Abstract
Helicobacter pylori is a Gram-negative, spiral-shaped microorganism associated with acute and chronic gastritis, peptic ulcer, gastric cancer and gastric lymphomas in humans. H. pylori neutrophil-activating protein (NAP) is a major virulence factor playing a central role in pathogenesis of mucosal inflammation by immune cell attraction and Th1 cytokine response polarization. NAP is protective antigen and promising vaccine candidate against H. pylori infection. Here we present the development of measles virus (MV) vaccine strain encoding the NAP antigen. In order to facilitate the extracellular transport and detection, NAP was inserted in the human lambda immunoglobulin chain replacing a major part of the variable domain. We generated two MV vectors expressing secretory NAP forms: MV-lambda-NAP encoding the full-length constant lambda light chain domain and MV-s-NAP encoding only the N-terminus of the lambda light chain with the leader peptide. Immunization of MV permissive Ifnarko-CD46Ge transgenic mice by a single intraperitoneal injection of the NAP-expressing strains induced a robust, long-term humoral and cellular immune response against MV. Nine months post vaccination measles-neutralizing antibody titers were above the serum level considered protective for humans. Furthermore, all animals immunized with MV strains expressing the secretory NAP antigen developed strong humoral immunity against NAP, reaching titers >1:10,000 within 2–4 weeks. IFN-γ ELISpot assay confirmed that NAP-encoding MV vectors can also stimulate NAP-specific cell-mediated immunity. Our data demonstrate that MV is an excellent vector platform for expression of bacterial antigens and development of vaccines for H. pylori immunoprophylaxis in humans.
Keywords: Attenuated measles virus, Helicobacter pylori, Neutrophil-activating protein
1. Introduction
Helicobacter pylori is a Gram-negative spiral-shaped motile microorganism associated with acute and chronic gastritis, peptic ulcer, gastric cancer and gastric MALT lymphomas in humans [1–3]. Epithelial colonization by the pathogen occurs early in life with more than 20% of the population in industrialized countries and over 80–90% in developing countries being chronic H. pylori carriers [4]. Combination of proton pump inhibitors, antibiotics and bismuth compounds is recommended as a first line therapy. The emerging antibiotic resistance and high rate of Helicobacter recurrence or re-infection however, make H. pylori eradication a challenging task [5].
The development of a successful vaccine strategy represents a promising alternative approach in prevention and control of Helicobacter infections. Multiple H. pylori virulence factors are involved in colonization of gastroduodenal mucosa and induction of detrimental inflammatory reaction [3,6,7]. H. pylori neutrophil-activating protein (NAP) is a dodecameric iron binding protein composed of identical monomers with molecular weight (MW) of approximately 17 kDa [8,9]. Once released in the gastroduodenal mucosa, NAP is transported via transcytosis across endothelial cells where it directly stimulates polymorphonuclear cells adherence and migration at the site of infection [10,11]. NAP is also a potent activator of other immune cells, including monocytes and mast cells [6,12]. A robust production of oxygen radicals and chemokines by attracted neutrophils instigates a strong mucosal inflammatory reaction [13–16]. As a toll-like receptor 2 (TLR-2) agonist, NAP is a potent immunomodulator stimulating interleukin-12 (IL-12) and IL-23 secretion and redirecting the immune response toward Th1 cytotoxic type [17–21].
Gene expression profile of H. pylori clinical isolates and analysis of the immune response in infected individuals have identified NAP, along with CagA, VacA, urease and flagellar proteins, as protective antigens [22]. Protective antigens are considered as the main candidates for vaccine development. Immunity to these factors confers protection against H. pylori in experimental models of infection [23]. Current approaches in H. pylori immunoprophylaxis include vaccination with whole bacteria and recombinant protective antigens administered with adjuvants or delivered by live vectors, such as attenuated Salmonella or polio virus vaccine strains [22,23]. Mucosal immunization with inactivated whole bacteria or recombinant urease combined with adjuvants induced detectable immune response but failed to eradicate infection in chronically infected individuals [24]. Therapeutic parenteral immunization of beagle dogs with adjuvant formulated combination of the CagA, VacA and NAP antigens induced humoral immune response and reduced bacterial colonization and gastric mucosa inflammation [25]. The safety and immunogenicity of CagA, VacA and NAP recombinant protein vaccine in humans has been recently evaluated in a phase I clinical study [26]. Highly conserved and expressed by virtually all clinical isolates, NAP is an excellent candidate for prophylactic vaccine development, targeting a crucial step in Helicobacter infection pathogenesis.
Measles virus (MV) is a paramyxovirus with a negative strand RNA genome and lipoprotein envelope [27]. Measles is considered to be one of the most contagious human infectious diseases. Vaccination with live attenuated MV Edmoston strain derivatives has an excellent safety record for decades and has dramatically reduced the incidence of measles infection. Reverse genetic techniques make insertion of foreign genes into MV genome possible [28] and MV vectors expressing protective antigens have been tested in the development of vaccines against viral pathogens such as flaviviruses, hepatitis B and HIV [29–32].
Here we present the generation and immunogenicity testing of a recombinant NAP-encoding MV vaccine based on the attenuated Edmonston strain platform. The engineered vectors induced long-lasting anti-measles immunity in MV susceptible mice. Furthermore, cellular immune response and high antibody titer against NAP were detected in all animals immunized with a single dose of the MV vaccine expressing a secretory form of the NAP antigen.
