Abstract
Objectives
The mechanism of action of, and resistance to, metronidazole in the anaerobic (or micro-aerotolerant) protozoan parasite Giardia lamblia has long been associated with the reduction of ferredoxin (Fd) by the enzyme pyruvate:ferredoxin oxidoreductase (PFOR) and the subsequent activation of metronidazole by Fd to toxic radical species. Resistance to metronidazole has been associated with down-regulation of PFOR and Fd. The aim of this study was to determine whether the PFOR/Fd couple is the only pathway involved in metronidazole activation in Giardia.
Methods
PFOR and Fd activities were measured in extracts of highly metronidazole-resistant (MTRr) lines and activities of recombinant G. lamblia thioredoxin reductase (GlTrxR) and NADPH oxidase were assessed for their involvement in metronidazole activation and resistance.
Results
We demonstrated that several lines of highly MTRr G. lamblia have fully functional PFOR and Fd indicating that PFOR/Fd-independent mechanisms are involved in metronidazole activation and resistance in these cells. Flavin-dependent GlTrxR, like TrxR of other anaerobic protozoa, reduces 5-nitroimidazole compounds including metronidazole, although expression of TrxR is not decreased in MTRr Giardia. However, reduction of flavins is suppressed in highly MTRr cells, as evidenced by as much as an 80% decrease in NADPH oxidase flavin mononucleotide reduction activity. This suppression is consistent with generalized impaired flavin metabolism in highly MTRr Trichomonas vaginalis.
Conclusions
These data add to the mounting evidence against the dogma that PFOR/Fd is the only couple with a low enough redox potential to reduce metronidazole in anaerobes and point to the multi-factorial nature of metronidazole resistance.
Keywords: metronidazole, ronidazole, tinidazole, Blastocystis, NADPH oxidase
Introduction
The flagellated, intestinal, protozoan, anaerobic parasite, Giardia lamblia, is the causative agent of giardiasis, with symptoms including diarrhoea, bloating, nausea, loss of appetite, vomiting and weight loss in chronic infections, but infection may also be asymptomatic.1 Giardiasis is treated primarily with the 5-nitroimidazole (5-NI) drug, metronidazole, but other drugs are also effective including the benzimidazole, albendazole, the 5-nitrothiazole, nitazoxanide, the 5-nitrofuran, furazolidone, and quinacrine, an acridine derivative.2 The prodrug metronidazole is thought to be activated to its toxic, radical state via reduction by ferredoxin (Fd), which is itself reduced by the key metabolic enzyme, pyruvate:ferredoxin oxidoreductase (PFOR) in Giardia.3,4 The 5-NI prodrug is presumed to undergo a series of reduction steps with the most likely toxic candidate being the highly active nitroso radical, which can react with a variety of cell components, ultimately disabling and killing the cell.3,5 However, other reduction reactions are possible, including the formation of an imidazole radical and nitrite.3 More recently, it has been proposed that metronidazole reduction in the anaerobes Trichomonas vaginalis and Entamoeba histolytica is via flavin-(flavin adenine dinucleotide—FAD) and β-nicotinamide adenine dinucleotide phosphate (NADPH)-dependent thioredoxin reductase (TrxR), which acts directly on metronidazole as a nitroreductase.6,7 The involvement of a G. lamblia nitroreductase (GlNR1), in the toxicity of 5-nitro drugs has also been raised in the context of direct inhibition of nitroreductase activity by the 5-nitrothiazole, nitazoxanide.8
Purified Giardia PFOR together with Fd is capable of reducing metronidazole in a cell-free assay containing pyruvate and the cofactor CoASH4,9 (Figure 1). Decrease in absorbance at 320 nm can be used to follow metronidazole reduction, and under ideal conditions complete reduction occurs.9 These data have supported the belief as stated by Edwards3 that the PFOR/Fd couple is the only one with a low enough redox potential capable of reducing metronidazole in anaerobic microbes, whereas aerobes are incapable of reducing metronidazole because they do not possess a couple with a low enough redox potential. Further supporting this hypothesis, down-regulation of PFOR and Fd was observed in metronidazole-resistant (MTRr) Giardia,4,10,11 decreased PFOR mRNA levels were reported in MTRr Giardia,12 an inverse correlation of PFOR activity and metronidazole susceptibility was seen in a variety of Giardia isolates13 and cells with suppressed PFOR expression due to transfection with hammerhead ribozymes successfully targeted against PFOR mRNA were significantly more resistant to metronidazole than control cells.14
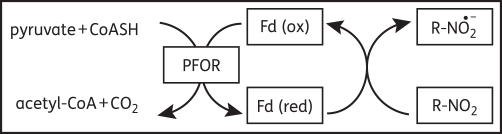
Figure 1.
Anaerobic reduction of 5-NI compounds by PFOR and Fd. 5-NI compounds (R-NO2) are reduced in an anaerobic cell-free assay by purified Fd, which accepts one electron from PFOR during the decarboxylation of pyruvate.9 Reduction of metronidazole and tinidazole can be followed by a decrease in absorbance at 320 nm, but reduction of other 5-NIs is not so readily monitored. In the intact cell and in the absence of 5-NIs, Fd is oxidized by Fd:NAD oxidoreductase with terminal electron acceptors being NAD(P)H:menadione oxidoreductase, DT diaphorase50 or NADH oxidases.51,52
Metronidazole is a 2-methyl, 5-NI with a short side chain at the 1-position of the imidazole ring. Other 5-NIs available to treat giardiasis include tinidazole and secnidazole (also with simple side chains in the 1-position and a methyl in the 2-position)15 although cross-resistance between these drugs is well documented.10,16,17 Recently, we showed that 5-NIs with extended side chains in the 2-position of the ring can be far more effective against Giardia.10,16,18 The compound C17, and other 2-position substituted 5-NIs have been used to demonstrate that MTRr Giardia and T. vaginalis exhibit susceptibility to some 5-NI drugs, indicating that cross-resistance among diverse 5-NIs is not absolute.16,18
In spite of the improved potency of C17 compared with metronidazole, we were able to develop C17r Giardia, which we found to be the most highly MTRr Giardia cells yet reported.10 Surprisingly, these C17r parasites demonstrated apparently normal PFOR expression10 conflicting with the dogma of the central importance of PFOR in metronidazole reduction.3 This apparent anomaly led us to investigate more closely the pathways of 5-NI reduction in Giardia and alternative mechanisms of antimicrobial resistance in laboratory-induced, 5-NI drug-resistant Giardia lines.
Materials and methods
Drugs and chemicals
Metronidazole and ronidazole were from Sigma-Aldrich (Australia). Tinidazole was from AK Scientific, Inc. (Mountain View, CA, USA) and from Sigma-Aldrich (Austria). The 2-position-substituted 5-NI compound, C17, was synthesized as previously described.10,16 All drugs were prepared as 0.1 M stock solutions in dimethyl sulphoxide (DMSO) (Sigma-Aldrich) and susceptibility assay working stocks for Blastocystis assays were prepared in complete pre-reduced media.
NADPH, flavin mononucleotide (FMN), ATP, cytochrome c, glucose oxidase from Aspergillus niger, catalase, bovine superoxide dismutase, 3-morpholinopropane-1-sulfonic acid (MOPS), sodium hydrosulphite (SHS) commonly known as sodium dithionite, and all other reagents unless otherwise stated were from Sigma-Aldrich.
