Abstract
Objectives
The development of daptomycin resistance in Staphylococcus aureus is associated with clinical treatment failures. The mechanism(s) of such resistance have not been clearly defined.
Methods
We studied an isogenic daptomycin-susceptible (DAPS) and daptomycin-resistant (DAPR) S. aureus strain pair (616; 701) from a patient with relapsing endocarditis during daptomycin treatment, using comparative transcriptomic and proteomic techniques.
Results
Minor differences in the genome content were found between strains by DNA hybridization. Transcriptomic analyses identified a number of genes differentially expressed in important functional categories: cell division; metabolism of bacterial envelopes; and global regulation. Of note, the DAPR isolate exhibited reduced expression of the major cell wall autolysis gene coincident with the up-regulation of genes involved in cell wall teichoic acid production. Using quantitative (q)RT–PCR on the gene cadre putatively involved in cationic peptide resistance, we formulated a putative regulatory network compatible with microarray data sets, mainly implicating bacterial envelopes. Of interest, qRT–PCR of this same gene cadre from two distinct isogenic DAPS/DAPR clinical strain pairs revealed evidence of other strain–dependent networks operative in the DAPR phenotype. Comparative proteomics of 616 versus 701 revealed a differential abundance of proteins in various functional categories, including cell wall-associated targets and biofilm formation proteins. Phenotypically, strains 616 and 701 showed major differences in their ability to develop bacterial biofilms in the presence of the antibacterial lipid, oleic acid.
Conclusions
Compatible with previous in vitro observations, in vivo-acquired DAPR in S. aureus is a complex, multistep phenomenon involving: (i) strain-dependent phenotypes; (ii) transcriptome adaptation; and (iii) modification of the lipid and protein contents of cellular envelopes.
Keywords: cell wall metabolism, antibiotic resistance, biofilms, δ-haemolysis, oleic acid, microarrays, virulence, quantitative proteomics
Introduction
Daptomycin (formerly LY146032) is a cyclic lipopeptide antimicrobial that was recently approved in the USA for the treatment of a wide variety of Staphylococcus aureus infections [both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA)], including skin and soft tissue infections, uncomplicated bacteraemia and right-sided endocarditis.1 In vitro, this agent is rapidly bactericidal against S. aureus in a concentration-dependent manner.2,3 However, clinical treatment failures due to the emergence of daptomycin-resistant (DAPR) strains during therapy have now been described, especially in subacute and chronic infections such as osteomyelitis and endocarditis, and with prolonged daptomycin exposure.2,4 Daptomycin absolutely requires Ca2+ for activity:5,6 while the native molecule is anionic, daptomycin is less active microbiologically (∼10 times) until it is heavily calcium decorated, making this agent a de facto cationic antimicrobial peptide functionally.7
To date, no specific genetic determinant(s) of DAPR in S. aureus have been universally defined in such strains, despite several well-known phenotypic correlates of DAPR [e.g. thickened cell walls, enhanced surface charge, alterations in cell membrane (CM) fluidity, cross-resistance to host defence cationic peptides, altered CM phospholipid synthesis and/or translocations].8 Also, previous investigations by our group and others suggested that alterations of CM fluidity may increase S. aureus resistance to antibacterial lipids such as oleic acid,9 i.e. natural compounds having structural characteristics similar to the lipid moiety of daptomycin.10 Of note, CM modification in S. aureus appears to affect the abilities of such strains to develop and maintain bacterial biofilms.11,12 Importantly, resistance to glycopeptide antibiotics such as vancomycin has been linked to a number of phenotypic perturbations, including bacterial envelope changes,13,14 the ability to produce biofilms15 and/or differences in the cell wall peptidoglycan composition.16 Concomitantly, we and others have confirmed that S. aureus DAPR acquired either during serial in vitro passage or, more relevant to this investigation, emerging during daptomycin therapy features a temporal and progressive ‘accumulation’ of genetic polymorphisms [single nucleotide polymorphisms (SNPs)].17 The acquisition of such SNPs has been most commonly observed within the mprF and/or yyc operons, with the former operon being important in surface positive charge maintenance, and the latter operon being a vital regulatory locus involved in CM lipid biosynthesis, cell wall homeostasis and biofilm formation.18,19 Interestingly, these SNPs have frequently been associated with genetic ‘gains in function’.20,21
Although such studies have been pivotal in disclosing potential phenotypic and genotypic correlates of DAPR, the precise interaction(s) among DAPR, CM lipid resistance, surface charge and biofilm dynamics, as well as putative DAPR genetic ‘pathways’, remain to be elucidated. The overarching goal of the current study was to gain new insights into putative genetic determinants and pathways involved in the emergence of DAPR in S. aureus in vivo. Thus, we undertook a combined transcriptomic–proteomic–phenotypic correlate approach to attempt further clarification of these issues.17
Materials and methods
Reagents and chemicals
All chemicals purchased were of the highest purity grade, unless otherwise stated. LiChrosolv water (Merck, Darmstadt, Germany) was used for the preparation of all buffers and solvents. Acetonitrile was purchased from Biosolve (Westford, MA, USA). Trifluoroacetic acid (TFA), α-cyano-4-hydroxycinnamic acid, 1,4-dithioerythritol, ammonium bicarbonate, iodoacetamide, glycine, porcine trypsin, Tris, BSA, rabbit phosphorylase b, chicken ovalbumin and bovine β-casein were from Sigma–Aldrich (St Louis, MO, USA). Immobilized pH gradient (IPG) strips and ampholines were purchased from GE Healthcare (Piscataway, NJ, USA). SDS–PAGE pre-cast gels and molecular mass markers were purchased from Bio-Rad (Hercules, CA, USA).
Bacterial strains and cultures
The two primary strains (methicillin susceptible) used in this study have been described in detail previously.22 Strain 616 is the parental (pre-therapy) daptomycin-susceptible (DAPS) bloodstream isolate initially obtained from a patient with endocarditis [daptomycin MIC (Etest) = 0.5 mg/L]; strain 701 is a DAPR organism recovered during daptomycin treatment (daptomycin MIC = 2 mg/L) [although the currently accepted terminology should be ‘daptomycin non-susceptibility’, ‘daptomycin resistance’ (DAPR) is used in this manuscript for ease of presentation]. These strains were isogenic by pulsogram as previously detailed, and were each agr type 2.22 Lastly, strain 701 (but not 616) contained an SNP within the mprF open reading frame (ORF) (S295L), which resulted in a phenotypic ‘gain in function’ of the mprF gene as previously described, localized to the putative ‘translocase’ domain for flipping lysyl-phosphotidylglycerol from the inner CM to the outer CM.23,24 Moreover, transmission electron microscopy (TEM) revealed this DAPR isolate to have significantly thicker cell walls than the DAPS parental strain.25
For selected and strain-dependent comparisons [multiple loci variable number tandem repeat analysis (MLVA) and quantitative (q)RT–PCR, see below], two additional DAPS/DAPR S. aureus strain pairs were utilized. Both strain pairs were obtained from patients with recalcitrant endocarditis; the clinical, phenotypic and selected genotypic details of these isolate pairs have been previously described.26,27 Both DAPS/DAPR strain pairs were identical by PFGE pulsograms. They included MSSA strain pair BOY755 and BOY300, and MRSA strain pair 11-11 and REF2145, respectively.24
Biofilm assays
The potential impact of adaptations to daptomycin upon biofilm characteristics was assessed. S. aureus strains were grown in trypticase soy broth (TSB; Becton Dickinson, Le Pont de Claix, France) supplemented with 1% (w/v) glucose. Oleic acid (cis-9-octadecenoic acid; Sigma–Aldrich, Basel, Switzerland), used to mimic the lipid tail of daptomycin, was emulsified with TSB–glucose media (TSBglucOleic) by overnight agitation at 220 rpm in a Lab-Shaker at 37°C. Biofilm development was performed in TSBgluc (or TSBglucOleic emulsions) with 20 μL of overnight culture/mL of fresh medium (6 mL). Bacterial colonies were counted on Mueller–Hinton agar plates (MHA; Bio-Rad, Marnes-La-Coquette, France). Biofilm staining assays were performed as described previously.11
Bacterial counting methods
Direct counting was performed in Neubauer chambers, as previously described.11 Determination of colony forming units was performed on MHA using a Countermat Flash colony counter (IUL, RB Scientific, Southampton, England).
Assessment of δ-haemolysin activity
As will be demonstrated below, we identified differences in agr expression (a key global regulon of S. aureus) between DAPS and DAPR strain pairs. To assess the phenotypic correlates of this genotypic difference, the functions of the agr operon were measured by δ-haemolysin production. The δ-haemolytic activities were determined by first streaking RN4220, a strain that only produces β–haemolysin, without the interference of α- and δ-haemolysins, on sheep blood agar plates.28 Then, the test strains were streaked perpendicularly to the RN4220 streak. The β- and δ-haemolysins of S. aureus act synergistically in the lysis of sheep red blood cells. Therefore, δ-haemolysin produced by any test strain results in a zone of enhanced haemolysis in areas where this haemolysin overlaps with the β-haemolysin zone of the RN4220 strain. The degree of synergistic haemolysis was graded from 0 to 4+ by one of the authors (S.-J. Y.), who was blinded as to the agr transcription data and strain identities.29 All experiments were conducted at least twice on separate days.
Genotyping of S. aureus strains by MLVA assay
The DAPS and DAPR isolate pairs (616 and 701, BOY755 and BOY300, and MRSA11-11 and REF2145), previously characterized by PFGE, were additionally genotyped using a well-described MLVA assay.30 This method assesses genomic elements that are different to those determined by PFGE. This technique affords higher resolution than traditional PFGE, as it is able to further discriminate into subclusters some isolates that appear clonal by multilocus sequence typing (MLST).31 For clonal cluster (CC) comparisons, we utilized the following strains with well-characterized ‘clonal complex’ profiles: N315 and Mu50 (CC = 5); COL, USA300 and NCTC 8325 (CC = 8); and MW2 and MSSA476 (CC = 1).