2. Materials and methods
2.1. H. pylori strains, NAP cloning and expression
H. pylori strain 26695 and genomic DNA from H. pylori 26695 were purchased from American Type Culture Collection (ATCC, Manassas VA). Genomic DNA from H. pylori ATCC 43504 (ATCC) was isolated using DNAzol reagent (Invitrogen). NAP gene from both strains was PCR amplified and cloned into pCRII vector using TA cloning kit (Invitrogen). For expression of H. pylori 43504 NAP fragment in pCRII plasmid was digested using BamHI and NotI restriction enzymes (New England Biolabs) and was subsequently inserted into pET28 vector (Novagen), creating N-terminus 6-histidine-tagged (6-his) protein (6H-NAP-43504) with 48 additional amino acids. NAP from H. pylori strain 26695 was amplified using a forward primer encoding a shorter NcoI-flanked 10 amino acid N-terminus peptide with 6-his epitope. The NcoI/NotI digested fragment was subsequently cloned into pET28 plasmid. Recombinant NAP proteins were expressed in pET28 vector transformed E. coli BL21 Star (DE3) chemically competent cells (Invitrogen) according to the manufacturer’s protocol (Invitrogen).
2.2. Purification of recombinant NAP
6H-tagged NAP proteins were isolated from bacterial inclusion bodies and purified using Ni-NTA Fast Start kit (QIAGEN). 6H-NAP-43504 eluted fractions were dialyzed overnight in Tris-buffered saline (TBS) pH 7.5. To reduce the lipopolysaccharide contamination the samples were passed through Detoxi-Gel Endotoxin Removing Columns (Pierce). Protein concentration was measured using BCA Protein Assay kit (Pierce). Purity of the product was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Bacterial lysates and inclusion bodies from pET28 empty vector-transformed E. coli BL21 Star (DE3) cells were purified in the same way and used as controls (pET28-control).
2.3. Generation of recombinant MV strains encoding NAP
NAP gene from H. pylori strains (strain 26695 and 43504) and 6H-NAP (N-terminus 10 amino acid peptide with 6-his epitope) were amplified using primers with MluI and AatII flanking restriction sites. PCR products were cloned into TA-cloning vector and the correct gene sequences were confirmed. MluI/AatII (New England Biolabs) digested NAP fragment was inserted into the full-length MV cDNA plasmid p(+)MVeGFP (kindly provided by Dr. R. Cattaneo, Mayo Clinic), replacing the green fluorescent protein (GFP) gene.
MV expressing human lambda light immunoglobulin protein (MV-lambda) has been characterized recently [33]. To facilitate the extracellular expression of NAP antigen, the NAP-26695 gene was amplified and cloned using the unique BstEII and AvrII restriction sites in the variable domain of the lambda light chain encoded by MV-lambda. We generated two MV constructs encoding a secretory form of the NAP antigen: MV-lambda-NAP expressing the full length of the constant lambda domain and MV-s-NAP expressing truncated variant of the chimeric protein with the 38-amino acid N-terminus of immunoglobulin molecule containing the leader peptide without the constant part of lambda chain. The recombinant MV strains were rescued using 293-3-46 cell system as previously described [34].
2.4. RT-PCR for MV encoded NAP
Total RNA was extracted from MV infected Vero cells by RNeasy mini kit (Qiagen). Forward and reverse primers, specific for sites located upstream (5′-GGATTAGGGATATCCCGACGCG-3′) and downstream (located in nucleoprotein (N) gene 5′-CTCTGATGGCTCCACCGGATCC-3′) of the MluI/AatII insertion and SuperScript One-Step RT-PCR kit (Invitrogen) were used to amplify the MV encoded NAP fragment. NAP insert was verified by sequencing of the RT-PCR product.
2.5. Test for mycoplasma and endotoxin levels
The Vero cells used for MV productions, viral stocks and supernatants from infected cells used in the cytokine assays were tested for the absence of endotoxin or mycoplasma contamination using the Limulus Amebocyte Lysate PYROGENT Plus test and the MycoAlert mycoplasma detection kit (Lonza).
2.6. MV propagation and growth kinetics
All recombinant MVs and control MV-GFP [35] and MV-lambda viruses were propagated on Vero cells (ATCC) as previously described [33]. Viral titer was determined by titration on Vero cells and calculated in both plaque-forming units (PFU) and tissue culture infectious does 50% (TCID50) per ml. For one-step MV growth kinetics, Vero cells were inoculated using multiplicity of infection (MOI) of 1 and incubated at 32 °C. Cell-associated and supernatant-released viruses were titrated following 24, 48, 72 and 96 h of incubation.
Human multiple myeloma (MM) line KAS-6/1 (kindly provided by Dr. D. Jelinek, Mayo Clinic) was maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and human recombinant IL-6 (R&D Systems). To demonstrate the expression of lambda-NAP chimeric protein, KAS-6/1 cells were infected with MV-lambda-NAP or MV-lambda (MOI of 1.0) and incubated in serum-free Opti-MEM medium with 2 ng/ml of IL-6. After 72 h of incubation culture supernatants were collected and analyzed for lambda light immunoglobulin expression by immunoblot.