Cell lines and culture
G. lamblia isolates BRIS/83/HEPU/106 (106) and BRIS/87/HEPU/713 (713), the MTRr lines 106-2ID10 (106-MTRr)19 and 713-M3 (713-MTRr),20 and the C17r lines 106-17A (106-C17r) and 713-M3-C17 (713-C17r)10 were maintained as previously described in TYI-S-33 medium with added bile and fetal bovine serum (FBS).10 Susceptibility assays of G. lamblia to drugs relied on trophozoite ATP levels as previously described by Dunn et al.10 using the BacTiter-Glo™ Microbial Cell Viability Assay System (Promega, Madison, WI, USA).
The axenic Blastocystis isolate WR1 (subtype 4) was grown as described by Mirza and Tan.21 Culture was in pre-reduced Iscove's modified Dulbecco's medium (IMDM) (Gibco-Invitrogen) with 10% heat inactivated horse serum (Gibco-Invitrogen). Cultures and pre-reducing media were incubated at 37°C for 48–120 h in a GasPak™ EZ gas generating system (Becton, Dickinson and Company) or an anaerobic jar (Oxoid). The sachets produce an anaerobic atmosphere with <1% O2 within 2.5 h, and with ≥15% carbon dioxide within 24 h.
Chemical reduction of 5-NI compounds
SHS was prepared as a 20 mM solution in 100 mM Tris–HCl, pH 7.5 in a nitrogen atmosphere. SHS at a final concentration of 500 μM was used to chemically reduce 100 μM metronidazole, tinidazole, ronidazole or 10 μM C17 (prepared from 0.1 M stocks in DMSO) in 100 mM Tris–HCl, pH 7.5 under nitrogen. After 5 min, metronidazole and tinidazole reduction was confirmed by a decrease in absorbance at 320 nm. Reduction reactions and SHS negative control assays were stored at −20°C for later testing in Blastocystis drug susceptibility assays.
Purification and activity of PFOR and Fd
Partial purification of PFOR was performed according to Townson et al.4 excluding the HiTrap Blue and Active Red column steps. Briefly, solubilized membrane fractions were loaded onto DE52 columns (pre-equilibrated with 50 mM Tris–HCl, pH 7.5, 200 μM thiamine pyrophosphate, 2.5 mM MgCl2) and eluted with 50 mM NaCl in the above buffer. Aliquots were assayed for peak activity. Purification of Fd was performed according to Townson et al.9 excluding the high-resolution liquid chromatography step. Briefly, cytosolic proteins were precipitated with acetone and then ammonium sulphate. The soluble fraction was bound to an ammonium sulphate-treated DEAE–cellulose column, eluted with 20 mM Tris–HCl, pH 7.5, and desalted through Sephadex G25. PFOR and Fd were prepared under anaerobic conditions under N2 from all six G. lamblia isolates and lines, and were tested in various combinations. The activities of PFOR and Fd preparations were determined in cell-free assays by following the reduction of metronidazole as a decrease in absorbance at 320 nm under anaerobic conditions.9 All assays contained 4 μg of purified PFOR preparation and 12 μg of purified Fd preparation in 50 mM Tris–HCl, pH 7.5, 2.5 mM pyruvate, 200 μM CoASH, 2 mM β-mercaptoethanol and 100 μM metronidazole in a total volume of 400 μL. Cell-free assay mixtures together with Fd negative controls were stored at −20°C for later testing in Blastocystis drug susceptibility assays.
Determination of 5-NI reduction by drug susceptibility assays
5-NI susceptibility of Blastocystis isolate WR1 was used to reveal the results of chemical and cell-free 5-NI reduction assays. Parasites were subjected to drug following prior drug reduction by SHS or Fd in cell-free assays. In all cases, drug concentrations of assay mixtures added to live cells were regarded as the concentration of the pre-reduced drug. Drugs in respective assay mixtures were added to white-walled, flat, clear-bottomed, 96-well plates in 100 μL of pre-reduced complete medium before serial 2-fold dilutions into 50 μL of medium. Each test was performed in triplicate. Typical drug concentrations ranged from 0.2 to 20 μM depending on predetermined drug toxicity. Blastocystis parasites (1.5 × 106) were added in 50 μL of pre-reduced media to each well, except for blank wells containing medium only.
Plates were incubated at 37°C for 48 h in the GasPak™ EZ gas generating container with GasPak™ EZ gas generating sachets. In each assay, ATP levels of viable drug-treated parasites compared with control-treated parasites were determined using the BacTiter-Glo™ Microbial Cell Viability Assay System. BacTiter-Glo™ substrate/reagent mixture (100 μL) was added to each well at room temperature, and luminescence was measured in a POLARStar Optima luminometer (BMG Labtech, Australia). Sample signal was compared with ATP standard signals resulting in determination of nM concentrations of ATP for each set of replicates. Sample nM ATP concentrations were compared with ATP concentrations of control wells. Drug concentrations were determined at which parasites (using ATP levels as read-out) were inhibited by 50% (ID50) and 90% (ID90) relative to levels in untreated parasites.
Cloning and expression of recombinant G. lamblia TrxR (GlTrxR)
The GlTrxR gene (XP_001707168)22 was amplified from G. lamblia BRIS/83/HEPU/106 genomic DNA using the forward primer 5′-TACGTACGCATATGTCCACTCAGCGCCACGTCAGGATC-3′ and the reverse primer 5′-TCATCCAGCTCGAGTTAGTGATGGTGATGGTGATGCTCCTGCATGGCAAGCCA-3′. The forward primer contains an NdeI restriction site and the reverse primer an XhoI restriction site and a 6× His-tag sequence. The fragment was cloned into a pET-17b plasmid vector (Novagen) via its NdeI and XhoI restriction sites. Recombinant expression of G. lamblia TrxR was performed in arabinose-inducible Escherichia coli BL21-AI according to the manufacturer's instructions. Recombinant GlTrxR was isolated with Ni-NTA spin columns (Qiagen) under aerobic, native conditions.
Nitroreductase assay with recombinant G. lamblia TrxR
Nitroreductase activity of recombinant GlTrxR was measured aerobically in a cytochrome c-coupled assay with either 1 mM or 100 μM 5-NI drug as previously described (Figure 2).6,7 Specific 5-NI reduction rates were obtained by subtracting the background oxygen reduction rate. In a second approach, 5-NI reduction rates were determined anaerobically by measuring NADPH consumption in the presence of 100 μM 5-NI drug. Anaerobic conditions in the assay buffer were attained in cuvettes by removal of molecular oxygen with glucose oxidase.23 Cuvettes were sealed with parafilm and prior to the addition of GlTrxR, reaction buffers were incubated in a heated spectrophotometer (Perkin Elmer PTP A) for 5 min at 37°C. The assay buffer contained 6 U/mL glucose oxidase, 6 U/mL catalase, 25 mM glucose, 0.2 mM NADPH, 10 mM Tris–HCl, pH 7.5, and 1 mM EDTA. In both assays 5 μg/mL GlTrxR was used. In order to measure the proportion of cytochrome c reduced by superoxide anions, bovine superoxide dismutase at a concentration of 1 μM was added to assay mixtures.
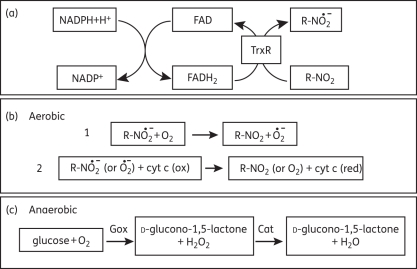
Figure 2.