Transcriptional analyses
Microarray manufacturing and design
The microarray was manufactured by the in situ synthesis of 10 807 60-mer long oligonucleotide probes (Agilent, Palo Alto, CA, USA), selected as previously described.32 It covers >98% of all ORFs annotated in strains N315 and Mu50,33 MW234 and COL,35 NCTC 8325 and USA300,36 as well as MRSA252 and MSSA476 (including their respective plasmids).37
Preparation of labelled nucleic acids for expression microarrays
Total RNA was purified from both early and late exponential phase bacteria grown in Mueller Hinton broth (MHB) and treated with DNase. Preparations of 5 μg of total S. aureus RNA were labelled with Cy-3 dCTP using the SuperScript II method (Invitrogen, Basel, Switzerland) and purified, as previously described.38
Purified genomic DNA from the reference sequenced strains used for the design of the microarray was labelled with Cy-5 dCTP and used in microarray normalization.39 Mixtures of Cy5-labelled DNA and Cy3-labelled cDNA were hybridized and scanned, as previously described.38
Microarray analysis
Hybridization fluorescence intensities were quantified using Feature Extraction software (Agilent, version 8). Local background subtracted signals were corrected for unequal dye incorporation or unequal load of the labelled product, using a rank consistency filter and a curve-fitting algorithm per the default LOWESS (locally weighted linear regression) method. Data from three independent biological experiments were analysed using GeneSpring 8.0 (Silicon Genetics, Redwood City, CA, USA), as previously described,38 with a 5% false discovery rate (P value cut-off, 0.05) and an arbitrary threshold of 1.5-fold for defining significant differences in expression ratios. The complete microarray data set is posted on the Gene Expression Omnibus database, available at http://www.ncbi.nlm.nih.gov/geo/, under accession numbers GPL7137 for the platform design and GSE28632 for the original data set.
Real-time PCR (qRT–PCR) validation
Gene-specific probes were designed using Primer Express 3.0 (Applied Biosystems) and are shown in Table 1. Oligonucleotide primers and probes obtained from Sigma or Applied Biosystems (minor groove binder coupled to dark quencher) were solubilized in water, and reactions were assembled in a one-step RT–PCR enzymatic mixture (Invitrogen, Carlsbad, Germany) in a final volume of 10 μL. Reactions were performed in a StepOne Plus instrument (Applied Biosystems), as described previously.40 Results were normalized using intensity levels recorded for the rRNA 16S gene, as described previously.41 These studies provided relative gene expression for DAPR strains 701, BOY300 and REF2145 as compared with their respective parental DAPS isolates listed above. The statistical significance of strain-specific differences in the normalized cycle threshold values for each transcript was evaluated by the paired t-test, and data were considered significant at P values <0.05.
Table 1.
List of primers/probes for qRT–PCR
| Primer/probe name | Sequence (5′→3′) | Dye/quencher | Length (bp) | Cf (μM) | NCBI accession no. |
|---|---|---|---|---|---|
| asp23_299_F | GTTAAGCCACCTTTCATGTCTAAGATAC | 28 | 0.2 | ||
| asp23_390_R | AAATTAACTTTCTCTGATGAAGTTGTTGA | 29 | 0.2 | NP_375295.1 | |
| asp23_333_T | CTTCACGTGCAGCGATACCAGCAATTT | FAM/TAMRA | 27 | 0.1 | |
| mprF_F | TCATTGCTGCATTATCAGGTTTAGTC | 28 | 0.2 | ||
| mprF_R | TTCCTCAGGGACACCTAAAGTTTT | 29 | 0.2 | NP_374473.1 | |
| mprF_P | ATTCCTGGTGGTTTCGGCG | FAM/3BQ1 | 27 | 0.1 | |
| hla_337_F | ATGAGTACTTTAACTTATGGATTCAACGG | 28 | 0.2 | ||
| hla_437_R | AGTGTATGACCAATCGAAACATTTG | 29 | 0.2 | M90536.1 | |
| hla_385_T | ACAGGAAAAATTGGCGGCCTTATTGGT | FAM/MGB | 27 | 0.1 | |
| rot_332_F | GAGTTAATGTCACCCAAAAGTGTTTCT | 28 | 0.2 | ||
| rot_418_R | TTGGGAGATGTTTAGCATGAAAAA | 29 | 0.2 | NP_374872.1 | |
| rot_MGB | CAAAATTCCAAATACAGTGTCGTT | FAM/MGB | 27 | 0.1 | |
| saeRS_F | AAGAACATGATACCATTTACGCCTTA | 28 | 0.2 | ||
| saeRS_R | CCTTGGACTAAATGGTTTTTTGACA | 29 | 0.2 | NP_373916.1 | |
| saeRS_P | CTTTAGGTGCAGATGACT | FAM/MGB | 27 | 0.1 | |
| sarA_17_F | ACATGGCAATTACAAAAATCAATGAT | 28 | 0.2 | ||
| sarA_167_R | TCTTTCTCTTTGTTTTCGCTGATG | 29 | 0.2 | NP_373827.1 | |
| sarA_45_T | CTTTGAGTTGTTATCAATGGT | FAM/MGB | 27 | 0.1 | |
| sarR_F | TGAGTCTAACGAAATCTCATCTAAAGAGA | 28 | 0.2 | ||
| sarR_R | CAATAACTGTTCTTTCGTCTTGTAAACTTC | 29 | 0.2 | NP_375408.1 | |
| sarR_P | TGCTAAGTGCTCAGAGTT | FAM/MGB | 27 | 0.1 | |
| sarS_552-576_F | CCACCATAAATACCCTCAAACTGTT | 28 | 0.2 | ||
| sarS_615-638_R | TCATCTTCAGTTGAGCGTTCTTTT | 29 | 0.2 | NP_373349.1 | |
| sarS_595-613_P | AAAAAGCAAGGCTATCTAA | FAM/MGB | 27 | 0.1 | |
| sarT_217-241_F | AGCGTAAAAGAATTATCAAAAAAGG | 28 | 0.2 | ||
| sarT_306-280_R | TTTTACAGAAACAACAATGATTACATT | 29 | 0.2 | NP_375610.1 | |
| sarT_243-265_P | TTACTTGAATAAATGTAGAGACC | FAM/MGB | 27 | 0.1 |
mprF, fmtC; sarS, sarH1; Cf, final concentration; NCBI, National Center for Biotechnology Information.
Proteomics analyses
The complete procedure is described by Vaezzadeh et al.42 Adaptations in the methods or experimental design for the current study are described below.
S. aureus strain growth conditions
Bacterial strains were grown in MHB, essentially as described previously.38 Cells were grown for 5 h and lysed with 20 mg/L lysostaphin (Ambicin; Applied Microbiology, Tarratown, NY, USA) for 15 min at 37°C, in Tris–EDTA buffer. For preparation of crude membrane extracts, 20 mL culture aliquots were washed in 1.1 M saccharose-containing buffer and then suspended in 2 mL aliquots of the same buffer containing 50 mg/L of the hydrolytic enzyme lysostaphin for 10 min at 37°C. Protoplasts were recovered after centrifugation (30 min at 8000 g) and membrane pellets were obtained after ultracentrifugation at 50 000 g for 50 min.
Sample preparation
Quantitative mass spectrometry-based proteomic experiments were performed on three independent replicates. Samples were prepared using isobaric tags iTRAQ (Applied Biosystems, Framingham, MD, USA), according to the manufacturer's protocol. Digestion was performed by trypsin at a protease-to-protein ratio of 1 : 25 using microwave catalysis (FUNAI, Hamburg, Germany). After digestion, the reaction was immediately quenched with 1 M formic acid.42 For Experiment 1 (PR1), strain 616 was labelled with iTRAQ 114 and 701 with iTRAQ 117. For Experiment 2 (PR2), strain tags were crossed; strain 616 was labelled with iTRAQ 117 and 701 with iTRAQ 116. Lastly, for Experiment 3 (PR3), strain 616 was labelled with iTRAQ 116 and 701 with iTRAQ 114.
IPG-isoelectric focusing (IEF)
IPG-IEF was performed under the following conditions, in sequence: (i) an initial 30 min step at 500 V; (ii) a linear gradient from 500 V to 4 kV over 90 min; (iii) a linear gradient from 4 kV to 8 kV in 30 min; and (iv) a final step-up cycle from 8 kV to 30 kV for 30 min. Samples (200 μL) were loaded by overnight in-gel rehydration. Next, IPG strips were washed three times for 10 s each in three distinct high-boiling point petroleum ether baths to remove the paraffin oil. Each strip was then manually cut and gel pieces were placed in polypropylene tubes containing 80 μL of 0.1% TFA. After three separate 30 min incubations with 0.1% TFA, all extracts were pooled. The samples were then cleaned using an Oasis HLB μ-Elution 96-Well Plate system as per the manufacturer's protocol (Waters, USA). Purified samples were then dried by evaporation, resuspended in 25 μL HPLC buffer A (0.1% formic acid in 3% acetonitrile) and stored at −20°C.
Liquid chromatography–mass spectrometry (LC–MS) and peptide analysis
A 5 μL peptide solution of each fraction was loaded in a 10 cm long column with an internal diameter of 100 μm. The elution gradient from 4% to 38% of the counter solvent (0.1% formic acid in 80% acetonitrile) was developed over 40 min and samples were eluted directly on a matrix-assisted laser desorption/ionization (MALDI) target using a spotting robot. An aqueous solution of matrix [α-cyano-4-hydroxycinnamic acid; 5 mg/mL (w/v) in 50% acetonitrile/0.1% TFA/10 mM NH4H2PO4] was applied and dried. Peptides were analysed in MS and MS/MS modes using a 4800 MALDI–time of flight (TOF)/TOF tandem mass spectrometer (Applied Biosystems) using a neodymium-doped yttrium aluminium garnet (Nd:YAG) laser at 355 nm, operating at 200 Hz. Eight hundred and 1500 consecutive laser desorptions were accumulated for MS and MS/MS spectra, respectively. Data-dependent MS/MS analysis was performed automatically on the 15 most intense ions from MS spectra, with lysozyme C as the external control.