2.7. Production of NAP specific monoclonal antibodies (MAbs)
The mouse myeloma line Sp2/0-Ag14 was purchased from ATCC and grown in ATCC recommended medium (ATCC). Inclusion bodies from E. coli BL21 expressing 6H-NAP (from H. pylori strain 43504) were used for immunization. Five-six week-old female BALB/c mice were injected intraperitoneally (i.p.) with 50 μg of the crude protein extract. Immunization was repeated twice on days 7 and 24 and NAP-specific serum antibody titers were determined by ELISA. Mice with the highest titer received a final intravenous (i.v.) boost with Ni-NTA purified 6H-NAP (H. pylori 43504). Three days later spleen cells were harvested and hybridomas were generated by fusion [36,37] with Sp2/0-Ag14 myeloma partner line using polyethylene glycol 4000 (EMD Chemicals). Hybridomas were grown in DMEM (ATCC) supplemented with 10% FBS and antibiotics (Invitrogen). Hybridoma culture supernatants were tested by antigen-mediated ELISA for MAb production. NAP-specific hybridomas were cloned from a single cell and MAb immunoglobulin class was determined using IsoStrip Monoclonal Antibody Isotyping kit (Santa Cruz Biotechnology).
MAb 16F4 (of IgG1 isotype) was purified from serum-free Hybridoma-SFM medium (Invitrogen) by affinity chromatography using Protein G columns (Pierce). Protein concentration was determined using BCA kit (Pierce) and purified MAb was coupled to horse-radish peroxidase (HRPO) using Lightning-Link HRP conjugation kit (Innova Biosciences, UK).
2.8. Enzyme-linked immunosorbent assay (ELISA)
For antigen-mediated ELISA, 96-well polystyrene plates (Nunc) were coated with 0.3 μg/well of purified 6H-NAP antigen in carbonate–bicarbonate buffer pH 9.6. After overnight incubation at room temperature the plates were washed with phosphate-buffered saline (PBS), containing 0.05% Tween 20 (PBS/T) and blocked for 2 h with 1% bovine serum albumin (BSA) in PBS. Mouse serum samples (serially diluted in PBS/T with 1% BSA) or hybridoma 16F4 culture supernatant dilutions were added to the wells and plates were incubated at room temperature for 1 h. After three washes in PBS/T a secondary anti-mouse polyvalent immunoglobulin (G, A, M) HRPO-conjugated antibody A0412 (from Sigma) was added for 1 h. Reaction was developed using TMB peroxidase substrate (Bethyl Laboratories). Total NAP-specific antibody concentration in mouse sera was measured by antigen-mediated ELISA using dilutions (from 400 ng/ml to 0.1 ng/ml) of purified MAb 16F4 as standards.
Quantitative ELISA for human lambda immunoglobulin was performed using ELISA kit (Bethyl Laboratories) following the manufacturer’s protocol.
For capture ELISA, plates were coated with 1 μg/well goat anti-lambda antibody (Bethyl Laboratories) or 0.5–1 μg/well purified MAb 16F4. After blocking samples with known concentrations of 6H-NAP or supernatant from Vero cells infected with MV encoding NAP constructs were added and incubated for 1 h at room temperature. Goat anti-human lambda HRPO-conjugated IgG (Bethyl Laboratories) or HRPO-conjugated MAb 16F4 were used as secondary antibodies. The reaction was developed using TMB peroxidase substrate as described above.
2.9. SDS-PAGE, immunoblot and dot-blot
Supernatants and cell lysates were prepared from Vero or KAS-6/1 cells infected (MOI of 1) with NAP encoding MV strains. MV-GFP and MV-lambda infected cells were used as controls. Proteins were resolved on polyacrylamide gels with different concentration (10%, 12.5%, 15% or 18%) using Criterion electrophoresis system (Bio-Rad). Protein bands were visualized by SimplyBlue SafeStain staining (Invitrogen).
For immunoblotting, SDS-PAGE fractionated proteins were blotted on PVDF membranes (Bio-Rad) using semi-dry transfer system (Bio-Rad). 5–10% non-fat dry milk (Bio-Rad) in PBS/T was used for blocking. Membranes were incubated with supernatant dilutions of MAb 16F4 or Penta-His (6-his-tag peptide specific) mouse MAb (Qiagen) for 4 h. Mouse specific polyvalent immunoglobulin (G, A, M) HRPO conjugate (diluted 1:2000 in 5% dry milk in PBS/T) was used as secondary antibody. Specific protein bands were visualized using chemiluminescent reagent (Pierce). For detection of lambda chain protein membranes were directly incubated for 2 h with HRPO conjugated goat antibody specific for human lambda light immunoglobulin chain (Bethyl Laboratories). Antibodies directed to the whole IgG or kappa light chain (Bethyl Laboratories) were also used in immunoblot experiments.
NAP transgene expression was also analyzed by dot-blot using a micro-sample filtration system (Schleicher & Schuell, Keene NH). Protein samples from MV infected Vero cells or purified 6H-NAP were transferred to PVDF or nitrocellulose membranes (Bio-Rad) and blocked in dry milk. Immobilized proteins were detected using MAb 16F4 or lambda chain specific antibody as described above.
2.10. Interferon-γ (IFN-γ) ELISpot assay
Cellular immunity to MV and NAP antigen in immunized animals was evaluated in isolated spleen lymphocytes using mouse IFN-γ ELISpot kit (Mabtech). Spleen cells were harvested, washed and plated (2 × 105 cells per well) into 96-well plates. MV-GFP at a MOI of 1.0 or 2.5 μg purified 6H-NAP were used for overnight stimulation. Non-stimulated cells or spleen cells treated with pET-control protein preparation were used as controls. The assay was performed following the ELISpot kit protocol (Mabtech). The plates were scanned and analyzed on an Immunospot S4 Pro Analyzer (Cellular Technology Ltd., Cleveland, OH) using Immunospot version 4.0 software (Cellular Technology).