In vitro reduction of 5-NI compounds by TrxR. Recombinant Giardia TrxR reduces 5-NI compounds (R-NO2), including metronidazole, to nitro radicals in the presence of NADPH (a). Under aerobic conditions, metronidazole is reduced by TrxR to a toxic state and can be recycled in the presence of O2 with production of a superoxide radical (b, reaction 1). To monitor the reduction reaction with 5-NI drugs, cytochrome c (cyt c) is included and its reduction by nitro radicals (or superoxide) is monitored at 550 nm (b, reaction 2). Superoxide dismutase (not shown in b) was added to remove superoxide radicals to demonstrate nitro radical reduction of cytochrome c. A more sensitive assay (anaerobic) employs glucose oxidase (Gox) to deplete O2 and catalase (Cat) to deplete H2O2 (c). In the latter case, lower concentrations of 5-NI drug can be assayed and this allows monitoring of NADPH turnover at 340 nm. In the intact cell, cytoplasmic NADPH and FAD-dependent TrxR maintain the redox balance of the cell by reducing its specific substrate, thioredoxin, which is subsequently involved in essential cell reactions including antioxidant defence, dNTP synthesis, sulphate reduction and redox control of chaperones.53
Protein analysis by two-dimensional gel electrophoresis (2DE)
G. lamblia cultures (2–5 × 107 cells) were harvested at room temperature by centrifugation at 750 g for 5 min. Cell pellets were washed in two centrifugation steps in phosphate buffered saline (PBS) (2.7 mM KCl, 1.8 mM KH2PO4, 137 mM NaCl, 10.1 mM Na2HPO4, pH 7.4). Cells were resuspended in 2 mL of 10% trichloroacetic acid, 20% ultrapure water and 70% acetone, and incubated at −20°C for at least 1 h. The resulting lysates were centrifuged at 20 000 g at 4°C and washed twice in 9 : 1 acetone:ddH2O followed by centrifugation at 20 000 g (at 4°C for 20 min). Pellets were resolubilized in an appropriate amount of sample buffer (300–600 μL) and insoluble material was removed by centrifugation at 20 000 g (at 20°C for 20 min). 2DE was performed on the supernatants as previously described.24 For isoelectric focusing in 17 cm IPG strips (Bio-Rad), 500 μg of protein was loaded. The second dimension was run in 20 × 20 cm gels in a Protean® II xi Cell (Bio-Rad). Gel images were evaluated using Melanie™ 4 software (GeneBio, Switzerland).
Protein identification
Major protein spots in 2DE separations in the range 32–38 kDa (GlTrxR is 35 kDa),22 were excised prior to digestion with 0.02 mg/mL trypsin (modified, sequencing grade; Roche). The acidified peptides were spotted on a Bruker AnchorChip™ target with matrix (α-cyano-4-hydroxy-cinnamic acid) 0.5 mg/mL in 90% acetonitrile, 0.1% trifluoroacetic acid (TFA). Sample spots were then washed with 0.5% TFA and recrystallized in 6 : 3 : 1 ethanol/acetone/10 mM ammonium phosphate in 0.1% TFA. Mass spectrometry (MS) and tandem mass spectrometry (MS/MS) data were acquired on a Bruker Daltonics Ultraflex III, MALDI TOF/TOF mass spectrometer. Mascot software (2.2.02) was used to search against the NCBInr database with mass tolerance of 0.4 Da for MS and 0.8 Da for MS/MS.
Measurement of flavin reduction by G. lamblia cell lysates
Cell lysates were prepared by adding 0.1% Triton X-100 to G. lamblia cells suspended in a buffer of 250 mM sucrose, 10 mM MOPS, pH 7.2, in the absence of protease inhibitors.
Insoluble material was removed by centrifugation at 20 000 g for 10 min. The supernatant was used for measuring reduction of FMN (10 μM), FAD (10 μM) or riboflavin (10 μM) in 100 mM Tris–HCl pH 7.5, 1 mM EDTA and 0.2 mM NADPH by determining the consumption of NADPH under aerobic conditions as change in absorbance at 340 nm (37°C). Cell extracts were used at a concentration of 20 μg of protein/mL of assay buffer.
Results
PFOR and Fd are active in C17r Giardia lines
Previously we showed by western blotting that PFOR is expressed at normal levels in 106-C17r.10 To extend these observations functionally, we prepared partially purified PFOR and Fd from 106 and 713 and their respective MTRr and C17r lines. Using PFOR and Fd prepared from 713 as controls, we assayed each PFOR preparation with 713 Fd to determine PFOR activity, and each Fd preparation with 713 PFOR to determine Fd activity in cell-free assays of metronidazole reduction. Assays were performed under the same conditions with the same concentration of partially purified protein. In all assays with PFOR and Fd from metronidazole-susceptible (MTRs) and C17r cells, >60% of the metronidazole was reduced within 30 min, as determined by a decrease in absorbance of metronidazole at 320 nm indicative of reduction to nitro radicals, indicating that PFOR and Fd from these cells are functional and active (Figure 1 and Table 1). In contrast, the assays with PFOR from 106-MTRr showed complete loss of activity. Consistent with this, during PFOR purification from these cells, the obvious brown-coloured band (due to iron–sulphur clusters) of PFOR protein was not observed bound to the DE52 column4 confirming that 106-MTRr express little if any active PFOR protein. The activities of partially purified PFOR, as well as Fd, from 713-MTRr were also decreased, albeit much less markedly, with 41% and 48% of metronidazole reduced in 30 min, respectively (Table 1). These data are consistent with an earlier report of partial PFOR down-regulation in 713-MTRr.4
Table 1.
Metronidazole (MTR) reduction with combinations of PFOR and Fd from MTRs and MTRr G. lamblia
| Source of PFOR (with 713 Fd) | Percentage reductiona of MTR (30 min) | Source of Fd (with 713 PFOR) | Percentage reductiona of MTR (30 min) |
|---|---|---|---|
| 106 (MTRs) | 62 | 106 | 80 |
| 106-MTRr | 0 | 106-MTRr | 64 |
| 106-C17r | 79 | 106-C17r | 76 |
| 713 (MTRs) | 64 | 713 | 59 |
| 713-MTRr | 41 | 713-MTRr | 48 |
| 713-C17r | 61 | 713-C17r | 60 |
aReduction is calculated as the percentage decrease in absorbance at 320 nm using 4 μg of purified PFOR protein and 12 μg of purified Fd in each standard assay.
Taken together, these results demonstrate that C17r cells express fully functional PFOR and Fd at similar levels to those expressed in MTRs cells.
PFOR and Fd can reduce different 5-NI drugs in cell-free assays
Only metronidazole and tinidazole can be reliably monitored for reduction, by measuring absorbance at 320 nm, in cell-free assays containing PFOR, Fd, pyruvate and CoASH (data not shown). No obvious decrease in absorbance occurred during reduction of the other 5-NIs used in this study, i.e. the experimental compound C17 and commercially available ronidazole, both of which are significantly more potent than metronidazole against Giardia (ID90 of 0.54 and 1.6 μM, respectively, compared with 9.4 μM for metronidazole) (Table 2). Because we could not directly measure reduction of these latter two compounds by purified PFOR and Fd, it was formally possible that the apparent normal reducing activity of PFOR and Fd in C17r cells is limited to metronidazole and not relevant to C17 reduction. In order to exclude this possibility, we subjected C17 and ronidazole, as well as the two control compounds metronidazole and tinidazole, to reduction in cell-free assays with purified Giardia PFOR and Fd and tested the spent assay mixtures and appropriate negative controls on live Blastocystis cells as an independent read-out for the antimicrobial activity remaining after reduction. The rationale behind these experiments was that once 5-NI compounds are reduced, the resulting radicals have a very short half-life and spontaneously degrade into inactive end products or form adducts with components of the assay mixture, which are harmless in whole-cell assays. We ensured that reduction assays proceeded reliably and that all assay components sustained activity by monitoring metronidazole reduction spectrophotometrically initially and again in a separate assay after all other reduction assays were completed. These additional controls were important because significant time elapsed while all assays were completed inside an anaerobic hood. We used Blastocystis as a biological assay system, since, using the ATP assay described here, susceptibility to metronidazole and C17 varied only 2-fold for the Blastocystis isolate WR1 (ID90 of 7.1 μM for metronidazole versus 3.6 μM for C17) compared with ∼15-fold for Giardia10 (9.4 μM metronidazole versus 0.54 μM C17) (Table 2). The latter large differences in susceptibility of Giardia to these two most relevant (for this work) drugs, resulted in large errors and unreliable data as incomplete reduction of C17 in cell-free assays still allowed significant inhibition of Giardia (data not shown). Control susceptibility assays were performed with the strong reducing agent SHS (which reduces all 5-NIs quantitatively) and with the same buffers used for cell-free drug reduction assays, but without SHS and Fd.
