable erosions and those who do not develop erosions.
Erosions appear early within the first 2–3 years and develop even with treatment [1]. Erosive disease also progresses in treated subjects in randomized controlled trials. In a trial comparing etanercept and MTX, alone and in combination [Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO)], subjects on MTX had a mean change in erosion score of 2.12 U after 2 years and those on etanercept had a mean increase of 0.36 U [4]. We therefore believe that 2 years was a reasonable length of follow-up to determine erosion-free status for our cohort where 81% have RA duration >2 years.
Several limitations in our methods are important to discuss. First, there is no clear definition for what score constitutes an erosion or erosive RA [21]. We employed a validated instrument, the Sharp/van der Heijde method to assess joint damage progression [14–16, 22]. Using a validated instrument allowed for scoring of radiographs by multiple readers. The inter-reader reliability (0.93) in our study was comparable with the published literature [16]. The SHS is a composite of a JSN score and erosion score for each joint on bilateral hand radiographs. We required a total erosion score of zero at baseline and 2 years, the strictest definition for defining erosion-free disease and the most sensitive measure for defining an erosion [17, 23, 24]. Alternatively, a more sensitive definition of erosion-free status can be employed such as the smallest detectable difference [24–26], two joints with erosions [21] or a total erosion score ≥1, to account for variation in reader interpretation. We opted to use the strictest definition for our primary outcome as there is no consensus in the literature of what erosion score constitutes a true bone erosion [23]. Secondly, the scoring method used in this analysis does exclude the feet as foot radiographs were not available. Erosive changes in the feet not seen in the hands have been observed in early disease (<2 years), with equivalent changes in the hands and feet in the ensuing years [1]. A misclassified subject in our study would have had erosions in the feet at baseline (mean disease duration of 3.4 years), and not developed erosions in the hands after ≥2 years. This is likely an uncommon occurrence.
Thirdly, subjects in our cohort had varying RA disease duration. Since joint damage progression has been shown to occur in the first years of the disease [1], comparing subjects with differing RA duration may not be a valid approach. Due to this concern, we limited this study to RA subjects with disease duration ≤10 years to account for secular trends in management and treatment availability. Although this allowed for a more homogeneous population, it decreased sample size and power for this study. To determine whether there were significant differences among subjects with <10 years of RA duration, we assessed the percentage of subjects who remained erosion free at differing intervals of disease duration: ≤2, >2–5, >5–10 years of RA and found no significant differences. Therefore, all disease durations were combined for the multivariate analysis and the model was further adjusted for disease duration. Finally, subjects in our study received various treatments, possibly blunting the role of specific variables on erosion-free status. It would be unethical to study untreated subjects to determine the natural history of erosions in RA. The majority of our subjects, even with short disease duration, were treated for RA before recruitment into the study. The first TNFi was approved for treatment of RA in the USA in 1998 [27], 11 years before the start of this study. Thus, we limited our analyses to subjects with disease duration of ≤10 years. However, even after additional adjustment for RA duration in the multivariable model, MTX and TNFi use at baseline were not significant predictors of erosion-free status nor did they appear to be confounders. Furthermore, the addition of these variables did not improve model goodness of fit, discrimination or calibration of risk. At 2-year follow-up, there remained no significant difference between the percentages of erosion-free subjects on TNFi’s compared with those with erosions. One explanation for these findings is that BRASS is an aggressively treated cohort. Another is that patients who remain erosion free may also have a clinical presentation similar to those who develop or have erosions. This is supported by our findings where there was no significant difference in the number of swollen and tender joints or the DAS-28 between the two groups at baseline. Therefore, medication use at baseline in the model was not a useful factor in differentiating between the two groups.
Strengths of this study are notable. We examined a clinically relevant subset of RA patients using a prospective cohort. These subjects were followed as part of typical care at a large academic centre. Erosions were read using a standardized system and readers were blinded to study hypotheses. Many variables were included as potential predictors and all variables were collected using standardized definitions.
In conclusion, we found that younger age at RA onset and shorter disease duration were the two consistent and significant predictors of erosion-free RA status after 2 years. Anti-CCP status was significant when the definition of erosion free was liberalized as a secondary outcome. These findings are consistent with work in two early arthritis cohorts [28]. Our study differs from prior studies of joint destruction in RA because we focused on understanding the factors that predict bone erosion alone rather than the composite of both erosions and JSN (total SHS) [5, 20, 29, 30]. Theoretically, distinct biologic processes may be responsible for bone erosions in contrast to JSN, which is primarily a consequence of cartilage destruction [31, 32]. Predictive models may serve as useful tools to inform clinicians on how much weight to place on specific clinical factors in deciding treatment. To further improve our understanding of erosion-free RA, a prospective study of early-onset inflammatory arthritis before the development of bone erosions with close follow-up and detailed information on medication use and disease activity is required. This study provides a foundation for understanding the clinical characteristics of RA subjects who remain erosion free in the context of a comprehensive clinical model. Findings from this study can inform future studies to better characterize this group in other cohorts and provide context for studies on the utility of novel serum and genetic markers in understanding erosion free and erosive disease in RA.
Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
We would like to thank Dr Desiree van der Heijde and Dr Vibeke Strand for sharing their expertise on bone erosions in RA, and Dr Robert Neer for his guidance on the approach to studying bone disease.