2.11. Interleukin-8 (IL-8) expression in THP-1 monocytic line
Supernatants from MV-s-NAP infected Vero cells cultured corresponding to the maximum level of NAP transgene expression (72–96 h of incubation at 32 °C) were collected and heat-inactivated at 60 °C for 30 min. MV-GFP was used as control strain in the experiment. Inactivation of infectious virions in the supernatants was confirmed by cell culture. THP-1 monocytic cells (ATCC) we treated by 1:4 diluted heat-inactivated Vero cell supernatants in 96-well plates. Samples were collected after 24–72 h of incubation and concentration of IL-8 in the supernatants was measured by human IL-8 ELISA kit according to the manufacturer’s protocol (Beckton Dickinson).
2.12. Virus neutralization (VN) test
Measles neutralizing antibody titer was determined using fluorescence based plaque-reduction microneutralization assay [38]. Mouse sera were diluted 2-fold (starting from 1:10–40 depending on the collected serum volume) in 96-well plates in reduced serum Opti-MEM medium (Invitrogen). Equal volume of diluted MV-GFP (adjusted to yield 30–50 PFU/well after inoculation) was mixed with the diluted sera and plates were incubated for 1 h at 37 °C. Vero cells were plated in different 96-well plates at a density of 1.5 × 104 per well in 50 μl Opti-MEM supplemented with 1–2% FBS. Following incubation, 50 μl of the samples were added to the plates with Vero cells and cultured at 37 °C in humidified tissue culture incubator. The number of syncytia per well was determined 48–72 h post-inoculation using fluorescent microscopy (Nikon). The controls of virus pre-incubated without serum were run in 8 wells. Plaque reduction neutralization titer 50% (PNT50) was calculated using the Karber’s formula [38].
2.13. In vivo experiments
All animal studies were approved by the Mayo Foundation Institutional Animal Care and Use Committee. Mice were maintained in the animal facilities of Mayo Clinic, Rochester MN.
2.14. Immunization and detection of NAP specific immune response in MV susceptible mice
Female 5–6-week-old interferon (IFN) type I receptor knockout (Ifnarko) mice and human CD46 transgenic Ifnarko (Ifnarko-CD46Ge) mice (5–7 per group) were inoculated i.p. with 106 PFU of MV-NAP or MV-lambda-NAP. Control groups received equivalent dose of MV-GFP. Mice were bled before virus injection and on day 14 and 28 after immunization. Serum samples were heat inactivated at 56 °C for 30 min and measles neutralization titer and NAP specific antibody titer were determined. Nine months post-inoculation all animals were terminally bled and sacrificed. Spleen cells were harvested and assayed for IFN-γ production following stimulation with MV or NAP as described above.
Antibody response kinetics to vector-encoded NAP antigen was determined in Ifnarko-CD46Ge mice. Briefly, the animals were inoculated i.p. with 106 PFU of MV-lambda-NAP. Serum samples were collected on days 1, 4, 7, 14, 21 and 28 of the study. On day 35 all mice were terminally bled and spleen cells were harvested. Kinetics of antibody response to NAP and MV was analyzed by ELISA and VN test as described above. Cell-mediated immunity against NAP and vector antigens was measured by IFN-γ ELISpot assay.
In the following immunization experiment, we compare the immune responses between the two MV strains expressing secretory forms of the NAP transgene. Female 5–6-week old Ifnarko-CD46Ge mice were injected i.p. with 5 × 105 PFU of MV-sNAP (10 mice) or MV-lambda-NAP (6 mice). The animals were bled on days 1, 4, 7, 15 and 29 and spleen cells were harvested on day 45. MV and NAP specific responses were determined as described above.
2.15. Statistical analyses
Statistical analysis was performed using the GraphPad Prism 5.0 software (GraphPad Software, San Diego CA).
3. Results
3.1. Cloning and expression of recombinant NAP
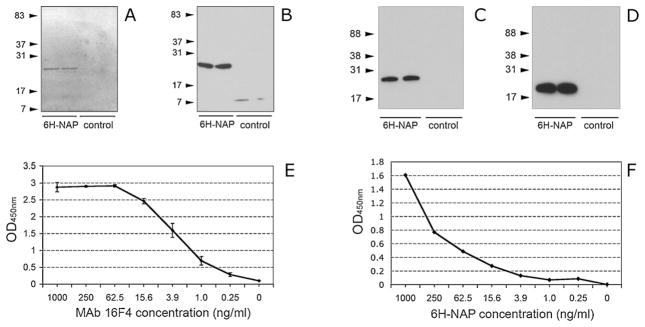
Two variants of the 6-his-tagged NAP antigen were cloned into pET28 expression vector. The additional N-terminus peptides consist of 48 amino acids for 6H-NAP H. pylori strain 43504 and 10 amino acids for 6H-NAP H. pylori 26695 (with a change of the NAP starting codon M → S) construct respectively. High yield of the recombinant proteins was obtained in an E. coli bacterial expression system. Correct MW of the products was confirmed by SDS-PAGE and Penta-His MAb immunoblot analysis of the crude inclusion body extracts or Ni-NTA affinity purified fractions (Fig. 1A and B).
Fig. 1.
