Abstract
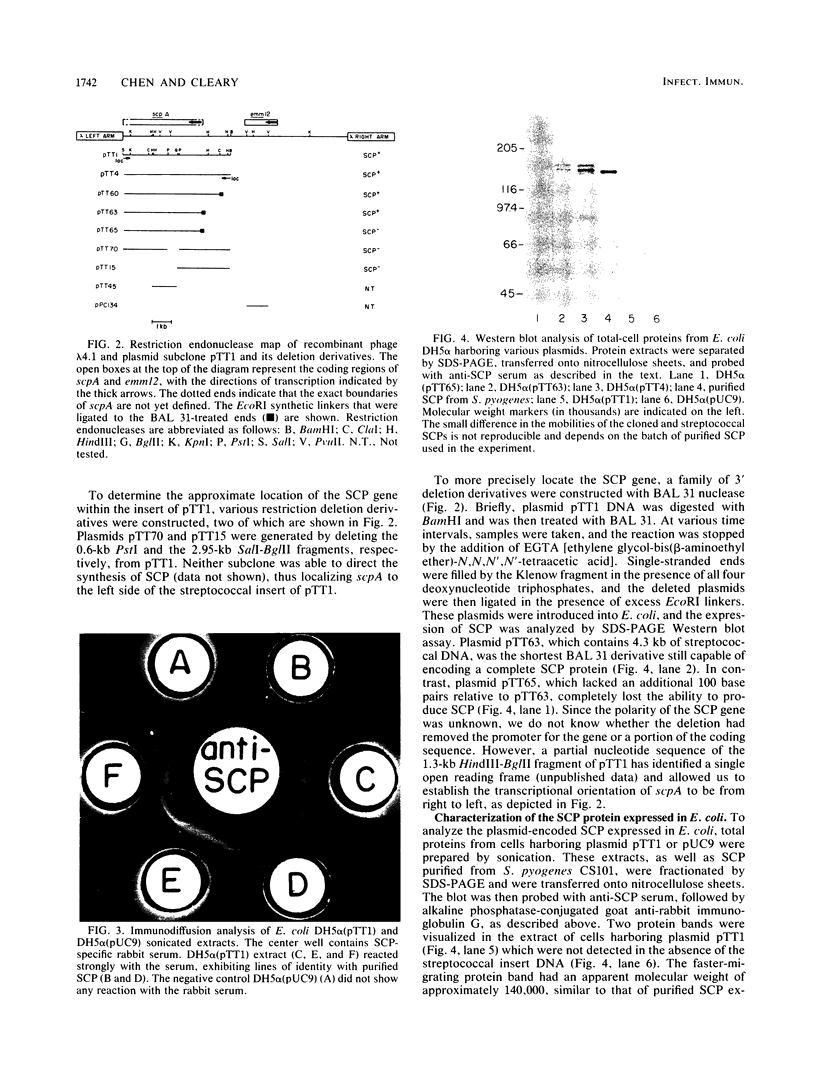
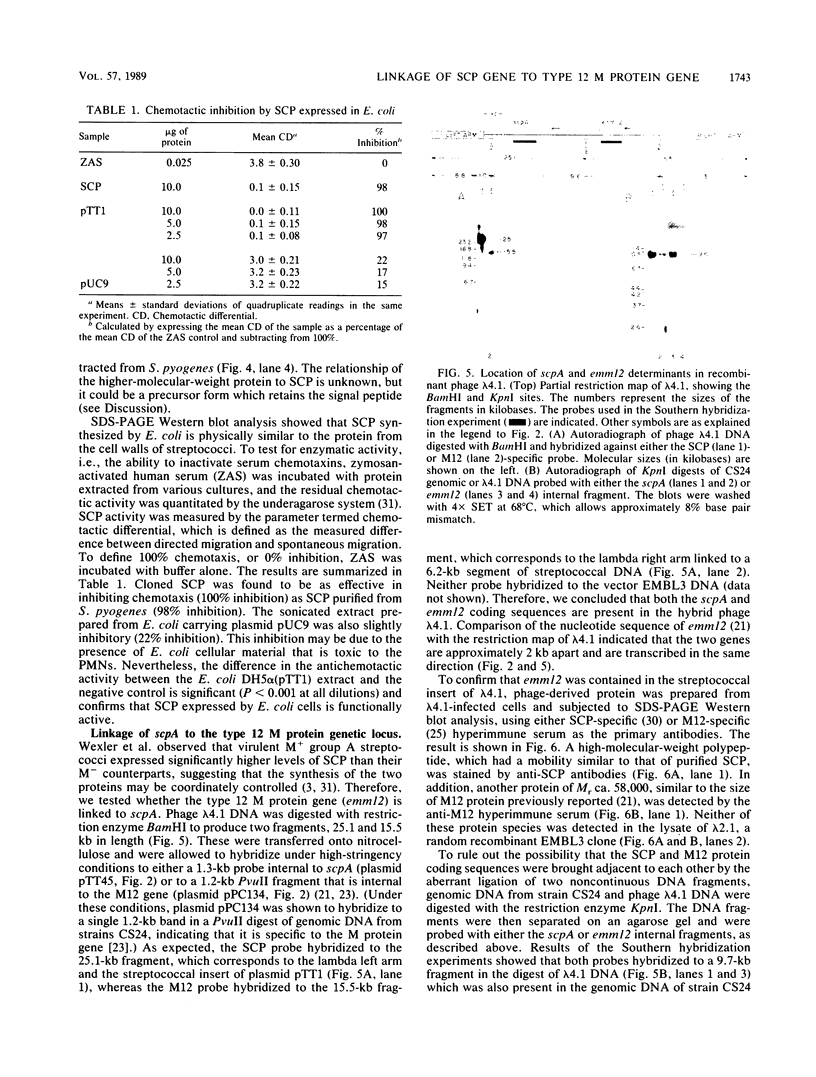
A genomic library of Streptococcus pyogenes CS24 DNA was constructed by cloning streptococcal DNA partially digested with Sau3A into the lambda replacement vector EMBL3. The expression of streptococcal C5a peptidase (SCP) was analyzed by radioimmunoassay with hyperimmune rabbit serum. Two clones, lambda 4.1 and lambda 4.2, were found to express the desired antigen, and various DNA fragments from the hybrid bacteriophage lambda 4.1 were subcloned into the plasmid vector pUC9 in Escherichia coli. One of the recombinant plasmids, designated pTT1, contained a 5.8-kilobase (kb) streptococcal DNA insert. Analysis of total cellular protein from this E. coli clone by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western (immuno-) blotting identified a 140,000-Mr protein, similar in size to the native protein purified from S. pyogenes. Cloned SCP was functionally active, as shown by its ability to inhibit C5a-mediated chemotaxis. By deletion analysis with both restriction endonucleases and BAL 31 nuclease, the SCP gene was localized to a 4.3-kb segment of DNA. Southern hybridization experiments showed that the type 12 M protein-coding sequence is also present in the hybrid phage lambda 4.1, at approximately 2 kb upstream of the SCP structural gene. Western blot analysis indicated that the cloned streptococcal DNA in lambda 4.1 directed the expression of both SCP and M12 protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Johnson Z., Wannamaker L. Genetic instability of M protein and serum opacity factor of group A streptocci: evidence suggesting extrachromosomal control. Infect Immun. 1975 Jul;12(1):109–118. doi: 10.1128/iai.12.1.109-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Bohnsack J. F., Morris E. Z., Augustine N. H., Parker C. J., Cleary P. P., Wu J. T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988 Nov 15;141(10):3551–3556. [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Connor S. P., Cleary P. P. In vivo Streptococcus pyogenes C5a peptidase activity: analysis using transposon- and nitrosoguanidine-induced mutants. J Infect Dis. 1987 Sep;156(3):495–504. doi: 10.1093/infdis/156.3.495. [DOI] [PubMed] [Google Scholar]