Table 2.
Effect of prior cell-free drug reduction on efficacy of 5-NI drugs against Blastocystis WR1
| ID90 (μM) |
||||||
|---|---|---|---|---|---|---|
| drug alonea |
drug treatment prior to Blastocystis susceptibility assay |
|||||
| 5-NI drug | Giardia | Blastocystis | controla (without SHS) | SHSb | controla (without Fd) | Fdb |
| Tinidazole | 2.3 | 2.7 | 2.3 | 18.5 | 7.4 ± 1.38(2) | >20(2) |
| Metronidazole | 9.44 ± 0.88(7) | 7.1 ± 1.49(4) | 6.75 | >20 | 9.3 ± 0.75(2) | >20(2) |
| Ronidazole | 1.6 | 0.9 ± 0.18(3) | 1.2 | >10 | 3.7 ± 0.35(2) | >10(2) |
| C17 | 0.54 ± 0.07(3) | 3.6 ± 1.0(4) | 2.3 | >5 | 2.6–>5(2)c | >5(2) |
A superscript number in parentheses refers to the number of times the experiment was performed. Where this value is ≥2, the data are averages of ID90 values (±SEM) obtained from plots of triplicate estimations of cell inhibition versus drug concentration (refer to Figure 3).
aSusceptibility assays with drug alone or controls (without SHS or Fd drug reduction) varied in the buffer system used.
bComplete reduction of 5-NI drugs by SHS or Fd resulted in non-toxic reduction products of the drugs. However, in all cases, drug concentrations of assay mixtures added to live cells were regarded as the concentration of the pre-reduced drug.
cWhere ‘>’ is used the indication is that the drug concentration on the plate was not high enough for an ID90 reading. In some cases, especially C17, high concentrations of drug are insoluble. Figure 3 demonstrates why values 2.6 and >5 μM apply to Blastocystis treated with C17 and >5 μM also applies to Blastocystis treated with Fd-reduced C17.
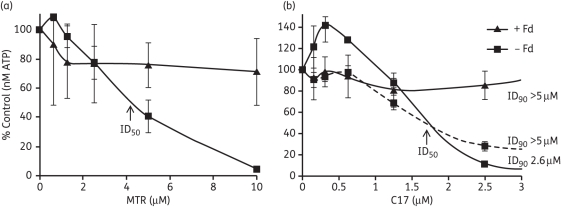
SHS reduced all the tested 5-NI drugs since drugs were ineffective subsequent to incubation with SHS in antimicrobial activity assays using Blastocystis (i.e. ID90 values were above what could be measured in the assays except for tinidazole with an ID90 of ∼18 μM) (Table 2). ID90 values of control assays without SHS were similar to assays of drug alone against Blastocystis with values ranging from ∼1 μM for ronidazole to 7 μM for metronidazole (Table 2). These data agree well with earlier values obtained using a [3H]hypoxanthine incorporation assay25 and thus validates the ATP/bioluminescence assay for Blastocystis susceptibility testing. However, Mirza et al.26 have pointed out the variability in metronidazole susceptibility among Blastocystis isolates using different assay methods to those described here. Partially purified Giardia PFOR/Fd also reduced all four 5-NIs in vitro since drugs reduced in cell-free assays were ineffective against Blastocystis with ID90 values beyond what could be determined in the assays (Table 2). In addition ID50 values could not be determined for cells treated with Fd-reduced drugs as shown for metronidazole and C17 in Figure 3. The figure clearly demonstrates that assay mixtures without Fd inhibited cells similarly in the two experiments performed, with comparable ID50 values of 4.25 μM for metronidazole and 1.7 μM for C17. Although an ID90 value for C17 in one experiment without Fd could not be determined, metronidazole was clearly active (Figure 3). Reduction of all 5-NIs tested was apparently to completion in the time allotted since little or no growth inhibition was observed with spent assay mixtures following reduction by SHS or PFOR/Fd (Figure 3 and Table 2). We conclude from these experiments that PFOR/Fd can effectively reduce all the 5-NI drugs tested in our system, further underlining that high levels of metronidazole and C17 resistance occur in the presence of normal levels and activity of PFOR and Fd.
Figure 3.
Blastocystis susceptibility to biologically (PFOR/Fd) reduced 5-NIs. Reduction assay mixtures were added to cells with appropriate controls and incubated for 48 h. The concentration of drug was regarded as that of the pre-reduced 5-NI of interest. The ability of the PFOR/Fd couple to reduce metronidazole (MTR) (a) and C17 (b) was determined by monitoring ATP production of cells subjected to spent reduction assay mixtures containing PFOR, Fd, pyruvate and other components. Controls omitting Fd behaved similarly to drug-alone susceptibility assays (refer to Table 2). The data for two experiments are shown. In all cases, except one (broken line), the data from the experiments were very similar.
Metronidazole reduction by TrxR in Giardia
Because purified PFOR/Fd can effectively reduce all tested 5-NIs under cell-free conditions, yet high-level resistance to metronidazole and C17 occurs in spite of normal expression and function of PFOR and Fd, we hypothesized that PFOR and Fd are not critical for reduction/activation of these drugs in C17r cells and that other pathways of 5-NI drug reduction must exist in Giardia. TrxR in Trichomonas and Entamoeba is a strong nitroreductase, which can reduce the nitro group of 5-NIs.6,7,27 This enzyme exists in Giardia,22 but its function has not been tested. Thus, we investigated whether GlTrxR has nitroreductase activity. We expressed recombinant GlTrxR in E. coli6,7 and used it in an aerobic nitroreductase assay with three different 5-NI substrates and cytochrome c reduction as readout (Figure 2). GlTrxR efficiently reduced metronidazole, ronidazole and tinidazole (81, 575 and 165 nmol of NADPH consumed/min/mg of protein—units of TrxR activity), when 5-NIs were used at 1 mM (Table 3). Further, the enzyme displayed considerable background reduction of oxygen in the buffer (785 nmol/min/mg of protein). The formation of superoxide by GlTrxR was verified by adding bovine superoxide dismutase (1 μM) to the assay mixtures, which diminished cytochrome c reduction to only 7% of the original reduction rate. 5-NI-mediated cytochrome c reduction was also diminished in the presence of superoxide dismutase, with approximately half of the original rate remaining in the case of metronidazole and tinidazole (49% and 47%, respectively) and about a third in the case of ronidazole (36%). These observations indicate that GlTrxR reduces 5-NIs to their respective nitroimidazole radical anions, which subsequently reduce cytochrome c either directly or indirectly, by first reducing oxygen to superoxide (Figure 2).