Production of recombinant NAP antigen and characterization of MAb 16F4. Recombinant 6-His-tagged NAP was produced using the E. coli BL21 protein expression system. A single protein band of Ni-NTA purified 6H-NAP (from H. pylori strain 43504) was detected by SDS-PAGE using 15% Criterion gel (A). Expression of 6H-NAP was confirmed by 6-His specific (Penta-His MAb) immunoblot (B). In immunoblot, Mab 16F4 recognized both homologous NAP antigen (6H-NAP from H. pylori 43504) used for immunization and hybridoma generation (C) and H6-NAP strain 26695 construct (D). Arrows indicate position and MW (in kDa) of the marker proteins. The detected protein bands correspond to the expected MW of the recombinant 6H-NAP strain 43504 (144 amino acid residues plus 48 amino acid 6-his-tagged peptide in the N-terminus) and 6H-NAP strain 26695 (144 amino acid protein with a 10 amino acid 6-his-tagged peptide at the N-terminus). Inclusion body extracts from E. coli BL21 transformed with empty pET-28 vector plasmid were used as controls. The samples were run in duplicate and positions and MW (in kDa) of the marker proteins are indicated by arrows. In antigen-mediated ELISA, affinity chromatography purified MAb 16F4 reacted with 6H-NAP at a concentration of 250 pg/ml (E). Capture ELISA using MAb 16F4 for both coating and detection was able to detect NAP antigen at concentration <1 ng/ml (F).
3.2. Production and characterization of NAP specific MAbs
Hybridoma clone 16F4 of IgG1 isotype demonstrated a strong reaction with both homologous NAP (H. pylori 43504) and heterologous NAP (H. pylori 26695) protein in antigen-mediated ELISA with supernatant antibody titer >1:81,920 (not shown). A single protein band corresponding to the MW of 6H-NAP recombinant antigen was detected by immunoblot with MAb 16F4 (Fig. 1C and D). In antigen-mediated ELISA, purified 16F4 antibody reacted with recombinant 6H-NAP at concentration of 250 pg/ml (Fig. 1E). Capture ELISA using purified MAb 16F4 for coating and MAb 16F4 HRPO conjugate as secondary antibody was able to detect NAP in a ng/ml protein concentration range (Fig. 1F) suggesting expression of repeated MAb epitopes on the antigen molecule. These results indirectly confirm the multimeric structure of NAP produced by prokaryotic cells.
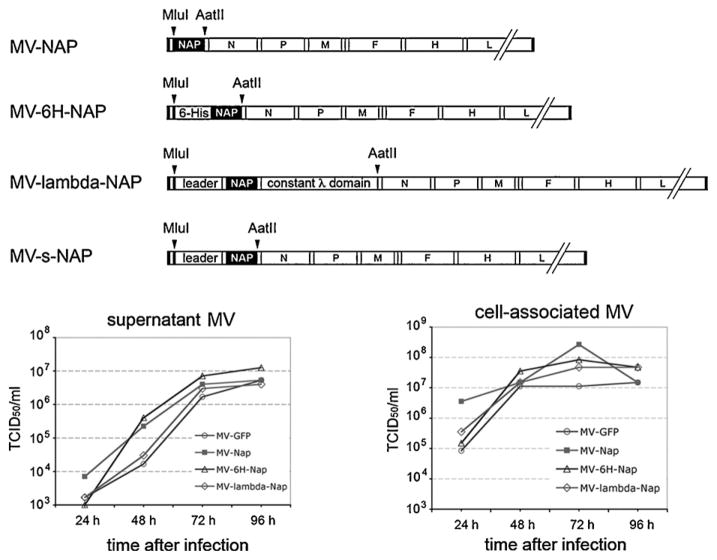
3.3. Generation and growth kinetics of MV encoding NAP and secretory NAP constructs
A 144 amino acid NAP from two H. pylori strains (26695 and 43504) was inserted into the additional transcription unit in the p(+)MVeGFP plasmid encoding the full-length infectious clone of attenuated MV Edmonston strain. The position upstream of N gene was chosen in order to support a high level of foreign gene transcription. Similarly, other NAP constructs: 6H-NAP (N-terminus 6-histidine-tagged NAP) and secretory NAP forms, such as lambda-NAP (NAP was inserted in the human lambda immunoglobulin chain replacing the main part of the variable domain) and s-NAP (encoding only the N-terminus of the lambda light chain with the leader peptide, without the constant part) were cloned in the same expression cassette replacing the GFP gene (Fig. 2A). All inserts were constructed following the “rule of six” to restore the hexameric MV genome length required for efficient replication of paramyxoviruses [39]. Recombinant viruses were successfully rescued and propagated in Vero cells. High titer viral stocks (passages 2 and 3) of MV-NAP (H. pylori 26695), MV-6H-NAP, MV-lambda-NAP and MV-s-NAP were used for the in vitro studies and animal experiments. RT-PCR amplification and sequencing of the third passage viruses confirmed the presence of correct NAP inserts (data not shown). The growth kinetics of the recombinant NAP encoding MVs was comparable to that of the control MV-GFP strain (Fig. 2B and C). The peak of viral production was observed 72 h post-inoculation. Both MV-NAP and MV-6H-NAP replicated faster than the control virus reaching 10-fold higher titer in the supernatant at 48 h. At 72 h the difference was approximately 2–4 fold indicating that NAP transgene does not affect significantly MV replication and infectivity. The MV preparations used for the in vitro and in vivo studies were tested and confirmed to be negative for endotoxin and mycoplasma contamination (data not shown).
Fig. 2.