Protein identification
Peak lists were generated from raw data using the PeaktoMascot software, as appropriate to the instrument. The combined peak lists of all fractions of the same IPG strip were merged into a single mascot generic file (mgf) format and searched against a database containing the 2581 genome-sequenced S. aureus strain N315 proteins (UniProt knowledgebase; release 15.12, 15 December 2009) using Phenyx (GeneBio, Geneva, Switzerland) with parent ion tolerance set to 100 ppm. A variable amino acid modification was oxidized methionine. Trypsin was selected as the enzyme, with one potential missed cleavage, and the normal cleavage mode was used. The peptide p value was 1 × 10−2 for linear ion trap-orbitrap data. False-positive ratios were estimated using a reverse decoy database.43 All data sets where searched once in the forward and once in the reverse database. Separate searches were used to keep the database size constant. Protein and peptide scores were then optimized to maintain a false-positive ratio <1%, biasing toward a conservative slight overestimation of the false-positive ratio.43 For all analyses, only proteins matching two different peptide sequences were prioritized for further consideration.
Data analysis using iTRAQ quantification
Reporter-ion abundances for each identified peptide were quantified directly from peak lists using the dedicated Phenyx export. Extracted ion abundance values were corrected from isotopic impurities44,45 and relative peptide ratios calculated by the quotient of corrected reporter-ion abundances at an m/z ratio corresponding to the respective channels. Protein ratios were then obtained by calculating the geometric mean of all peptide ratios corresponding to a given protein.
Results
DAPR and biofilm formation
A first step in studying the potential in vivo characteristics of S. aureus DAPR was to investigate the potential correlation between this resistance phenotype and biofilm formation. S. aureus strains 616 (DAPS) and 701 (DAPR) were exposed to 0.1% oleic acid in microtitre plates in a biofilm assessment assay.11 We chose oleic acid because of its similar biochemical nature to the lipid tail of daptomycin, in which the first seven carbene groups (CH2) are similar to the first eight carbene groups (CH2) of the daptomycin lipid tail, following the methyl terminal group in each molecule.
In the presence of oleic acid in the nutrient medium during growth in planktonic suspension, parental strain 616 cells outnumbered DAPR 701 cells (Figure 1a and b). Both isolates produced an abundant biofilm in oleic acid-free conditions on polystyrene plates (Figure 1c). The total amount of biofilm assessed after solubilization of crystal violet appears similar for the two strains (Figure 1d). However, in the presence of oleic acid, the adherent bacterial population appeared significantly larger in the DAPR strain than in the DAPS strain (Figure 1d). Consequently, the proportion of bacteria in suspension appears larger for strain 616 than for strain 701, indicating an abundant release of adherent bacteria (Figure 1a and b). We also observed a totally different biofilm organization between the two isolates: while strain 616 showed a homogeneous colonization of the surface, strain 701 demonstrated large macroscopic aggregates (Figure 1c).
Figure 1.
Biofilms of strains 616 and 701 under oleic acid stress and bacterial quantification. (a) Serial dilutions of planktonic cells spotted on agar plates. (b) Quantification of planktonic cells by OD540 for two independent experiments. (c) Biofilms stained with crystal violet in a 6-well multititre plate. The box shows a magnification of strain 701 grown in the presence of oleic acid and the arrows show spots stained with crystal violet. (d) Adherence in the presence of oleic acid during two independent experiments. Error bars show the range of duplicate experiments.
agr function
As shown in Figure S1 (available as Supplementary data at JAC Online), for the 616 versus 701 comparisons, both strains elaborated δ-haemolysin, although the parental strain appeared to produce somewhat more. In comparing BOY755 versus BOY300, δ-haemolysin production was equivalent. Of interest, parental MRSA11-11 produced extensive δ-haemolysin, while DAPR REF2145 elaborated very little δ-haemolysin.
Genotypic characterization of the isolate set
MLVA revealed that the three strain sets were strictly clonal, but differed from each other in dendrogram analysis (data not shown). Further analyses of the genome content of strain 616 versus strain 701 showed that the two isolates belonged to the same cluster and were most similar to the N315/Mu50 lineage.32,38 Note also that strains COL, USA300 and NCTC 8325, belonging to clonal complex 8, segregated into the same cluster, as did the two CC1 isolates, MW2 and MSSA476 (Figure 2). Interestingly, the comparison of the 616–701 DAPS/DAPR strain pair confirmed the acquisition of genes belonging to pUSA300, underscoring the notion that genetic elements may be acquired in vivo.36 However, on a more global basis, the list of regions or probes showing divergence following ‘genomotyping’ analysis software (GACK)46 after hybridization of genomic DNA revealed only a limited number of putative hits when the strain pair isolates were compared. Our microarray design allowed mapping of the S. aureus genome using an average of one probe every 450 nucleotides (Table 2).
Figure 2.
Molecular genotyping of the set of strains. Genotyping tree obtained using microarray covering whole S. aureus genomes of eight sequenced strains revealed that our isolates are genetically comparable while N315 appears as the more related reference strain. Each probe is represented by a single row of grey-scaled boxes and each sample corresponds to a single column. The grey areas correspond to genes present, whereas white bars indicate missing genes (N315 used as the reference). The dendrogram (top and left part of the figure) represents the similarity matrix of probe sets.
Table 2.
List of genes responsible for difference during complete genome hybridization
| Gene | Present only in strain |
|---|---|
| arsB | 701 |
| ccrB | 701 |
| ileS | 701 |
| nes | 701 |
| qacR | 701 |
| repA | 701 |
| SA1763 | 701 |
| SA1824 | 701 |
| SACOL0332 | 701 |
| SACOL0902 | 701 |
| SACOL1582 | 701 |
| SAUSA300_pUSA030027 | 701 |
| SAUSA300_pUSA030030 | 701 |
| SAUSA300_pUSA030034 | 701 |
| SAUSA300_pUSA030035 | 701 |
| SAUSA300_pUSA030036 | 701 |
| tnpF | 616 |
| traA | 701 |
| traB | 701 |
| traC | 701 |
| traI | 701 |
| traJ | 701 |
| traK | 701 |
| traM | 701 |
In addition, given the known major role of the global regulon, agr, in staphylococcal virulence factor expression, biofilm formation, persistent bacteraemia and glycopeptide resistance,47–51 the entire agr locus was sequenced in the two principal study strains. Both isolates revealed agr locus sequences identical to that of strain Mu3, i.e. with silent point mutations in agrA (A→G at position 264; data not shown).
Transcriptomic analysis
The results summarized in Table 3 were obtained from the average values of three independent replicate experiments showing at least a difference of ±1.5-fold between the DAPS and DAPR strains. In general, when comparing strain 616 with strain 701, although often statistically significant, most of the fold change values observed for the differentially expressed genes were moderate (2–4-fold range). The total number of genes showing differential expression at 5 h (late exponential phase) was ∼120. For the majority of these genes, the trends in differential expression seen at 5 h were also observed at 3 h (early exponential phase; data not shown). Overall, among these differentially expressed genes, 42% were up-regulated in the DAPR strain as compared with the DAPS strain, while 58% were down-regulated. Genes involved in metabolic functions constituted nearly one-half (47%) of those differentially expressed (e.g. sugar metabolism, such as lac A-B-D-E-F, or amino acid metabolism, such as arcA-B-B1-B2-D or argF-H for ornithine degradation and pH homeostasis). Among this category of genes, 74% were up-regulated in the DAPR isolate as compared with the DAPS isolate, whereas 26% showed the opposite trend. In addition, ∼15% of the differentially regulated genes belonged to gene families involved in the processes of translation or transcription, with 38% down-regulated and 62% up-regulated in the DAPR strain versus the DAPS strain. Cell wall metabolism genes also appeared as an important category distinguishing the two strains, showing only up-regulated genes in the DAPR isolate (n = 9/9). Genes that were ‘highly up-regulated’ (i.e. ≥5-fold) in the DAPR strain as compared with the DAPS strain were observed in the following categories: (i) metabolic functions, such as the tre and lac operons (>10-fold); (ii) putative virulence factors, such as the egc cluster encoding clinically important enterotoxins (>5-fold), hlb (>6-fold) and von Willebrand factor-binding protein (MW 766 kDa; >7-fold); and (iii) several ABC transporters (>5-fold). Selected gene grouping differences are further detailed below.
Table 3.