Table 3.
Aerobic nitroreductase activity of GlTxrR in a cytochrome c-coupled assay
| 5-NI drug | TrxR nitroreductase activity (nmol of NADPH consumed/min/mg of protein) |
|---|---|
| Metronidazole | |
| 100 µM | 0 |
| 1 mM | 81 ± 30 |
| Ronidazole | |
| 100 µM | 102 ± 29 |
| 1 mM | 575 ± 30 |
| Tinidazole | |
| 100 µM | 0 |
| 1 mM | 165 ± 31 |
Values were determined by at least three measurements and are averages (±SEM).
In order to determine whether GlTrxR reduces 5-NIs at similar concentrations to those employed for the PFOR/Fd couple (Figure 1), we also assayed nitroreductase activity of GlTrxR with each 5-NI at a concentration of 100 μM. Unfortunately, the high background reduction of oxygen by GlTrxR made the measurement of 5-NIs at this concentration difficult and only ronidazole clearly reduced cytochrome c compared with control reactions without added 5-NI, whereas 100 μM metronidazole and tinidazole did not (Table 3). Because of this, we conducted an anaerobic nitroreductase assay according to a protocol developed for the measurement of metronidazole reduction by the RdxA nitroreductase from Helicobacter pylori23 in which nitroreduction is monitored as a function of NADPH consumption in the buffer. Although nitroimidazoles display very strong absorbance at 340 nm, making measurements of NADPH concentrations difficult, the absence of oxygen abolished reoxidation of nitroimidazole radical anions (Figure 2) and resulted in a baseline that was stable and low enough to detect metronidazole reduction at a concentration of 100 μM metronidazole (16 ± 7 U TrxR activity) and ronidazole (30 ± 1 U of TrxR activity). However, for as yet unknown reasons, ronidazole was less efficiently reduced in this assay at a concentration of 100 μM as compared with the aerobic assay.
No nitroreductase activity of TrxR could be measured using C17 under either aerobic or anaerobic conditions. In contrast, C17 even diminished the background reduction of cytochrome c by TrxR, possibly by interfering with the reduction of oxygen in the assay buffer (data not shown). However, due to its poor solubility in water, it was not possible to add C17 to the assay mixtures at a concentration higher than 100 μM. Thus, it is possible that reduction of C17 by GlTrxR can occur at higher drug concentrations.
TrxR in G. lamblia drug-resistant lines
Because GlTrxR could reduce metronidazole and other 5-NIs under cell-free conditions, we examined whether TrxR activity was diminished in MTRr G. lamblia cell lines. We were unable to directly measure TrxR activity in G. lamblia cell extracts because Giardia thioredoxin was not available as a substrate. A candidate G. lamblia thioredoxin, XP_001709207, produced as a recombinant protein in E. coli, failed to be reduced by G. lamblia TrxR. As no other obvious thioredoxin candidates could be identified in the G. lamblia genome we also tested recombinant E. histolytica thioredoxin7 and recombinant T. vaginalis thioredoxin,6 again to no avail, leading us to discontinue our attempts to measure TrxR activity in crude G. lamblia extracts. Instead, we focused on assessing expression levels of TrxR in 106-MTRr and 713-MTRr using 2DE of cell proteins. Analysis of isolate 106 cell proteins within the 26–38 kDa range in 2DE by MS identified TrxR by five matched peptides giving 27% peptide coverage and peptide ion scores ranging from 76 to 172. Subsequent densitometry analysis revealed that both MTRr lines displayed unaltered expression of TrxR as compared with their respective MTRs parent strains, 106 and 713 (data not shown). In addition, TrxR was expressed similarly to MTRs isolates in both C17r lines, comprising ∼0.5% of total protein visualized (data not shown). Thus, diminished TrxR expression did not appear to account for metronidazole resistance in Giardia.
Role of flavin cofactors in 5-NI reduction in Giardia
In laboratory-induced, highly MTRr T. vaginalis, TrxR is normally expressed, but is functionally inactive, because the cells lack free flavins required for TrxR activity.6
TrxR activity in cell extracts was restored by the addition of FAD.6 In addition, highly MTRr trichomonads are incapable of reducing free flavins.6 The latter T. vaginalis flavin-reducing enzyme activity has been ascribed to a genetically uncharacterized NADPH-dependent flavin reductase, termed ‘NADPH oxidase’.28
The ability of Giardia NADPH oxidase to reduce FMN (Figure 4), FAD (data not shown) and riboflavin (data not shown) was confirmed using extracts of strain 106. Flavin reduction (FMN) was also detected in extracts of both Giardia MTRr lines, 106-MTRr and 713-MTRr, but at markedly lower levels than in the MTRs parent isolates (Figure 4). Compared with the respective parent strains, FMN reduction rates were diminished by 30% in 106-MTRr and by as much as 80% in 713-MTRr (Figure 4). We also determined that Giardia NADPH oxidase activity, as measured by flavin reduction, was decreased by ∼70% in both 106-C17r and 713-C17r compared with their parent isolates (Figure 4). Because free flavins are needed for the reducing activity of TrxR, these data suggest that decreased Giardia NADPH oxidase activity leads to impaired GlTrxR activity in vivo, which is likely to be a contributing factor in metronidazole resistance.
Figure 4.
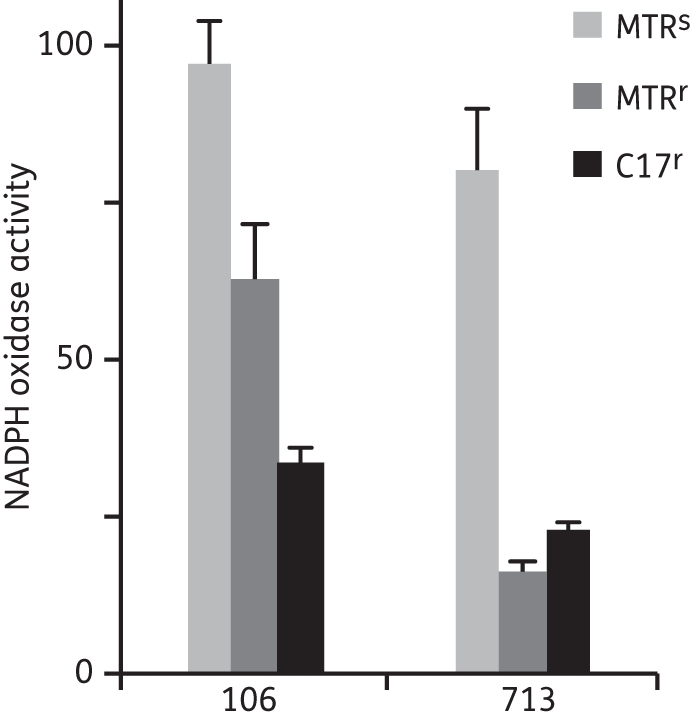
NADPH oxidase activity in drug-susceptible (MTRs) and drug-resistant (MTRr and C17r) G. lamblia. The units of NADPH oxidase activity are nmol of FMN (10 μM) reduced/min/mg of protein. All values were determined by at least three measurements and are averages (±SEM). The NADPH oxidase activity of 106-MTRr and 106-C17r extracts was decreased by 36% and 66%, respectively, compared with extracts of the MTRs parent isolate, 106. For 713-MTRr and 713-C17r the values were 80% and 72%, respectively, of the MTRs isolate 713. MTR, metronidazole.