Construction and growth kinetics of NAP expressing MV vectors. MluI/AatII flanked fragments of NAP constructs were ligated in the corresponding digestion site of an additional transcription unit upstream of the N gene in MV. For MV-lambda-NAP generation, NAP was first inserted in BstEII/AvrII digested lambda light chain gene and was subsequently cloned into MV genome (A). Kinetics of viral replication of MV strains encoding NAP was determined in Vero cells at different time points for both supernatant (B) and cell-associated viruses (C).
3.4. Characterization of the NAP transgene expression by MV strains encoding NAP
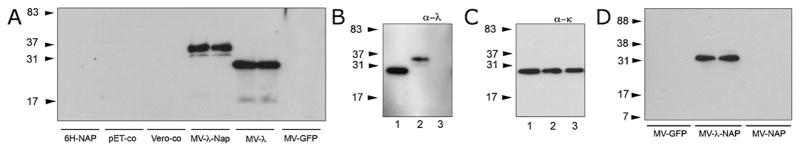
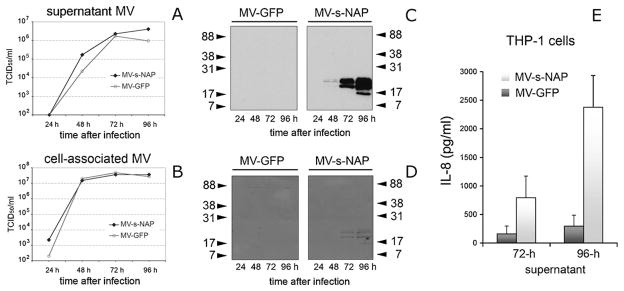
In order to detect the NAP expression by infected cells, we generated a monoclonal antibody (MAb) clone 16F4 with NAP-specific reactivity in ELISA and immunoblot. In order to monitor NAP transgene expression in supernatants and cell lysates from infected cells, we also generated a “trackable” lambda-NAP chimeric construct. Insertion of NAP in the variable domain of the lambda light chain, while keeping the leader peptide and its constant part created a unique protein that follows the synthesis and extracellular secretion of the natural immunoglobulin molecule. Concentration of the chimeric protein was quantified using a human lambda immunoglobulin-specific ELISA kit. The secretory lambda-NAP reached a peak of more than 4 μg/ml (4.18–4.49 μg/ml per 106 cells) in the culture supernatant from Vero cells infected for 72 h with MOI = 1.0 of MV-lambda-NAP. The hybrid molecule expression was confirmed by ELISA using lambda capturing and MAb 16F4 HRPO-conjugated detection antibody. The test was also performed with MAb 16F4 coating and anti-lambda secondary antibody (Table 1). In contrast to recombinant NAP expressed by E. coli BL21 Star (DE3) cells (Fig. 1), we were not able to detect NAP, 6H-NAP or lambda-NAP protein expressed by infected Vero cells by capture ELISA using MAb 16F4 as both capture and detection antibody. These results suggest that NAP antigen is processed and secreted by mammalian cells in monomeric form. A positive reaction in capture ELISA would require the presence of repeated epitopes or formation of multimeric protein structures or aggregates. Single bands with MW corresponding to human light immunoglobulin chain and chimeric lambda-NAP protein were detected in the supernatants from MV-lambda control or MV-lambda-NAP infected Vero cells (Fig. 3A) and human MM KAS-6/1 cells (Fig. 3B and C) by immunoblot using antibodies specific for human immunoglobulin molecules. Immunoblot with MAb 16F4 confirmed the high level of NAP antigen expression by MV-lambda-NAP inoculated cells but not by MV-NAP or control MV-GFP infected cells (Fig. 3D). Intracellular NAP and 6H-NAP was found only in concentrated lysates from MV-NAP and MV-6H-NAP infected Vero cells in dot-blots probed with MAb 16F4 (data not shown). A possible explanation for this observation could be a non-optimal low level of NAP synthesis or post-translational modification of the protein that affected the MAb 16F4 epitopes. Growth kinetics of MV-s-NAP demonstrated that supernatant MV-s-NAP virions accumulated faster to higher titers (5-fold higher at 48 h) than the control MV-GFP. The titers of cell associated viruses were similar for both strains (Fig. 4A and B). H. pylori antigen expression by MV-s-NAP infected cells was confirmed using SDS-PAGE and immunoblot with MAb 16F4. The corresponding protein band was isolated from Coomassie R250 stained gels and NAP specificity was confirmed by mass-spectrometry (data not shown). Transgene production followed the virus growth kinetics and a high level of secretory NAP accumulated in the supernatants 72–96 h post inoculation (Fig. 4C and D). Capture ELISA using MAb 16F4 and 6H-NAP as a standard detected more than 1 μg/ml specific protein concentration indicating that s-NAP is secreted partly as a multimeric form (not shown). Biological activity of s-NAP was confirmed by stimulation of IL-8 production by THP-1 monocytic cells following treatment with heat-inactivated supernatants from infected Vero cells (Fig. 4E).
Table 1.
| Coating Antibody | HRPO conjugate
|
|
|---|---|---|
| Anti-λ | MAb 16F4 | |
| Anti-λ | 3.727 | 2.008 |
| MAb 16F4 | 2.275 | 0.118 |
Plates were incubated with 1 μg/ml of 6H-NAP from H. pylori 43504.
Results are in OD450nm absorbance.
Fig. 3.