List of genes showing differential expression between daptomycin-susceptible and -non-susceptible isolates
| Gene | Function | Fold change 701 versus wild-type (5 h) | COG functional category |
|---|---|---|---|
| Transport and metabolism | |||
| arcA | arginine deiminase | 0.518 | E |
| arcB | ornithine carbamoyltransferase | 0.456 | E |
| arcB1 | ornithine carbamoyltransferase | 0.415 | E |
| arcB2 | ornithine carbamoyltransferase | 0.552 | E |
| arcD | arginine/ornithine antiporter | 2.037 | E |
| arg | arginase | 0.466 | E |
| argF | ornithine carbamoyltransferase | 0.448 | E |
| argH | argininosuccinate lyase | 4.777 | E |
| SACOL0408 | glyoxalase family protein | 2.032 | E |
| SACOL1916 | amino acid ABC transporter, permease/substrate-binding protein | 10.621 | E |
| SAV2440 | similar to amino acid permease | 2.057 | E |
| lacA | galactose-6-phosphate isomerase subunit LacA | 12.630 | G |
| lacB | galactose-6-phosphate isomerase subunit LacB | 11.730 | G |
| lacD | tagatose 1,6-diphosphate aldolase | 10.539 | G |
| lacE | PTS system, lactose-specific IIBC component | 7.762 | G |
| lacF | PTS system, lactose-specific IIA component | 7.590 | |
| lacG | 6-phospho-β-galactosidase | 8.629 | |
| lldP2 | l-lactate permease | 2.052 | C |
| manA | mannose-6-phosphate isomerase | 4.969 | G |
| SA0208 | maltose ABC transporter, permease protein | 2.642 | G |
| scrA | PTS system lactose-specific IIBC component | 7.931 | G |
| tagG | teichoic acid ABC transporter permease protein | 1.625 | G |
| treP | phosphoenolpyruvate and trehalose-specific PTS enzyme II | 18.050 | G |
| treC | α-amylase family protein | 19.550 | |
| SAV0734 | FecCD transport family protein | 3.969 | P |
| SAV2417 | cation efflux family protein | 4.536 | P |
| MW0149 | hypothetical protein | 10.694 | I |
| sirC | putative siderophore transport system permease | 9.215 | P |
| fadB | 3-hydroxyacyl-CoA dehydrogenase | 4.310 | I |
| Cell wall/membrane/envelope biogenesis | |||
| capB | capsular polysaccharide biosynthesis protein CapB | 1.788 | D |
| capC | capsular polysaccharide biosynthesis protein CapC | 2.433 | G |
| lytN | cell wall hydrolase | 3.915 | |
| lytH | N-acetylmuramoyl-l-alanine amidase | 2.139 | M |
| pbp2 | penicillin binding protein 2 | 1.528 | M |
| pbp4 | penicillin binding protein 4 | 1.841 | M |
| sgtA | transglycosylase domain protein | 3.477 | M |
| tagA | teichoic acid ABC transporter permease protein | 3.516 | M |
| tagG | teichoic acid ABC transporter permease protein | 2.232 | |
| Defence mechanisms and virulence factors | |||
| SACOL2356 | ABC transporter, ATP-binding protein | 11.740 | V |
| SAV0198 | ABC transporter, ATP-binding protein | 3.895 | V |
| SAV2360 | ABC transporter, permease protein | 7.640 | V |
| coa | coagulase precursor | 7.618 | |
| fnbB | fibronectin binding protein B | 23.575 | |
| SAV0812 | similar to secreted von Willebrand factor-binding protein | 7.469 | |
| SAV0945 | secreted von Willebrand factor-binding protein | 10.824 | P |
| sdrC | SdrC protein | 1.645 | |
| seg | enterotoxin SEG | 5.043 | |
| sei | enterotoxin SEI | 5.427 | |
| sem | enterotoxin SEM | 4.349 | |
| seo | enterotoxin SEO | 10.160 | |
| sep | enterotoxin SEP | 3.616 | |
| pls | methicillin-resistant surface protein | 10.950 | |
| clpL | ATP-dependent Clp protease | 0.610 | O |
| hla | α-haemolysin precursor | 1.470 | |
| hlb | β-haemolysin | 6.584 | |
| hld | δ-haemolysin | 0.243 | |
| hlgC | γ-haemolysin | 2.087 | |
| Signal transduction mechanisms | |||
| agrA | accessory gene regulator A | 0.634 | K |
| agrB | accessory gene regulator B | 0.625 | O |
| agrD | accessory gene regulator D | 0.574 | |
| saeR | DNA-binding response regulator SaeR | 1.966 | T |
| saeS | sensor histidine kinase SaeS | 1.856 | T |
| vraR | DNA-binding response regulator VraR | 2.219 | T |
| vraS | sensor histidine kinase VraS | 2.071 | T |
| lytR | autolysin response regulator protein | 4.424 | K |
| rot | repressor of toxins Rot | 1.793 | |
| sarT | staphylococcal accessory regulator T | 4.177 | K |
| treR | transcriptional regulator, GntR family | 10.891 | K |
| Uncharacterized | |||
| SACOL0739/SA0634 | acetyltransferase, GNAT family | 7.287 | J |
| MW0047 | hypothetical protein | 3.014 | |
| MW0203/SACOL0206 | hypothetical protein | 5.728 | |
| MW0372 | hypothetical protein | 9.550 | |
| MW0638/SACOL0736/SA0631 | acetyltransferase, GNAT family | 5.764 | |
| SACOL0478/SA0393 | superantigen-like protein | 6.094 | |
| SACOL1656/SA1428 | hypothetical protein | 4.880 | |
| SAV0868 | hypothetical protein | 15.320 | |
| ywpF/SACOL2090/SA1900 | ywpF protein | 2.080 | |
| yycI/SACOL0022/SA0020 | yycI protein | 1.675 | S |
| yycJ/SACOL0023/SA0021 | metallo-β-lactamase yycJ protein | 1.735 | R |
| Various | |||
| SAV2513/SACOL2522/SA2301 | DedA family protein | 4.169 | S |
| spsA/SACOL0968/SA0825 | signal peptidase IA | 3.539 | U |
| SACOL0872/SA0755 | OsmC/Ohr family protein | 0.444 | O |
| rnhC/SACOL1150/SA0987 | ribonuclease HIII | 5.306 | L |
COG (cluster of orthologous groups) categories are: C, energy production and conversion; D, cell division and chromosome partitioning; E, amino acid transport and metabolism; G, carbohydrate transport and metabolism; I, lipid metabolism; J, translation, ribosomal structure and biogenesis; K, transcription; L, DNA replication, recombination and repair; M, cell envelope biogenesis, outer membrane; O, post-translational modification, protein turnover, chaperones; P, inorganic ion transport and metabolism; R, general function prediction; S, function unknown; T, signal transduction mechanisms; U, secretion; V, defence mechanism; PTS, phosphotransferase system. Values <1 correspond to downregulated genes.
Metabolism and cell wall-related genes
The main categories of metabolic genes that were found to be differentially regulated are involved in the transport of amino acids, carbohydrates, coenzymes and lipids. The vast majority of these genes belonged to amino acid metabolism and transport families (e.g. arginine and ornithine metabolism), and were generally down-regulated by 2–4-fold in the DAPR versus DAPS strains. Of note, most of the lac operon appeared to be up-regulated in the DAPR isolate.
Among the genes involved in the biogenesis of the bacterial cell wall, some components of the lyt operon (e.g. lytN and lytH) were up-regulated in DAPR versus DAPS isolates. The tag genes (tagA and tagG) involved in the biogenesis and transport of teichoic acids were found to be significantly up-regulated in the DAPR isolate. A similar observation was noted for penicillin–binding proteins, pbp2 and pbp4. Moreover, genes belonging to the yycG/yycF operon (yycI and yycJ) were found to be moderately up-regulated in the DAPR strain. This system has been described as an important regulator of virulence (through the alteration of ssaA and lytM expression), as well as cell wall biosynthesis (through its action on tagA-D expression) and biofilm production. Interestingly, yycG has been linked to the DAPR phenotype in a recent report on such mutants obtained from in vitro passages and also following daptomycin exposure in vivo.52,53
Regulators and virulence factors
Numerous regulatory genes were found to be differentially expressed between the DAPR and DAPS strains. Two specific examples were components of the agr locus (as well as the downstream hld locus responsible for δ-haemolysin production) and the two-component regulatory system, saeRS. There were notable trends towards down-regulation of these loci in the DAPR versus DAPS strains. Consequently, differential expression of numerous virulence factors that are either agr-regulated and/or saeRS-regulated was also observed, i.e. spa, fnbA/B, hla and coa.54
qRT–PCR validation of microarray data for selected genes: comparisons with other DAPS–DAPR strain pairs
As an independent, but complementary metric, we utilized qRT–PCR to validate the relative expression levels of selected genes, including several found to be differentially regulated by microarray analysis. This list of genes was specifically prioritized by virtue of known or expected effects on virulence,17,24 as well as their potential for being part of an interactive regulatory network involved in the DAPR phenotype [e.g. agr; mprF (fmtC)].24,55 As previously established by a number of other studies, the absolute magnitude of the normalized amplification signals reflected a broader dynamic range in qRT–PCR as compared with microarray measurements.32,56
Overall, the results of the qRT–PCR analyses (Table 4) paralleled the microarray determinations in terms of up- or down-regulation, except for the down-regulated expression of yycI in 701. Importantly, qRT–PCR data confirmed the substantial down-regulation of agr and hld expression in the principal DAPR versus the DAPS strain pair, as also detected by microarray analysis. Similarly, a significant increase in the expression of rot and hla as well as sarT and saeRS was noted by qRT–PCR comparing the DAPR versus DAPS strains, in agreement with the microarray data. However, there was an apparent disconnection between hla abundance when compared with agrA expression. In strain 701, the low level of agrA (activator of hla) and the high level of rot (repressor of hla) transcripts would not normally be compatible with high hla transcription. This observation suggests either a mutation/dysfunction in Rot and/or a mutation in the promoter region of hla. Alternatively, the increased hla may be explained by the increase in SaeRS, increased MgrA and increased SarZ (data not shown). They all increase hla even in the absence of RNAIII that acts upon Rot (Figure S2, available as Supplementary data at JAC Online, shows some of the potential regulator interactions). Lastly, the levels of asp23, sarA and mprF (fmtC) transcription were significantly elevated in comparing the 616–701 DAPS versus DAPR isolates, while those for asp23 and sarA were only slightly increased in the DAPR strain.
Table 4.
Expression of selected genes in each DAPR from our three strain pairs, and comparison with quantitative transcriptomics and proteomics
| RQ DAPR versus DAPS | rot | sarA | sarS | sarT | hla | agrA | mprF (fmtC) | asp23 | hld | sarR | yycF | yycI | saeRS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOY300 | 1.37* | 0.92 | 1.28 | 0.78 | 1.00 | 1.28* | 1.12 | 0.53 | 1.49* | 1.00 | 0.79* | 1.27* | 1.66* | |
| REF2145 | 1.29* | 1.00 | 1.05 | 0.77 | 0.06* | 0.58* | 0.71* | 0.94 | 0.86 | 1.04 | 0.66* | 0.72* | 0.24* | |
| qRT–PCR | 701 | 2.53* | 1.09 | 1.84* | 6.01* | 1.82* | 0.25* | 1.47* | 1.49 | 0.04* | 0.74* | 0.81* | 0.79* | 2.84* |
| Microarray results | 1.90 | — | — | 4.20 | 1.50 | 0.63 | — | — | 0.24 | — | — | 1.67 | 2.00 | |
| Proteomic results | — | 1.4 | — | — | — | — | — | 2.2* | — | — | — | — | 1.00 |
*Statistically significant (t-test value <0.01); RQ, relative quantity.