Discussion
5-NI prodrugs can be activated to their toxic radical products in Giardia by at least two distinct mechanisms. The first is the well-known reduction via the PFOR/Fd couple in the electron transport pathway.4,9 The other, as shown here, involves reduction via the nitroreductase activity of GlTrxR, an activity similar to that demonstrated in Trichomonas and Entamoeba.6,7 Giardia clearly has the capacity to use both mechanisms of 5-NI activation, since cell-free extracts of purified PFOR and Fd can reduce all 5-NIs tested, and recombinant flavin-dependent GlTrxR displays nitroreductase activity for metronidazole, tinidazole and ronidazole, but no evidence exists to indicate which system is more important in live cells. In T. vaginalis, prodrug entering the cell first encounters cytoplasmic Trichomonas TrxR before moving into the hydrogenosome organelle where Fds are physically separated from the cytoplasm.29,30 This may be relevant to the Trichomonas TrxR system as the proposed primary metabolic pathway for 5-NI reduction.6 In Giardia, PFOR is largely plasma membrane associated4 while both Fd and GlTrxR are located in the cytoplasm.9,22 In the absence of any evident physical separation between the electron transport and GlTrxR pathways, no obvious cell biological argument can be made for one system or the other being preferentially, or perhaps exclusively, involved in drug reduction.
Metronidazole resistance can be associated with down-regulation of PFOR, as observed, in the MTRr line 106-MTRr described here and previously.4 Furthermore, PFOR down-regulation alone can be sufficient for mediating metronidazole resistance, as suggested by specific targeting of PFOR by hammerhead ribozymes.14 ‘PFOR’ is used generically here, with no distinction made between the two Giardia PFOR gene products, PFOR1 and PFOR2, GL50803_114609 and GL50803-17063, respectively (www.GiardiaDB.org). We originally sequenced the PFOR1 gene (GenBank accession number L27221) and purified it4 without knowledge of the existence of PFOR2. PFOR1 is the same gene that Dan et al.14 used in their hammerhead ribozyme studies. It is possible, but not known at this stage, that PFOR2 plays a role in metronidazole activation and resistance. Partially purified Fd used here9 is not annotated in the Giardia genome database (www.GiardiaDB.org), but the sequence of FdI of 59 amino acids matching that reported by Townson et al.9 can be found on ctg02_27 : 73612–73789 (www.GiardiaDB.org) or at NCBI XP778874. There are at least two other Fds as reported by Townson et al.9 of lesser concentration than, but which co-purify with, FdI. These are likely to be the products of the open reading frame (ORF) annotated in the database as ferredoxin, GL5083_9962, and the ORF expressing a hypothetical protein, GL5083_4081, both of which are the same size as FdI. Similarly to PFOR2, the latter two Fds may also be involved in metronidazole resistance. The relative importance of the alternative PFOR and Fds needs to be resolved before a complete understanding of metronidazole activation and resistance mechanisms can be reached.
Despite the importance of PFOR down-regulation in some MTRr Giardia lines, our data show that this is not necessary, since metronidazole resistance can occur in the absence of PFOR down-regulation, with the most highly MTRr Giardia cells having fully functional PFOR and Fd. This is consistent with the report of Argüello-Garcia et al.31 who showed up-regulation of PFOR mRNA in two MTRr lines. These cells also have apparently normal levels of GlTrxR, but significantly decreased NADPH oxidase/flavin reduction (alternatively referred to as NADPH:flavin oxidoreductase or NADPH-dependent flavin reductase) activity. The latter may not by itself fully account for the resistance mechanisms in these highly MTRr cells, because the MTRr lines 106-C17r and 713-C17r (with ID90 values of >200 μM metronidazole),10 have similar levels of PFOR and Fd to MTRs isolates and similarly decreased levels of NADPH oxidase activity to 713-MTRr, which show more modest levels of resistance to metronidazole (ID90 of 90 μM metronidazole).10 Furthermore, 106-MTRr have no PFOR activity and a 36% reduction in NADPH oxidase activity, while the PFOR and NADPH oxidase activities of 713-MTRr are decreased by ∼35% and 80%, respectively. These findings and previously published data2,6,10,12,14,31 indicate that diverse and multiple changes occur in the development of 5-NI drug resistance, although the relative importance of these changes for metronidazole resistance, as well as their ultimate clinical occurrence and relevance, remain to be established. Nonetheless, we have argued that the use of laboratory-induced, isogenic drug-resistant lines to follow changes in enzyme regulation involved in drug resistance, is a powerful tool to assess the full capability of the parasite in developing clinical drug resistance.32
Fully functional PFOR and Fd in the most highly MTRr Giardia lines may, nevertheless, be inactive in metronidazole reduction in vivo. Such a situation is evident in clinically, highly MTRr T. vaginalis, which have fully functional hydrogenosomes, but no obvious metronidazole resistance mechanisms.33,34 Further, it is also possible that metronidazole is reduced in these highly MTRr cells, but the cell has developed mechanisms to detoxify the nitro radicals, e.g. by adduct formation with non-protein thiols7 and efflux of these adducts from the cell. Finally, the cells may efflux metronidazole as we have shown for rhodamine 123,35 a well-described substrate for multidrug resistance transporter efflux proteins.36 This active efflux of rhodamine by Giardia can be readily and repeatedly reversed and reinstated.
Similar to highly MTRr Giardia, metronidazole resistance mechanisms in other organisms are not uniformly resolved. For example, nitroreductases, including the product of the RdxA37 and FrxA38 genes in H. pylori, are able to reduce metronidazole to its toxic form, but metronidazole resistance occurs in the presence of fully functional nitroreductases.39 In MTRr Bacteroides, drug reduction via nim genes, which produce nitroreductases that render the reduction products of metronidazole non-toxic, occurs,40 but other changes include decreased drug uptake,41 increased rhamnose catabolism42 and overexpression of the DNA repair RecA protein.43 MTRr Enterococcus spp. similarly have nitroreductases that reduce metronidazole to unidentified non-toxic products.44 The above indicates the variety of mechanisms microorganisms employ in metronidazole (and other 5-NI) activation and resistance.
GlTrxR reduces 5-NIs to nitroradical anions (under aerobic or anaerobic conditions) and also generates superoxide under aerobic conditions. In addition, like Trichomonas TrxR, GlTrxR is expressed in MTRr cells at levels similar to those in MTRs parent isolates, although our inability to identify the specific GlTrxR substrate, thioredoxin, prevented us from assessing whether endogenous TrxR activity is decreased in MTRr cells. In addition, we were unable to demonstrate reduction of C17 by either GlTrxR or Trichomonas TrxR (data not shown), but do not believe this is related to the general inability of 2-position-substituted 5-NIs (e.g. C17 and ronidazole) to be activated by GlTrxR since we have shown here that ronidazole is reduced by GlTrxR.