Immunoblotting analysis of NAP antigen expression in cells infected with the NAP-encoding MV vectors. Chimeric NAP expression was detected in MV-lambda-NAP infected Vero cells by immunoblot using a human lambda immunoglobulin chain specific antibody (A). Serum-free supernatant from MV-lambda (MV-λ) infected Vero cells was used as a positive control. There was no lambda protein detection in uninfected control cells (Vero-co), MV-GFP infected cells (MV-GFP) and the recombinant 6H-NAP and pET28 control extracts (pET-co). The samples were run in duplicates. The arrows indicate position and MW (in kDa) of the marker proteins. KAS-6/1 MM cells were infected with MV-lambda-NAP and the secretion of lambda light immunoglobulin chain or chimeric NAP in the culture supernatant was demonstrated by anti-lambda (a-λ) specific immunoblot (B). Anti-kappa (a-κ) light chain reaction (C) shows the equal presence of kappa light chain of MM IgG in the samples (lane 1 – MV-lambda infected cells; lane 2 – MV-lambda-NAP infected cells; and lane 3 – uninfected KAS-6/1 cells). NAP expression was confirmed by MAb 16F4 immunoblot analysis for MV-lambda-NAP (MV-λ-NAP) but not for MV-NAP (D). Samples were run in duplicate lanes and MV-GFP infected Vero cells were used as control.
Fig. 4.
Growth kinetics of supernatant (A) and cell-associated (B) MV-s-NAP was compared to the control MV-GFP strain in Vero cells. Expression of secretory NAP antigen in the culture supernatants at the corresponding time points was confirmed by MAb16F4 immunoblot (C). PVDF membranes from the immunoblot analysis were subsequently stained with Comassie R250 demonstrating high levels of s-NAP secreted in the supernatant (D). Virus inactivated supernatants from MV-s-NAP-infected cells (72-h and 96-h time points with the maximal NAP expression) induce significantly higher IL-8 production in THP-1 cells compared to MV-GFP control (E). Experiment was run in 4 wells and the data are presented as stimulated IL-8 concentration (mean ± SD in pg/ml) in the supernatant above the control of non-stimulated THP-1 cells. MV-s-NAP vs. MV-GFP – 72-h supernatant p = 0.0193; MV-s-NAP vs. MV-GFP – 96-h supernatant p = 0.0004; MV-s-NAP 72-h vs. 96 h supernatant p = 0.0033.
3.5. MV expressing secretory lambda-NAP induced strong long-lasting anti-measles and NAP specific response in immunized animals
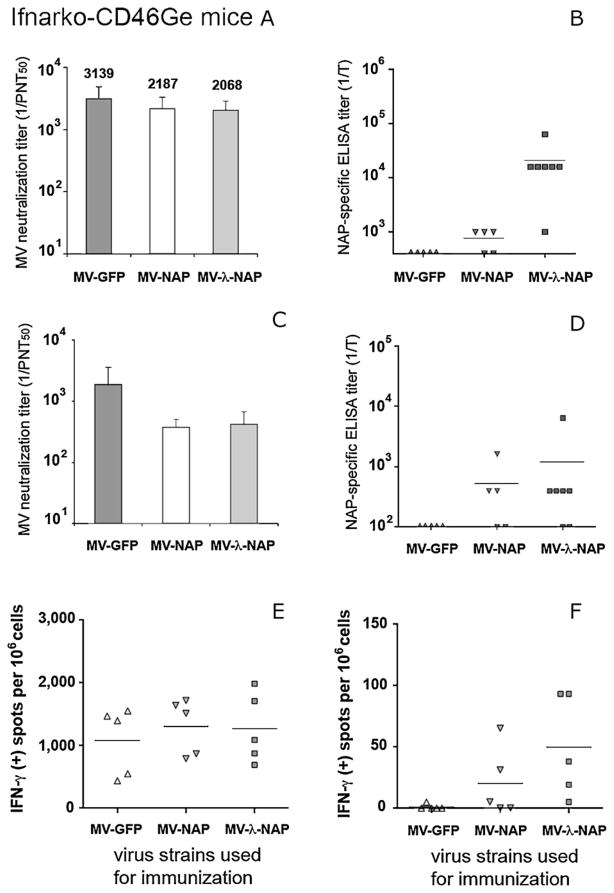
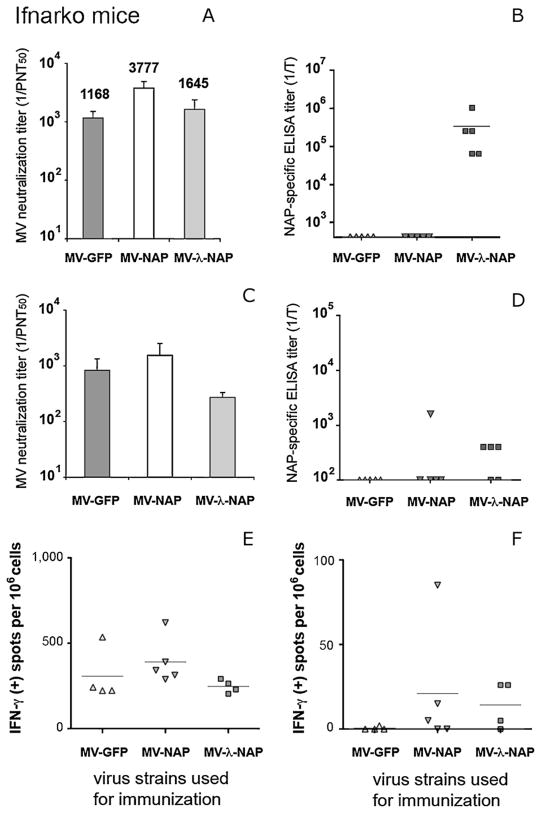
MV susceptible, IFN type I receptor knockout (Ifnarko) and Ifnarko-CD46Ge transgenic mice are relevant small animal models for studying viral pathogenesis and anti-measles host immune mechanisms [40]. Immune responses to both transgene and vector were monitored in Ifnarko-CD46Ge mice following a single i.p. injection of 106 PFU of MV-NAP or MV-lambda-NAP. MV-GFP was used as control. Parallel groups of Ifnarko animals, an IFN receptor knockout but CD46 negative derivative line of IfnarKo-CD46Ge mice [41], were treated identically. Humoral immunity against NAP and MV was analyzed by ELISA and VN test respectively. Serum from non-immunized animals was used as control. Two weeks post-inoculation all Ifnarko-CD46Ge animals had protective (PNT50 > 1:120) anti-measles antibodies. The neutralizing titer increased and on day 28 reached mean levels higher than 1:2000 in all groups regardless of the vector used for immunization (Fig. 5A). Similarly, Ifnarko mice developed strong anti-measles response (Fig. 6A) with significantly (p < 0.01) higher neutralizing titer in MV-NAP inoculated group (mean PNT50 titer 1:3777) compared to MV-lambda-NAP (1:1645) or MV-GFP control (1:1168). Robust antibody response to NAP was detected in all MV-lambda-NAP immunized mice. Serum titer in antigen-mediated ELISA varied from 1:1000 to over 1:1,000,000. The mean titer was calculated to be above 1:16,000 for the Ifnarko-CD46Ge group (Fig. 5B) and above 1:100,000 for the Ifnarko group