We compared the qRT–PCR profiles of the prioritized genes of interest above for our prototype strain pair (616–701) with those of two other recent clinical DAPS–DAPR strain pairs (BOY755–BOY300; and MRSA11-11–REF2145) (Table 4). Of note, the differential expression profiles for rot, sarS and yycF were quite consistent across the three strain sets, although the net profiles differed significantly among the other genes queried. These data suggested a strong strain-dependent impact on the overall specific genetic networks responsible for the ultimate DAPR phenotype. These findings are similar to distinct strain-to-strain expression profiling analyses among diverse vancomycin-intermediate S. aureus (VISA) strains of S. aureus.13,57,58
Quantitative proteomics analysis
Three independent replicates (PR1, PR2 and PR3) identified 771 individual proteins (Figure S3a, available as Supplementary data at JAC Online) by combining all proteins identified at least once (see Table S1, available as Supplementary data at JAC Online). More than 30% of the identified proteins had more than one predicted transmembrane segment; on average, four to five peptides were identified per protein, confirming the robustness of the quantitative results. To further enhance the accuracy, relative quantification was performed on 546 proteins commonly identified in at least two measurements. Overall, the number of proteins identified in at least two replicates reached >70%, also supporting the robustness of the analysis for the most abundant proteins. Figure S3(b) (available as Supplementary data at JAC Online) shows the proportion of proteins in each cluster of orthologous groups (COG) considering the annotation of N315 genome and in our study. Both profiles appear similar except for one category, ‘J’ (consisting of translation, ribosomal structure and biogenesis), which covers the most abundant bacterial proteins.
Following our conservative quantitative proteomic analysis, only 27 proteins were found to be differentially produced between the DAPR and DAPS strains (Table 5). Thus, comparison of this relatively short list with the more extensive differential microarray results failed to detect significant overlap, as is often observed for such analysis.59,60 Moreover, a number of proteins homologous to those involved in RNA turnover (degradosome) in Bacillus subtilis were overexpressed in strain 701, offering a potential explanation for the above lack of overlap between ‘omics’ techniques.61 Thus, Eno, RNaseJ1, RNAseJ2, PfkA and Pnp exhibited 1.76-, 1.31-, 1.21-, 1.47- and 1.12-fold increases in the DAPR strain (Table 5 and Table S1), likely increasing RNA degradation.
Table 5.
Proteins differentially expressed between 701 and 616
| Fold change DAPR versus DAPS | Description | Common name | Gene name |
|---|---|---|---|
| METABOLISM | |||
| energy production and conversion C | |||
| 1.62 | acetate kinase | AckA | SA1533 |
| 0.59 | succinate dehydrogenase flavoprotein subunit | SdhA | SA0995 |
| 0.61 | succinate dehydrogenase iron–sulphur protein subunit | SdhB | SA0996 |
| carbohydrate transport and metabolism G | |||
| 1.76 | enolase (2-phosphoglycerate dehydratase) | Eno | SA0731 |
| 2.17 | glyceraldehyde-3-phosphate dehydrogenase 1 | GapA | SA0727 |
| 0.36 | maltose transmembrane transporter activity | SA0207 | |
| 0.44 | multiple sugar-binding transport ATP-binding protein | MsmX | SA0206 |
| amino acid transport and metabolism E | |||
| 1.54 | bifunctional purine biosynthesis protein | PurH | SA0925 |
| lipid transport and metabolism I | |||
| 2.10 | 3-oxoacyl-(acyl-carrier-protein) synthase 2 | FabF | SA0843 |
| secondary metabolites biosynthesis, transport and catabolism Q | |||
| 0.40 | dehydrosqualene desaturase | CrtN | SA2348 |
| inorganic ion transport and metabolism P | |||
| 0.56 | lipoprotein similar to streptococcal adhesin PsaA | SA0587 | |
| INFORMATION STORAGE AND PROCESSING | |||
| translation, ribosomal structure and biogenesis J | |||
| 1.80 | 30S ribosomal protein S10 | RpsJ | SA2048 |
| 1.81 | 30S ribosomal protein S13 | RpsM | SA2025 |
| 1.72 | 30S ribosomal protein S2 | RpsB | SA1099 |
| 2.52 | 30S ribosomal protein S3 | RpsC | SA2041 |
| 1.68 | 30S ribosomal protein S4 | RpsD | SAS052 |
| 1.71 | 30S ribosomal protein S5 | RpsE | SA2031 |
| 2.08 | 30S ribosomal protein S6 | RpsF | SA0352 |
| 1.88 | 30S ribosomal protein S7 | RpsG | SA0504 |
| 1.51 | 50S ribosomal protein L7/L12 | RplL | SA0498 |
| 1.63 | elongation factor Tu (EF-Tu) | TufA | SA0506 |
| 1.56 | lysyl-tRNA synthetase | LysRS | SA0475 |
| 1.71 | prolyl-tRNA synthetase | ProRS | SA1106 |
| CELLULAR PROCESS AND SIGNALLING | |||
| signal transduction mechanisms T | |||
| 0.44 | stress response protein | SA1528 | |
| UNKNOWN | |||
| 2.18 | alkaline shock protein 23 | Asp23 | SA1984 |
| 1.64 | GTP-sensing transcriptional pleiotropic repressor | CodY | SA1098 |
| 0.54 | hypothetical protein | SA0269 |
After strategic categorization, the ascribed functions of genes or proteins found to be differentially abundant were quite similar. The main protein categories that overlapped with microarray analyses represented target genes involved in membrane metabolism (across all metabolic categories) as well as putative virulence genes (PurH, PsaA, enolase or GapA), stress response genes or genes involved in biofilm formation (MsmX, FabF, enolase, PurH, SdhA-B or Asp23).41,62,63 Extensive query of the SAMMD database (http://www.bioinformatics.org/sammd/) found that the majority of the protein set (92%) showing differential abundance between our two principal strains has been previously documented to be involved in stress response at the transcriptional level.64–66 We also found that 13 of the differentially produced proteins (48%) are involved in biofilm regulation,67–69 such as SdhA-B. Additionally, an important number of proteins appearing in this list (n = 12; 44%) are involved in translation processes (COG J).
Discussion
S. aureus (especially MRSA) has been considered for decades as a prototypic hospital-acquired pathogen. However, secular trends have demonstrated that MRSA strains are now responsible for many severe community-acquired infections.70,71 S. aureus has a propensity to rapidly develop resistance to many antimicrobial classes22,72–76 and this predominantly occurs by: (i) acquisition of resistance determinants;77 (ii) phenotypic variation;78 or (iii) profound alterations of its genetic repertoire.79 This latter category is likely the most difficult to study, as it requires the deployment of extensive and parallel analytical methods (e.g. whole transcriptomic and quantitative proteomic profiling) to uncover discrete genome-scale modifications potentially involved in a given resistance mechanism.13,14,16,80 In addition, only limited correlations have been reported between a given gene transcript level and the abundance of its respective encoded protein.59,60 With these issues in mind, our group has recently used several robust analytical approaches to document differential protein abundance between isogenic MRSA strains and their spontaneous glycopeptide-intermediate S. aureus (GISA) derivatives.38 Moreover, we have used such strategies to identify targets involved in cell wall biogenesis or in cell division that were not decipherable by transcriptomic analysis.59,81 The current study extends these approaches to analyse potential genotypic and phenotypic mechanisms underlying the DAPR phenotype.
The spontaneous emergence of S. aureus with decreased susceptibility to daptomycin can be rapidly obtained in vitro,82 suggesting an early adaptive mechanism(s) induced by exposure to daptomycin. Cui et al.83 reported a correlation between co-evolution of the DAPR and GISA phenotypes in S. aureus by in vitro passage in daptomycin-containing environments, suggesting that resistance to these two structurally distinct molecules was triggered by a common pathway(s). More recently, the same group identified putative pathways associated with DAPR using a transcriptomic approach, highlighting the potential contribution of the vraSR two-component regulatory system in this phenotype.17
Despite these interesting findings, fundamental insights remain to be identified regarding potential genetic mechanisms of DAPR in strains that were directly isolated from daptomycin-treated patients. Thus, in the current investigation, we used one principal pair of isolates obtained from a patient with endocarditis, in which the initially DAPS strain (616) emerged with a DAPR phenotype (701) during daptomycin treatment.22 Two additional pairs of isolates showing similar evolution (also obtained from patients with endocarditis), which evolved from DAPS parental strains during daptomycin therapy, were used in qRT–PCR experiments to evaluate whether or not putative regulatory events leading to DAPR in S. aureus were strain-dependent or more universal.
A number of pivotal and interesting findings emerged from our complementary analytical strategies, which revealed specific changes in gene expression or protein abundance between the DAPR and DAPS isolates. Firstly, genomic analysis revealed the presence of the plasmid pUSA300 in the principal DAPR strain, but not in the DAPS parental strain. Of note, USA300 clinical isolates, which carry this plasmid, are DAPS.36 Thus, the presence of the pUSA300 plasmid does not appear to directly confer the DAPR phenotype.
Secondly, extensive differences in the expression of several central metabolic function genes were observed in the DAPS versus DAPR strains, along with the differential expression of numerous genes involved in important regulatory processes. For example, the agr locus appears to be involved in the DAPR mechanism. Evidence for this interpretation comes from RNAIII and hld transcripts showing moderate decreases, as corroborated by reduced δ-haemolysin production in two of the three DAPR isolates queried (Figure S1). Simultaneously, we observed increased saeRS expression in the DAPR strain, a finding also compatible with reduced agr function.84 Moreover, expression of vraSR, a key two-component regulatory system involved in the control of cell wall synthesis in S. aureus, was enhanced in the DAPR strain. This latter profile has been previously identified in GISA isolates14 and in strains exposed to daptomycin in vitro.85 We also noticed a slight increase of the asp23 transcript levels and its real increase at the protein level in the DAPR; this locus is a reliable surrogate marker for expression of the key global regulator, SigB.86 Collectively, these observations are consistent with a recent report showing that daptomycin impacts genes involved in both the cell wall stress stimulon and in membrane depolarization.85
Thirdly, the notion that daptomycin can target the staphylococcal cell wall as well as its CM was underscored in the evolution towards DAPR. Thus, the abundance of differentially expressed genes (as well as their translation products) involved in the metabolism of the bacterial cell wall was notably distinct between the principal strain pair. A number of such genes (e.g. pbp2 and pbp4, lytN and lytH, tagA, and sgtA) have been previously reported to be differentially regulated in the following conditions: stress response to cell wall-active antibiotics;87 mild acidic shock;88 or during the stringent response.64 Likewise, our recent findings of thickened cell walls in this same DAPR strain (701) by TEM,25 as well as in an in vitro-selected DAPR strain are also consistent with this paradigm.8,13 Current studies in our laboratories are focused upon a detailed comparative evaluation of the cell wall compositions of our DAPS–DAPR strain pair.25
Fourthly, we employed qRT–PCR to confirm the differential expression profiles of a number of critical S. aureus global regulators and structural virulence genes that were disclosed in the transcriptomic analyses. The specific genes assessed by qRT–PCR were selected based on their putative roles in endovascular infections (related to their individual or combined impacts upon surface adhesins, exotoxins and/or exoproteins likely involved in one or more pathogenetic phases of such infections).89 As in the microarray analyses above, qRT–PCR analysis showed that the expression of most of these genes was up-regulated in the DAPR strain (701) as compared with the DAPS parental strain (616), with the notable exceptions of down-regulation of sarR and agr expression (as evidenced by reductions in both agrA and hld expression levels). The finding of reduced agr expression in the DAPR strain is of interest in the context of VISA strains. Several studies have confirmed the relationship of agr deletions or point mutations, reduced agr function phenotypically and glycopeptide resistance.15 Despite down-regulation of agr in strain 701, hla expression was significantly up-regulated in the face of rot up-regulation. One logical explanation for this seemingly paradoxical interactive network of gene expressions is the acquisition of a loss-in-function mutation in rot;90,91 sequence analysis of the rot locus is in progress.