The marked decrease in NADPH oxidase activity, i.e. reduction of free flavins, in all MTRr cells suggests inhibition of flavin metabolism and possible inhibition of FAD-dependent GlTrxR. It is consistent with data from a laboratory-induced, MTRr T. vaginalis line6 indicating that this enzyme activity is involved in the establishment of metronidazole resistance. The NADPH oxidase activity in 106-MTRr very closely mirrors that measured earlier by Ellis et al.45 although absolute values reported here for 106-MTRr and 106-MTRs are somewhat lower, i.e. 97 versus 240 nmol/min/mg of protein for 106-MTRs and 63 versus 160 nmol/min/mg of protein for 106-MTRr. This discrepancy is probably due to the lower concentration of substrate (FMN) used here (10 μM versus 100 μM). The physiological role of NADPH oxidase and its contribution to the activation of 5-NI drugs are unclear, but several interpretations are possible. As a flavin reductase, NADPH oxidase could be involved in iron acquisition as suggested by the role of reduced riboflavin, a flavin precursor, in the acquisition of iron in microaerophilic bacteria.46,47 Reduction of flavins is important for antioxidative defence in G. lamblia and T. vaginalis and NADPH oxidase may be directly involved in metronidazole reduction via reduced flavins as described by Clarke et al.48 Recent publication of a Giardia flavohaemoglobin demonstrating NADH or NADPH oxidase activity49 may indicate the identity of the NADPH oxidase referred to here. In addition, other candidate NADPH oxidases include a NAD(P)H:menadione oxidoreductase or DT diaphorase thought to be essential for maintaining NAD(P)H cycling under anaerobic conditions50 and NADH oxidase, which can also use NADPH, described by Brown et al.51 A highly efficient oxygen-scavenging Giardia flavodiiron enzyme, however, is reportedly NADH specific.52
In conclusion, at least two likely mechanisms of 5-NI activation exist in live Giardia cells—activation by reduced Fd and by the nitroreductase activity of flavin-dependent GlTrxR. 5-NI resistance involves PFOR down-regulation (in some cases) and decreased NADPH oxidase/flavin reducing activity, but not apparently altered GlTrxR expression. In addition, resistance is likely to be more complex than inhibition of flavin metabolism or a combination of down-regulation and inhibition of 5-NI reduction pathways, since neither down-regulation of PFOR nor decreased NADPH oxidase activity was specific to the most highly MTRr lines. This dissection and discovery approach to the mechanisms of 5-NI activation and resistance has led to a greater understanding and appreciation of in vitro drug resistance in Giardia. These insights are essential to defining new drug targets and designing new drugs to bypass clinical resistance, not only in Giardia but in a much wider range of anaerobes.2,32
Funding
This study was supported by: U01 Cooperative Research Agreement AI75527 and DK80506 from the National Institutes of Health, USA; by the National Health and Medical Research Council of Australia grant 496640; and the project grants P22546 and P22037 from the Austrian Science Fund (FWF).
Transparency declarations
None to declare.
Acknowledgements
This study was facilitated by the commissioning of synthesis of C17 by NIH from Southern Research Institute USA.
References
- 1.Buret AG. Pathophysiology of enteric infections with Giardia duodenalis. Parasite. 2008;15:261–5. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- 2.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. doi:10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards DI. Nitroimidazole drugs - action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. doi:10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Townson SM, Upcroft JA, Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol Biochem Parasitol. 1996;79:183–93. doi: 10.1016/0166-6851(96)02661-8. doi:10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- 5.Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–71. [PubMed] [Google Scholar]
- 6.Leitsch D, Kolarich D, Binder M, et al. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol Microbiol. 2009;72:518–36. doi: 10.1111/j.1365-2958.2009.06675.x. doi:10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- 7.Leitsch D, Kolarich D, Wilson IBH, et al. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5:e211. doi: 10.1371/journal.pbio.0050211. doi:10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller J, Wastling J, Sanderson S, et al. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob Agents Chemother. 2007;51:1979–86. doi: 10.1128/AAC.01548-06. doi:10.1128/AAC.01548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townson SM, Hanson GR, Upcroft JA, et al. A purified ferredoxin from Giardia duodenalis. Eur J Biochem. 1994;220:439–46. doi: 10.1111/j.1432-1033.1994.tb18641.x. doi:10.1111/j.1432-1033.1994.tb18641.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunn LA, Burgess AG, Krauer KG, et al. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int J Antimicrob Agents. 2010;36:37–42. doi: 10.1016/j.ijantimicag.2010.03.004. doi:10.1016/j.ijantimicag.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SM, Brown DM, O'Donoghue P, et al. Ferredoxin involvement in metronidazole resistance of Giardia duodenalis. Mol Biochem Parasitol. 2000;108:137–40. doi: 10.1016/s0166-6851(00)00194-8. doi:10.1016/S0166-6851(00)00194-8. [DOI] [PubMed] [Google Scholar]
- 12.Müller J, Sterk M, Hemphill A, et al. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J Antimicrob Chemother. 2007;60:280–7. doi: 10.1093/jac/dkm205. doi:10.1093/jac/dkm205. [DOI] [PubMed] [Google Scholar]
- 13.Smith NC, Bryant C, Boreham PF. Possible roles for pyruvate:ferredoxin oxidoreductase and thiol-dependent peroxidase and reductase activities in resistance to nitroheterocyclic drugs in Giardia intestinalis. Int J Parasitol. 1988;18:991–7. doi: 10.1016/0020-7519(88)90183-x. doi:10.1016/0020-7519(88)90183-X. [DOI] [PubMed] [Google Scholar]
- 14.Dan M, Wang AL, Wang CC. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol Microbiol. 2000;36:447–56. doi: 10.1046/j.1365-2958.2000.01863.x. doi:10.1046/j.1365-2958.2000.01863.x. [DOI] [PubMed] [Google Scholar]
- 15.Cimerman B, Camilo Coura L, Salle JMC, et al. Evaluation of secnidazole gel and tinidazole suspension in the treatment of giardiasis in children. Braz J Infect Dis. 1997;1:241–7. [PubMed] [Google Scholar]
- 16.Upcroft JA, Dunn LA, Wright JM, et al. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother. 2006;50:344–7. doi: 10.1128/AAC.50.1.344-347.2006. doi:10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voolmann T, Boreham P. Metronidazole resistant Trichomonas vaginalis in Brisbane. Med J Aust. 1993;159:490. doi: 10.5694/j.1326-5377.1993.tb137978.x. [DOI] [PubMed] [Google Scholar]
- 18.Valdez CA, Tripp JC, Miyamoto Y, et al. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J Med Chem. 2009;52:4038–53. doi: 10.1021/jm900356n. doi:10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boreham PF, Phillips RE, Shepherd RW. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med Hyg. 1988;82:104–6. doi: 10.1016/0035-9203(88)90278-7. doi:10.1016/0035-9203(88)90278-7. [DOI] [PubMed] [Google Scholar]
- 20.Townson SM, Laqua H, Upcroft P, et al. Induction of metronidazole and furazolidone resistance in Giardia. Trans R Soc Trop Med Hyg. 1992;86:521–2. doi: 10.1016/0035-9203(92)90095-t. doi:10.1016/0035-9203(92)90095-T. [DOI] [PubMed] [Google Scholar]
- 21.Mirza H, Tan KS. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol Res. 2009;104:355–61. doi: 10.1007/s00436-008-1203-1. doi:10.1007/s00436-008-1203-1. [DOI] [PubMed] [Google Scholar]