- O'Connor S. P., Cleary P. P. Localization of the streptococcal C5a peptidase to the surface of group A streptococci. Infect Immun. 1986 Aug;53(2):432–434. doi: 10.1128/iai.53.2.432-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Dale J. B., Timmis K. N., Beachey E. H. Expression of protective and cardiac tissue cross-reactive epitopes of type 5 streptococcal M protein in Escherichia coli. Infect Immun. 1985 Apr;48(1):198–203. doi: 10.1128/iai.48.1.198-203.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Spanier J. G., Jones S. J., Simpson W. J., Cleary P. P. Streptococcus pyogenes type 12 M protein gene regulation by upstream sequences. J Bacteriol. 1987 Dec;169(12):5633–5640. doi: 10.1128/jb.169.12.5633-5640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Cleary P. P. Expression of M type 12 protein by a group A streptococcus exhibits phaselike variation: evidence for coregulation of colony opacity determinants and M protein. Infect Immun. 1987 Oct;55(10):2448–2455. doi: 10.1128/iai.55.10.2448-2455.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Robbins J. C., Cleary P. P. Evidence for group A-related M protein genes in human but not animal-associated group G streptococcal pathogens. Microb Pathog. 1987 Nov;3(5):339–350. doi: 10.1016/0882-4010(87)90004-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spanier J. G., Cleary P. P. Bacteriophage control of antiphagocytic determinants in group A streptococci. J Exp Med. 1980 Nov 1;152(5):1393–1406. doi: 10.1084/jem.152.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier J. G., Jones S. J., Cleary P. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science. 1984 Aug 31;225(4665):935–938. doi: 10.1126/science.6089334. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Law S. K., Levine R. P., Cleary P. P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985 Jan;134(1):500–505. [PubMed] [Google Scholar]
- Wexler D. E., Chenoweth D. E., Cleary P. P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. E., Cleary P. P. Purification and characteristics of the streptococcal chemotactic factor inactivator. Infect Immun. 1985 Dec;50(3):757–764. doi: 10.1128/iai.50.3.757-764.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. E., Nelson R. D., Cleary P. P. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect Immun. 1983 Jan;39(1):239–246. doi: 10.1128/iai.39.1.239-246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]