In formulating potential DAPR interactive pathways, it seems clear that there are strain-to-strain variations. For the 616–701 strain pair, in addition to increased rot and reduced agr in 701, sarT (encoding for a negative regulators of hla) is also up-regulated. Thus, increased hla production may be explained by the increase in saeRS, increased mgrA and increased sarZ expression (our microarray results showed a trend towards increased expression in 701). Indeed they will all increase hla even in the absence of RNAIII acting upon Rot. Hence, we propose that 701 shows increased hla even in the presence of Rot and SarT repressor functionalities, i.e. it is either autonomous or SaeRS, MgrA and SarZ are stimulating increased hla transcripts.51 In the 11-11/REF2145 strain pair, reduced hla transcript levels in REF2145 are associated with reduced agr expression and increased rot transcription. However, in BOY300, hla transcripts are decreased despite the increase in agr, hld and saeRS, without any increase in rot transcripts, suggesting that the production of hla has become autonomous. Finally, in terms of mprF (fmtC) expression involved in saeRS regulation,92 this tends to vary from strain to strain, ranging from unchanged (BOY300), to increased (701), to reduced (REF2145). It has been well chronicled that in daptomycin-resistant staphylococci, mutations in the mprF gene usually produce gains in function.22,24 Thus, it is quite possible that strains BOY300 and REF2145 have reduced transcript levels, but increased activity of MprF due to gain-in-function mutations (regulator interactions are summarized in Figure S2).
Our studies above support a hypothesis that the DAPR phenotype results from coordination among multiple adaptive response circuits. For example, our observed reduction in sarR expression in the DAPR isolate can be linked to an increase in sarA expression. In turn, an increase in sarA expression could modify biofilm formation as well as increase hla expression via an agr-independent pathway.93 Supporting this concept, the current studies demonstrated a clear difference in biofilm dynamics and phenotype in the DAPS versus DAPR strains.
Finally, a pivotal observation in our investigation was that the key regulatory locus, yycFGHI, was differentially expressed when our DAPS and DAPR strain sets were compared. This operon is a key regulator affecting CM lipid homeostasis, cell wall metabolism and biofilm formation.19,53,94,95 The expression of this regulator contributes to modification of the net surface charge via both mprF and dlt pathways; thus, likely impacting daptomycin binding as well as the initial attachment phases in biofilm formation.19 These latter interpretations are substantiated by our findings that our principal study strain pair (616–701) differed in their capacity to form biofilm in the presence of the antimicrobial lipid, oleic acid. Oleic acid has been reported previously as a microbicidal agent against S. aureus, presumably through a mechanism involving membrane fluidity and the lipid composition of the cytoplasmic membrane.9 We used oleic acid instead of daptomycin, because: (i) they share similarities in their lipid tail (7–8 CH2 following a CH3); and (ii) we were able to use similar concentrations of this compound for both strains. Moreover, another chemical compound called friulimicin B that shares similar structure and chemical properties with daptomycin also possesses a lipid tail of 8 CH2 (+2 CH3 terminus) before a C=C double bond, like the oleic acid lipid tail.96 Importantly, like daptomycin, friulimicin B absolutely requires Ca2+ for antimicrobial activity. In the present study, the DAPR isolate retained the ability to produce an abundant and structurally distinct biofilm, with greater adhesive properties, even in the setting of an antibacterial lipid environment (i.e. oleic acid exposure). It is tempting to speculate that an adaptation to the lipid moiety of daptomycin in DAPR strains may be involved in the relative resistance of this strain to oleic acid, especially within biofilms. If so, this modification of biofilm formation could be linked to an increase in sarA expression in the DAPR strain. For example, Weiss et al.97 showed an increased susceptibility to daptomycin linked to a decreased biofilm adherence (in vivo) in an S. aureus sarA mutant.
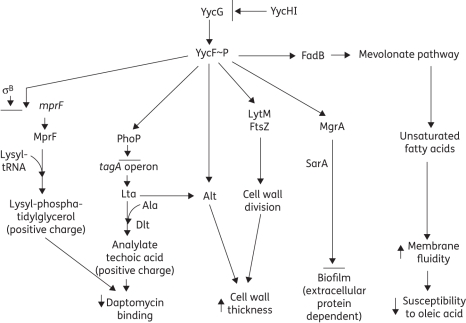
Examination of our composite data sets from our principal strain pair suggested that changes in YycF and YycI could account for many of the changes found in the DAPR strain. Increased positive surface charge (e.g. via gain-in-function mutations in mprF) and increased membrane fluidity have been associated with DAPR.18 In addition, some DAPR strains show thickened cell walls and altered biofilm formation. Activated YycF positively regulates genes involved in cell division, and ultimately cell wall thickness and division planes. Thus, a reduction in YycF activity due to either increased YycI activity (an inhibitor of YycF) or to a mutation in yycF could account for alterations in the cell wall structure. When yycI transcripts are increased, then YycFG will have reduced activity (i.e. strain BOY300). When yycF is reduced, then there is no need to have higher levels of yycI, as either high yycI or low yycF will result in the same phenotype. This implies that more than one mutation can provide the same phenotype, i.e. reduced YycF∼P. Moreover, YycF regulates mprF, as well as MgrA, which is the sole regulator of sarZ and a repressor of sarS and sarT,98 which are differentially regulated in our three backgrounds (Table 4 and Figure S2). MgrA represses icaABDC, which is essential for biofilm formation. Thus, reduced activation of mgrA due to decreased YycF activity could result in increased biofilm formation. Lastly, activated YycF is a positive regulator of PhoP, which negatively regulates the tagA operon. As TagA is the first committed step in the lipoteichoic acid (LTA) pathway, a reduction in YycF activity would reduce the repressive effect of PhoP, thereby leading to more LTA. LTA inhibits the autolysin (Alt) and increases the positive charge on the cell wall, thereby influencing both cell wall thickness and daptomycin susceptibility, respectively.8 These interactive paradigms are summarized in a putative network model that allows integration of biofilm formation, changes in positive surface charge, cell wall structure, daptomycin binding and cell wall susceptibility to lipids (Figure 3). We view this model as a working hypothesis upon which future experiments can be performed in the examination of DAPR. Another explanation for the daptomycin-resistant phenotype would be that there are multiple mutations in distinct regulatory pathways in such strains. This hypothesis is compatible with the stepwise development of resistance that is found both in patients receiving long-term daptomycin, as well as in strains undergoing serial in vitro passage in daptomycin in the laboratory (Figure S2). Clearly, not all our data sets fit into a single cohesive model; this observation is entirely consistent with the stepwise development of DAPR, as well as a summation of multiple mutations producing this resistance phenotype.
Figure 3.
Potential pathways leading to DAPR. Cell wall metabolism and regulators appear as major targets of mechanisms leading to DAPR acquired in vivo during therapy. The biosynthesis of bacterial envelopes is involved in the process leading to major phenotypic changes between DAPS and DAPR S. aureus. Black bars represent inhibition.
It should be emphasized that in comparing qRT–PCR data sets between our three DAPS–DAPR strain pairs, there were several consistent observations in the DAPR strains; up-regulation of rot and sarS, and down-regulation of yycF. However, expression patterns of the other 10 genes queried in our focused analysis yielded no universally consistent profile. This finding would underscore the concepts that: (i) DAPR networks are complex and strain specific; and (ii) the accumulation of diverse mutations during daptomycin exposures, as confirmed for many reported DAPR strains,22 may well be critical in ultimate DAPR ‘pathways’.
Conclusions
In vivo emergence of DAPR in S. aureus during daptomycin treatment is the result of multiple adaptations in metabolic functions, global regulatory pathways, as well as the biogenesis of the cellular envelope. The evolution of DAPR in the clinical strain set 616–701 corresponded with an increase in the DAPR strain's capability to generate a pronounced and structurally distinct biofilm, and to resist toxic membrane-targeting antimicrobial lipids. Combined transcriptomic and proteomic analyses provided a global view of the complex process corresponding to the adaptive DAPR phenotype. Several genes and proteins, potentially involved in the emergence of DAPR in vivo, were identified in this study, and appear quite distinct from in vitro-selected DAPR organisms. Finally, comparison between three genotypically distinct strain pairs revealed a strain-dependent, multifactorial regulation in the DAPR phenotype. It appears likely that the global regulatory locus, yycFGHI, plays a key role in the development of DAPR.
Funding
This research was supported by grants from the NIH to A. S. B. (AI-039108) and M. R. Y. (AI-039001), grants 3100A0-112370/1 (to J. S.) and 3100A0-116075 (to P. F.) from the Swiss National Science Foundation.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We thank Dr Ambrose Cheung (Dartmouth Medical College, Hanover, NH, USA) for assistance in the genotypic interpretations and Dr Andreas Peschel (University of Tubingen, Germany) for many helpful discussions.