- 22.Brown DM, Upcroft JA, Upcroft P. A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol Biochem Parasitol. 1996;83:211–20. doi: 10.1016/s0166-6851(96)02776-4. doi:10.1016/S0166-6851(96)02776-4. [DOI] [PubMed] [Google Scholar]
- 23.Olekhnovich IN, Goodwin A, Hoffman PS. Characterization of the NAD(P)H oxidase and metronidazole reductase activities of the RdxA nitroreductase of Helicobacter pylori. FEBS J. 2009;276:3354–64. doi: 10.1111/j.1742-4658.2009.07060.x. doi:10.1111/j.1742-4658.2009.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitsch D, Radauer C, Paschinger K, et al. Entamoeba histolytica: analysis of the trophozoite proteome by two-dimensional polyacrylamide gel electrophoresis. Exp Parasitol. 2005;110:191–5. doi: 10.1016/j.exppara.2005.02.016. doi:10.1016/j.exppara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Dunn LA, Boreham PF. The in-vitro activity of drugs against Blastocystis hominis. J Antimicrob Chemother. 1991;27:507–16. doi: 10.1093/jac/27.4.507. doi:10.1093/jac/27.4.507. [DOI] [PubMed] [Google Scholar]
- 26.Mirza H, Teo JD, Upcroft J, et al. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob Agents Chemother. 2011;55:637–48. doi: 10.1128/AAC.00900-10. doi:10.1128/AAC.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitsch D, Kolarich D, Duchêne M. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol Biochem Parasitol. 2010;171:17–24. doi: 10.1016/j.molbiopara.2010.01.001. doi:10.1016/j.molbiopara.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Linstead DJ, Bradley S. The purification and properties of two soluble reduced nicotinamide:acceptor oxidoreductases from Trichomonas vaginalis. Mol Biochem Parasitol. 1988;27:125–33. doi: 10.1016/0166-6851(88)90032-1. doi:10.1016/0166-6851(88)90032-1. [DOI] [PubMed] [Google Scholar]
- 29.Hrdý I, Cammack R, Stopka P, et al. Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes. Antimicrob Agents Chemother. 2005;49:5033–6. doi: 10.1128/AAC.49.12.5033-5036.2005. doi:10.1128/AAC.49.12.5033-5036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marczak R, Gorrell TE, Müller M. Hydrogenosomal ferredoxin of the anaerobic protozoon, Tritrichomonas foetus. J Biol Chem. 1983;258:12427–33. [PubMed] [Google Scholar]
- 31.Argüello-García R, Cruz-Soto M, Romero-Montoya L, et al. In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect Genet Evol. 2009;9:1057–64. doi: 10.1016/j.meegid.2009.05.015. doi:10.1016/j.meegid.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Upcroft P. Drug resistance in Giardia: clinical versus laboratory isolates. Drug Resist Updat. 1998;1:166–8. doi: 10.1016/s1368-7646(98)80035-6. doi:10.1016/S1368-7646(98)80035-6. [DOI] [PubMed] [Google Scholar]
- 33.Wright JM, Dunn LA, Kazimierczuk Z, et al. Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2′-deoxyadenosine. Parasitol Res. 2010;107:847–53. doi: 10.1007/s00436-010-1938-3. doi:10.1007/s00436-010-1938-3. [DOI] [PubMed] [Google Scholar]
- 34.Wright JM, Webb RI, O'Donoghue P, et al. Hydrogenosomes of laboratory-induced metronidazole-resistant Trichomonas vaginalis lines are downsized while those from clinically metronidazole-resistant isolates are not. J Eukaryot Microbiol. 2010;57:171–6. doi: 10.1111/j.1550-7408.2009.00455.x. doi:10.1111/j.1550-7408.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 35.Ross JA, Zvyagin AV, Heckenberg NR, et al. Measurement of action spectra of light-activated processes. J Biomed Opt. 2006;11:014008. doi: 10.1117/1.2161172. doi:10.1117/1.2161172. [DOI] [PubMed] [Google Scholar]
- 36.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–83. doi: 10.1146/annurev.pa.36.040196.001113. doi:10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman PS. Antibiotic resistance mechanisms of Helicobacter pylori. Can J Gastroenterol. 1999;13:243–9. doi: 10.1155/1999/838072. [DOI] [PubMed] [Google Scholar]
- 38.Kwon DH, El-Zaatari FA, Kato M, et al. Analysis of rdxA and involvement of additional genes encoding NAD(P)H flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:2133–42. doi: 10.1128/aac.44.8.2133-2142.2000. doi:10.1128/AAC.44.8.2133-2142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wouden EJ, Thijs JC, Kusters JG, et al. Mechanism and clinical significance of metronidazole resistance in Helicobacter pylori. Scand J Gastroenterol Suppl. 2001;234:10–4. doi: 10.1080/003655201753265055. [DOI] [PubMed] [Google Scholar]
- 40.Carlier JP, Sellier N, Rager MN, et al. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob Agents Chemother. 1997;41:1495–9. doi: 10.1128/aac.41.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang H, Edlund C, Hedberg M, et al. New findings in β-lactam and metronidazole resistant Bacteroides fragilis group. Int J Antimicrob Agents. 2002;19:361–70. doi: 10.1016/s0924-8579(02)00019-5. doi:10.1016/S0924-8579(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 42.Patel EH, Paul LV, Casanueva AI, et al. Overexpression of the rhamnose catabolism regulatory protein, RhaR: a novel mechanism for metronidazole resistance in Bacteroides thetaiotaomicron. J Antimicrob Chemother. 2009;64:267–73. doi: 10.1093/jac/dkp203. doi:10.1093/jac/dkp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steffens LS, Nicholson S, Paul LV, et al. Bacteroides fragilis RecA protein overexpression causes resistance to metronidazole. Res Microbiol. 2010;161:346–54. doi: 10.1016/j.resmic.2010.04.003. doi:10.1016/j.resmic.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafii F, Wynne R, Heinze TM, et al. Mechanism of metronidazole resistance by isolates of nitroreductase-producing Enterococcus gallinarum and Enterococcus casseliflavus from the human intestinal tract. FEMS Microbiol Lett. 2003;225:195–200. doi: 10.1016/S0378-1097(03)00513-5. doi:10.1016/S0378-1097(03)00513-5. [DOI] [PubMed] [Google Scholar]
- 45.Ellis JE, Wingfield JM, Cole D, et al. Oxygen affinities of metronidazole-resistant and -sensitive stocks of Giardia intestinalis. Int J Parasitol. 1993;23:35–9. doi: 10.1016/0020-7519(93)90095-g. doi:10.1016/0020-7519(93)90095-G. [DOI] [PubMed] [Google Scholar]
- 46.Crossley RA, Gaskin DJH, Holmes K. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl Environ Microbiol. 2007;73:7819–25. doi: 10.1128/AEM.01919-07. doi:10.1128/AEM.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worst DJ, Gerrits MM, Vandenbroucke-Grauls CM, et al. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J Bacteriol. 1998;180:1473–9. doi: 10.1128/jb.180.6.1473-1479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke ED, Wardman P, Goulding KH. Anaerobic reduction of nitroimidazoles by reduced flavin mononucleotide and by xanthine oxidase. Biochem Pharmacol. 1980;29:2684–7. doi: 10.1016/0006-2952(80)90087-8. doi:10.1016/0006-2952(80)90087-8. [DOI] [PubMed] [Google Scholar]
- 49.Rafferty S, Luu B, March RE, et al. Giardia lamblia encodes a functional flavohemoglobin. Biochem Biophys Res Commun. 2010;399:347–51. doi: 10.1016/j.bbrc.2010.07.073. doi:10.1016/j.bbrc.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Wang CC. A likely molecular basis of the susceptibility of Giardia lamblia towards oxygen. Mol Microbiol. 2006;59:202–11. doi: 10.1111/j.1365-2958.2005.04896.x. doi:10.1111/j.1365-2958.2005.04896.x. [DOI] [PubMed] [Google Scholar]
- 51.Brown DM, Upcroft JA, Upcroft P. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur J Biochem. 1996;241:155–61. doi: 10.1111/j.1432-1033.1996.0155t.x. doi:10.1111/j.1432-1033.1996.0155t.x. [DOI] [PubMed] [Google Scholar]
- 52.Di Matteo A, Scandurra FM, Testa F, et al. The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J Biol Chem. 2008;283:4061–8. doi: 10.1074/jbc.M705605200. doi:10.1074/jbc.M705605200. [DOI] [PubMed] [Google Scholar]
- 53.Arnér ES, Holmgren AD. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. doi:10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]