Funding: This study was supported by the National Institutes of Health (NIH) (T32AR055885 to K.P.L.; K24AR055989 to D.H.S.). K.P.L. also receives support from the American College of Rheumatology REF Rheumatology Scientist Development Award and the Katherine Swan Ginsburg Fund. The Brigham and Women’s Arthritis Sequential Study (BRASS) is funded by Biogen IDEC and Crescendo Biosciences.
Disclosure statement: M.E.W. receives research funding and serves as a consultant to Abbott, Bristol Myers Squibb, Roche, Biogen/IDEC and Crescendo Biosciences. He also serves as a consultant to Amgen, Centocor, Union Chimique Belge, Pfizer and has received support from the Raymond P. Lavietes Foundation. D.H.S. receives salary support from NIH, Agency for Healthcare Research and Quality, the Arthritis Foundation, Amgen and Abbott. He has also run an educational course supported by Bristol-Myers Squibb. N.A.S. receives research grant funding from AMGEN, Biogen IDEC and Crescendo Biosciences. All other authors have declared no conflicts of interest.
References
- 1.Lindqvist E, Jonsson K, Saxne T, Eberhardt K. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis. 2003;62:611–6. doi: 10.1136/ard.62.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 3.Syversen SW, Gaarder PI, Goll GL, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis. 2008;67:212–7. doi: 10.1136/ard.2006.068247. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 5.Courvoisier N, Dougados M, Cantagrel A, et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2008;10:R106. doi: 10.1186/ar2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strand V, Sharp JT. Radiographic data from recent randomized controlled trials in rheumatoid arthritis: what have we learned? Arthritis Rheum. 2003;48:21–34. doi: 10.1002/art.10683. [DOI] [PubMed] [Google Scholar]
- 7.Berglin E, Johansson T, Sundin U, et al. Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis. 2006;65:453–8. doi: 10.1136/ard.2005.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP) Ann Rheum Dis. 2004;63:1090–5. doi: 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22:335–8. [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42:2220–30. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–60. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannaccone CK, Lee YC, Cui J, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology. 2011;50:40–6. doi: 10.1093/rheumatology/keq263. [DOI] [PubMed] [Google Scholar]
- 14.Landewe R, van der Heijde D. Radiographic progression in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl. 39):S63–8. [PubMed] [Google Scholar]
- 15.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–3. [PubMed] [Google Scholar]
- 16.Boini S, Guillemin F. Radiographic scoring methods as outcome measures in rheumatoid arthritis: properties and advantages. Ann Rheum Dis. 2001;60:817–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–65. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 18.Raza K, Breese M, Nightingale P, et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol. 2005;32:231–8. [PMC free article] [PubMed] [Google Scholar]
- 19.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–58. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–6. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thabet MM, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH. The prognostic value of baseline erosions in undifferentiated arthritis. Arthritis Res Ther. 2009;11:R155. doi: 10.1186/ar2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ory PA. Interpreting radiographic data in rheumatoid arthritis. Ann Rheum Dis. 2003;62:597–604. doi: 10.1136/ard.62.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulus HE, Oh M, Sharp JT, et al. Classifying structural joint damage in rheumatoid arthritis as progressive or nonprogressive using a composite definition of joint radiographic change: a preliminary proposal. Arthritis Rheum. 2004;50:1083–96. doi: 10.1002/art.20270. [DOI] [PubMed] [Google Scholar]
- 24.Lassere MN, van der Heijde D, Johnson K, et al. Robustness and generalizability of smallest detectable difference in radiological progression. J Rheumatol. 2001;28:911–3. [PubMed] [Google Scholar]
- 25.Lassere MN, van der Heijde D, Johnson KR, Boers M, Edmonds J. Reliability of measures of disease activity and disease damage in rheumatoid arthritis: implications for smallest detectable difference, minimal clinically important difference, and analysis of treatment effects in randomized controlled trials. J Rheumatol. 2001;28:892–903. [PubMed] [Google Scholar]
- 26.Bruynesteyn K, van der Heijde D, Boers M, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum. 2002;46:913–20. doi: 10.1002/art.10190. [DOI] [PubMed] [Google Scholar]
- 27.FDA. United States Food and Drug Administration Approved Drug Products. Drugs @ FDA, 2010. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. [Google Scholar]
- 28.van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum. 2009;60:2262–71. doi: 10.1002/art.24661. [DOI] [PubMed] [Google Scholar]
- 29.Combe B, Dougados M, Goupille P, et al. Prognostic factors for radiographic damage in early rheumatoid arthritis: a multiparameter prospective study. Arthritis Rheum. 2001;44:1736–43. doi: 10.1002/1529-0131(200108)44:8<1736::AID-ART308>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Drossaers-Bakker KW, Zwinderman AH, Vliet Vlieland TP, et al. Long-term outcome in rheumatoid arthritis: a simple algorithm of baseline parameters can predict radiographic damage, disability, and disease course at 12-year followup. Arthritis Rheum. 2002;47:383–90. doi: 10.1002/art.10513. [DOI] [PubMed] [Google Scholar]
- 31.Mulherin D, Fitzgerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol. 1996;35:1263–8. doi: 10.1093/rheumatology/35.12.1263. [DOI] [PubMed] [Google Scholar]
- 32.Schett G. Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity. 2008;41:224–9. doi: 10.1080/08916930701694717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