References
- 1.Mortara LA, Bayer AS. Staphylococcus aureus bacteremia and endocarditis. New diagnostic and therapeutic concepts. Infect Dis Clin North Am. 1993;7:53–68. [PubMed] [Google Scholar]
- 2.Hobbs JK, Miller K, O'Neill AJ, et al. Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J Antimicrob Chemother. 2008;62:1003–8. doi: 10.1093/jac/dkn321. doi:10.1093/jac/dkn321. [DOI] [PubMed] [Google Scholar]
- 3.Sakoulas G, Eliopoulos GM, Alder J, et al. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1714–8. doi: 10.1128/AAC.47.5.1714-1718.2003. doi:10.1128/AAC.47.5.1714-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–79. doi: 10.1016/S0140-6736(04)16727-5. doi:10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 5.Eliopoulos GM, Thauvin C, Gerson B, et al. In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1985;27:357–62. doi: 10.1128/aac.27.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu JQ, Nguyen KT, Gandhi C, et al. Structural characterization of daptomycin analogues A21978C1-3(d-Asn11) produced by a recombinant Streptomyces roseosporus strain. J Nat Prod. 2007;70:233–40. doi: 10.1021/np0605135. doi:10.1021/np0605135. [DOI] [PubMed] [Google Scholar]
- 7.Lakey JH, Ptak M. Fluorescence indicates a calcium-dependent interaction between the lipopeptide antibiotic LY146032 and phospholipid membranes. Biochemistry. 1988;27:4639–45. doi: 10.1021/bi00413a009. doi:10.1021/bi00413a009. [DOI] [PubMed] [Google Scholar]
- 8.Mishra NN, Yang SJ, Sawa A, et al. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2312–8. doi: 10.1128/AAC.01682-08. doi:10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain NR, Mehrtens BG, Xiong Z, et al. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect Immun. 1991;59:4332–7. doi: 10.1128/iai.59.12.4332-4337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell IM, Crozier DN, Pawagi AB. Effect of hypobaric oxygen and oleic acid on respiration of Staphylococcus aureus. Eur J Clin Microbiol. 1986;5:622–8. doi: 10.1007/BF02013285. doi:10.1007/BF02013285. [DOI] [PubMed] [Google Scholar]
- 11.Stenz L, Francois P, Fischer A, et al. Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. FEMS Microbiol Lett. 2008;287:149–55. doi: 10.1111/j.1574-6968.2008.01316.x. doi:10.1111/j.1574-6968.2008.01316.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. doi:10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 13.Cui L, Ma X, Sato K, et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol. 2003;41:5–14. doi: 10.1128/JCM.41.1.5-14.2003. doi:10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda M, Kuroda H, Oshima T, et al. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49:807–21. doi: 10.1046/j.1365-2958.2003.03599.x. doi:10.1046/j.1365-2958.2003.03599.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–502. doi: 10.1128/AAC.46.5.1492-1502.2002. doi:10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehl JL, Muthaiyan A, Jayaswal RK, et al. Cell wall composition and decreased autolytic activity and lysostaphin susceptibility of glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:3749–57. doi: 10.1128/AAC.48.10.3749-3757.2004. doi:10.1128/AAC.48.10.3749-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo IL, Neoh HM, Cui L, et al. Serial daptomycin selection generates daptomycin-nonsusceptible Staphylococcus aureus strains with a heterogeneous vancomycin-intermediate phenotype. Antimicrob Agents Chemother. 2008;52:4289–99. doi: 10.1128/AAC.00417-08. doi:10.1128/AAC.00417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin PK, Li T, Sun D, et al. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J Bacteriol. 1999;181:3666–73. doi: 10.1128/jb.181.12.3666-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubrac S, Boneca IG, Poupel O, et al. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol. 2007;189:8257–69. doi: 10.1128/JB.00645-07. doi:10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwangi MM, Wu SW, Zhou Y, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci USA. 2007;104:9451–6. doi: 10.1073/pnas.0609839104. doi:10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta T, Hirakawa H, Morikawa K, et al. Nucleotide substitutions in Staphylococcus aureus strains, Mu50, Mu3, and N315. DNA Res. 2004;11:51–6. doi: 10.1093/dnares/11.1.51. doi:10.1093/dnares/11.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Jones T, Yeaman MR, Sakoulas G, et al. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother. 2008;52:269–78. doi: 10.1128/AAC.00719-07. doi:10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst CM, Staubitz P, Mishra NN, et al. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. doi:10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SJ, Xiong YQ, Dunman PM, et al. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus strains. Antimicrob Agents Chemother. 2009;53:2636–7. doi: 10.1128/AAC.01415-08. doi:10.1128/AAC.01415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SJ, Nast CC, Mishra NN, et al. Cell wall thickening is not a universal accompaniment of the daptomycin nonsusceptibility phenotype in Staphylococcus aureus: evidence for multiple resistance mechanisms. Antimicrob Agents Chemother. 2010;54:3079–85. doi: 10.1128/AAC.00122-10. doi:10.1128/AAC.00122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SJ, Kreiswirth BN, Sakoulas G, et al. Enhanced expression of dltABCD is associated with the development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J Infect Dis. 2009;200:1916–20. doi: 10.1086/648473. doi:10.1086/648473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy MH, Olson ME, Wickert RW, et al. Daptomycin non-susceptible meticillin-resistant Staphylococcus aureus USA 300 isolate. J Med Microbiol. 2008;57:1036–8. doi: 10.1099/jmm.0.2008/000588-0. doi:10.1099/jmm.0.2008/000588-0. [DOI] [PubMed] [Google Scholar]
- 28.Traber K, Novick R. A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate δ- and α-haemolysins. Mol Microbiol. 2006;59:1519–30. doi: 10.1111/j.1365-2958.2006.04986.x. doi:10.1111/j.1365-2958.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 29.McCalla C, Smyth DS, Robinson DA, et al. Microbiological and genotypic analysis of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:3441–3. doi: 10.1128/AAC.00357-08. doi:10.1128/AAC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francois P, Huyghe A, Charbonnier Y, et al. Use of an automated multiple-locus, variable-number tandem repeat-based method for rapid and high-throughput genotyping of Staphylococcus aureus isolates. J Clin Microbiol. 2005;43:3346–55. doi: 10.1128/JCM.43.7.3346-3355.2005. doi:10.1128/JCM.43.7.3346-3355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francois P, Harbarth S, Huyghe A, et al. Methicillin-resistant Staphylococcus aureus, Geneva, Switzerland, 1993–2005. Emerg Infect Dis. 2008;14:304–7. doi: 10.3201/eid1402.070229. doi:10.3201/eid1402.070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charbonnier Y, Gettler BM, Francois P, et al. A generic approach for the design of whole-genome oligoarrays, validated for genomotyping, deletion mapping and gene expression analysis on Staphylococcus aureus. BMC Genomics. 2005;6:95. doi: 10.1186/1471-2164-6-95. doi:10.1186/1471-2164-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroda M, Ohta T, Uchiyama I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–40. doi: 10.1016/s0140-6736(00)04403-2. doi:10.1016/S0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 34.Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–27. doi: 10.1016/s0140-6736(02)08713-5. doi:10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 35.Gill SR, Fouts DE, Archer GL, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–38. doi: 10.1128/JB.187.7.2426-2438.2005. doi:10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. doi:10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 37.Holden MT, Feil EJ, Lindsay JA, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 2004;101:9786–91. doi: 10.1073/pnas.0402521101. doi:10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherl A, Francois P, Charbonnier Y, et al. Exploring glycopeptide resistance in Staphylococcus aureus: a combined proteomics and transcriptomics approach for the identification of resistance related markers. BMC Genomics. 2006;7:296. doi: 10.1186/1471-2164-7-296. doi:10.1186/1471-2164-7-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talaat AM, Howard ST, Hale W, et al. Genomic DNA standards for gene expression profiling in Mycobacterium tuberculosis. Nucleic Acids Res. 2002;30:e104. doi: 10.1093/nar/gnf103. doi:10.1093/nar/gnf103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garzoni C, Francois P, Huyghe A, et al. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics. 2007;8:171. doi: 10.1186/1471-2164-8-171. doi:10.1186/1471-2164-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renzoni A, Francois P, Li D, et al. Modulation of fibronectin adhesins and other virulence factors in a teicoplanin-resistant derivative of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:2958–65. doi: 10.1128/AAC.48.8.2958-2965.2004. doi:10.1128/AAC.48.8.2958-2965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaezzadeh AR, Simicevic J, Chauvet A, et al. Imaging mass spectrometry using peptide isoelectric focusing. Rapid Commun Mass Spectrom. 2008;22:2667–76. doi: 10.1002/rcm.3658. doi:10.1002/rcm.3658. [DOI] [PubMed] [Google Scholar]
- 43.Elias JE, Gygi SP. Target–decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–14. doi: 10.1038/nmeth1019. doi:10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 44.Dayon L, Hainard A, Licker V, et al. Relative quantification of proteins in human cerebrospinal fluids by MS/MS using 6-plex isobaric tags. Anal Chem. 2008;80:2921–31. doi: 10.1021/ac702422x. doi:10.1021/ac702422x. [DOI] [PubMed] [Google Scholar]
- 45.Shadforth IP, Dunkley TP, Lilley KS, et al. i-Tracker: for quantitative proteomics using iTRAQ. BMC Genomics. 2005;6:145. doi: 10.1186/1471-2164-6-145. doi:10.1186/1471-2164-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CC, Joyce EA, Chan K, et al. Improved analytical methods for microarray-based genome-composition analysis. Genome Biol. 2002;3:0065.1–0065.17. doi: 10.1186/gb-2002-3-11-research0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunman PM, Murphy E, Hanney S, et al. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–53. doi: 10.1128/JB.183.24.7341-7353.2001. doi:10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantrangi M, Singh VK, Wolz C, et al. Staphylococcal superantigen-like genes, ssl5 and ssl8, are positively regulated by Sae and negatively by Agr in the Newman strain. FEMS Microbiol Lett. 2010;308:175–84. doi: 10.1111/j.1574-6968.2010.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beenken KE, Mrak LN, Griffin LM, et al. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS One. 2010;5:e10790. doi: 10.1371/journal.pone.0010790. doi:10.1371/journal.pone.0010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majerczyk CD, Dunman PM, Luong TT, et al. Direct targets of CodY in Staphylococcus aureus. J Bacteriol. 2010;192:2861–77. doi: 10.1128/JB.00220-10. doi:10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamber S, Cheung AL. SarZ promotes the expression of virulence factors and represses biofilm formation by modulating SarA and agr in Staphylococcus aureus. Infect Immun. 2009;77:419–28. doi: 10.1128/IAI.00859-08. doi:10.1128/IAI.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn B, Hussain S, Malik M, et al. Daptomycin inoculum effects and mutant prevention concentration with Staphylococcus aureus. J Antimicrob Chemother. 2007;60:1380–3. doi: 10.1093/jac/dkm375. doi:10.1093/jac/dkm375. [DOI] [PubMed] [Google Scholar]
- 53.Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:2137–45. doi: 10.1128/AAC.00039-06. doi:10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung AL, Bayer AS, Zhang G, et al. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9. doi: 10.1016/S0928-8244(03)00309-2. doi:10.1016/S0928-8244(03)00309-2. [DOI] [PubMed] [Google Scholar]
- 55.Sakoulas G, Eliopoulos GM, Fowler VG, Jr, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005;49:2687–92. doi: 10.1128/AAC.49.7.2687-2692.2005. doi:10.1128/AAC.49.7.2687-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramakrishnan R, Dorris D, Lublinsky A, et al. An assessment of Motorola CodeLink microarray performance for gene expression profiling applications. Nucleic Acids Res. 2002;30:e30. doi: 10.1093/nar/30.7.e30. doi:10.1093/nar/30.7.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wootton M, MacGowan AP, Walsh TR. Expression of tcaA and mprF and glycopeptide resistance in clinical glycopeptide-intermediate Staphylococcus aureus (GISA) and heteroGISA strains. Biochim Biophys Acta. 2005;1726:326–7. doi: 10.1016/j.bbagen.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Wootton M, Bennett PM, MacGowan AP, et al. Strain-specific expression levels of pbp4 exist in isolates of glycopeptide-intermediate Staphylococcus aureus (GISA) and heterogeneous GISA. Antimicrob Agents Chemother. 2005;49:3598–9. doi: 10.1128/AAC.49.8.3598-3599.2005. doi:10.1128/AAC.49.8.3598-3599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scherl A, Francois P, Bento M, et al. Correlation of proteomic and transcriptomic profiles of Staphylococcus aureus during the post-exponential phase of growth. J Microbiol Methods. 2005;60:247–57. doi: 10.1016/j.mimet.2004.09.017. doi:10.1016/j.mimet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Corbin RW, Paliy O, Yang F, et al. Toward a protein profile of Escherichia coli: comparison to its transcription profile. Proc Natl Acad Sci USA. 2003;100:9232–7. doi: 10.1073/pnas.1533294100. doi:10.1073/pnas.1533294100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehnik-Habrink M, Pfortner H, Rempeters L, et al. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07264.x. doi:10.1111/j.1365-2958.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- 62.Conlon KM, Humphreys H, O'Gara JP. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J Bacteriol. 2004;186:6208–19. doi: 10.1128/JB.186.18.6208-6219.2004. doi:10.1128/JB.186.18.6208-6219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goerke C, Esser S, Kummel M, et al. Staphylococcus aureus strain designation by agr and cap polymorphism typing and delineation of agr diversification by sequence analysis. Int J Med Microbiol. 2005;295:67–75. doi: 10.1016/j.ijmm.2005.01.004. doi:10.1016/j.ijmm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Anderson KL, Roberts C, Disz T, et al. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol. 2006;188:6739–56. doi: 10.1128/JB.00609-06. doi:10.1128/JB.00609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlag S, Nerz C, Birkenstock TA, et al. Inhibition of staphylococcal biofilm formation by nitrite. J Bacteriol. 2007;189:7911–9. doi: 10.1128/JB.00598-07. doi:10.1128/JB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol. 2006;61:927–39. doi: 10.1111/j.1365-2958.2006.05290.x. doi:10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- 67.Brady RA, Leid JG, Camper AK, et al. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74:3415–26. doi: 10.1128/IAI.00392-06. doi:10.1128/IAI.00392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beenken KE, Dunman PM, McAleese F, et al. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–84. doi: 10.1128/JB.186.14.4665-4684.2004. doi:10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Resch A, Rosenstein R, Nerz C, et al. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol. 2005;71:2663–76. doi: 10.1128/AEM.71.5.2663-2676.2005. doi:10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. doi:10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charlebois ED, Perdreau-Remington F, Kreiswirth B, et al. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;39:47–54. doi: 10.1086/421090. doi:10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–93. doi: 10.1136/jcp.14.4.385. doi:10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang S, Sievert DM, Hageman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–7. doi: 10.1056/NEJMoa025025. doi:10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 74.Hiramatsu K, Okuma K, Ma XX, et al. New trends in Staphylococcus aureus infections: glycopeptide resistance in hospital and methicillin resistance in the community. Curr Opin Infect Dis. 2002;15:407–13. doi: 10.1097/00001432-200208000-00009. doi:10.1097/00001432-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Cambau E, Gutmann L. Mechanisms of resistance to quinolones. Drugs. 1993;45(Suppl 3):15–23. doi: 10.2165/00003495-199300453-00005. doi:10.2165/00003495-199300453-00005. [DOI] [PubMed] [Google Scholar]
- 76.Tsiodras S, Gold HS, Sakoulas G, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358:207–8. doi: 10.1016/S0140-6736(01)05410-1. doi:10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 77.Ryffel C, Kayser FH, Berger-Bachi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992;36:25–31. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Proctor RA, Kahl B, von Eiff C, et al. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin Infect Dis. 1998;27(Suppl 1):S68–74. doi: 10.1086/514906. doi:10.1086/514906. [DOI] [PubMed] [Google Scholar]
- 79.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–55. doi: 10.1016/S1473-3099(01)00091-3. doi:10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 80.Boyle-Vavra S, Carey RB, Daum RS. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J Antimicrob Chemother. 2001;48:617–25. doi: 10.1093/jac/48.5.617. doi:10.1093/jac/48.5.617. [DOI] [PubMed] [Google Scholar]
- 81.Cui L, Lian JQ, Neoh HM, et al. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:3404–13. doi: 10.1128/AAC.49.8.3404-3413.2005. doi:10.1128/AAC.49.8.3404-3413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liebowitz LD, Saunders J, Chalkley LJ, et al. In vitro selection of bacteria resistant to LY146032, a new cyclic lipopeptide. Antimicrob Agents Chemother. 1988;32:24–6. doi: 10.1128/aac.32.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cui L, Tominaga E, Neoh HM, et al. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:1079–82. doi: 10.1128/AAC.50.3.1079-1082.2006. doi:10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rose WE, Rybak MJ, Tsuji BT, et al. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus in reference to accessory gene regulator (agr) polymorphism and function. J Antimicrob Chemother. 2007;59:1190–3. doi: 10.1093/jac/dkm091. doi:10.1093/jac/dkm091. [DOI] [PubMed] [Google Scholar]
- 85.Muthaiyan A, Silverman JA, Jayaswal RK, et al. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob Agents Chemother. 2008;52:980–90. doi: 10.1128/AAC.01121-07. doi:10.1128/AAC.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mongodin E, Finan J, Climo MW, et al. Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J Bacteriol. 2003;185:4638–43. doi: 10.1128/JB.185.15.4638-4643.2003. doi:10.1128/JB.185.15.4638-4643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Utaida S, Dunman PM, Macapagal D, et al. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology. 2003;149:2719–32. doi: 10.1099/mic.0.26426-0. doi:10.1099/mic.0.26426-0. [DOI] [PubMed] [Google Scholar]
- 88.Weinrick B, Dunman PM, McAleese F, et al. Effect of mild acid on gene expression in Staphylococcus aureus. J Bacteriol. 2004;186:8407–23. doi: 10.1128/JB.186.24.8407-8423.2004. doi:10.1128/JB.186.24.8407-8423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. doi:10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 90.Geisinger E, Adhikari RP, Jin R, et al. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol. 2006;61:1038–48. doi: 10.1111/j.1365-2958.2006.05292.x. doi:10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 91.Goerke C, Fluckiger U, Steinhuber A, et al. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of α-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol Microbiol. 2001;40:1439–47. doi: 10.1046/j.1365-2958.2001.02494.x. doi:10.1046/j.1365-2958.2001.02494.x. [DOI] [PubMed] [Google Scholar]
- 92.Sievers S, Ernst CM, Geiger T, et al. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics. 2010;10:1685–93. doi: 10.1002/pmic.200900772. doi:10.1002/pmic.200900772. [DOI] [PubMed] [Google Scholar]
- 93.Chien Y, Manna AC, Projan SJ, et al. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem. 1999;274:37169–76. doi: 10.1074/jbc.274.52.37169. doi:10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 94.Dubrac S, Msadek T. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J Bacteriol. 2004;186:1175–81. doi: 10.1128/JB.186.4.1175-1181.2004. doi:10.1128/JB.186.4.1175-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szurmant H, White RA, Hoch JA. Sensor complexes regulating two-component signal transduction. Curr Opin Struct Biol. 2007;17:706–15. doi: 10.1016/j.sbi.2007.08.019. doi:10.1016/j.sbi.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneider T, Gries K, Josten M, et al. The lipopeptide antibiotic friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob Agents Chemother. 2009;53:1610–8. doi: 10.1128/AAC.01040-08. doi:10.1128/AAC.01040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weiss EC, Zielinska A, Beenken KE, et al. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother. 2009;53:4096–102. doi: 10.1128/AAC.00484-09. doi:10.1128/AAC.00484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ballal A, Ray B, Manna AC. sarZ, a sarA family gene, is transcriptionally activated by MgrA and is involved in the regulation of genes encoding exoproteins in Staphylococcus aureus. J Bacteriol. 2009;191:1656–65. doi: 10.1128/JB.01555-08. doi:10.1128/JB.01555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.