respectively (Fig. 6B). In contrast, only three of the MV-NAP-inoculated Ifnarko-CD46Ge mice developed antibodies to the transgene. A long-lasting (9 months post-vaccination) measles protective (PNT50 > 1:120) humoral response was demonstrated in 100% of the animals (Figs. 5C and 6C). At the end of the study, a high titer of anti-NAP antibodies was detected in 5 of 7 MV-lambda-NAP immunized Ifnarko-CD46Ge mice (Figs. 5D and 6D. IFN-γ ELISpot analysis demonstrated a strong long-lasting cellular immunity against MV, 9 months post immunization (Figs. 5E and 6E). Spleen cells from all MV-lambda-NAP-injected CD46-transgenic mice responded to the stimulation with recombinant NAP. Increased NAP-specific IFN-γ production was also observed in three animals from the MV-NAP-injected group (Fig. 5F). In addition, immunization with MV-lambda-NAP or MV-NAP induced cell-mediated immune memory to NAP antigen in most of the Ifnarko mice (Fig. 6F). These results suggest that recombinant MV vectors replicate efficiently in vivo and transgene expression does not affect the development of anti-measles immunity. Vaccination with MV strains encoding the secretory form of NAP antigen (such as MV-lambda-NAP) induces strong antibody response and T-cell immune memory against NAP. Low level of NAP synthesis (including a possible post-translational modification and rapid NAP degradation) and intracellular localization of the antigen could explain the poor humoral immune response against NAP in MV-NAP injected animal groups.
Fig. 5.
Immune response to MV and NAP following immunization of MV permissive Ifnarko-CD46Ge mice with the NAP-encoding MV vectors. Mice were immunized by a single i.p. injection of 106 PFU of MV-NAP, MV-lambda-NAP or MV-GFP control virus. All animal groups (5–7 per group) developed strong humoral anti-measles immunity on day 28 post-vaccination (A). The virus neutralization (VN) titers were determined by plaque-reduction microneutralization assay. Results are presented as mean titer ± SD. Neutralization titer (in PNT50 ) >1:120 is considered protective for humans. The NAP-specific response was determined by antigen-mediated ELISA. All mice injected with MV-lambda-NAP encoding secretory form of the antigen (MV-λ-NAP in the figure) developed NAP-specific antibodies as determined by antigen-mediated ELISA (B). Bars indicate the mean titer of each group. NAP-specific antibody titer is significantly higher for MV-lambda-NAP compared to MV-NAP (p < 0.05). Nine months after immunization all groups had anti-measles antibody titer >1:120 PNT50 (C) and NAP-specific humoral immunity was detected in most of the MV-lambda-NAP immunized mice (D). Immunization with the MV vectors induced long-term cell-mediated immunity in genetically modified Ifnarko-CD46Ge mice. Isolated spleen cells were stimulated either with MV-GFP (E) or recombinant 6H-NAP (F). The frequencies of IFN-γ-producing cells were determined by ELISpot assay. Results are presented as a number of IFN-γ positive spots per 106 cells for the individual animals and the bars indicate the mean level for each group.
Fig. 6.
Detection of immune response against MV and NAP in Ifnarko mice (5 per group) immunized with the NAP-encoding MV strains. Mice were injected i.p. with 106 PFU of MV-NAP, MV-lambda-NAP or MV-GFP control virus. Serum samples were analyzed for MV neutralization and NAP-specific ELISA titer on day 28 (A and B) and 9 months post-vaccination (C and D). On day 28, the MV-NAP-immunized group had significantly higher neutralization titer, as compared to MV-GFP and MV-lambda-NAP immunized groups (p < 0.01). All MV-lambda-NAP injected mice (5 of 5) developed strong antibody response against NAP antigen. Nine months after immunization, the MV neutralization titer in all animals was >1:120 PNT50. Cell-mediated immunity against MV (E) or NAP antigen (F) was determined by IFN-γ ELISpot assay 9 months post-vaccination.
3.6. Kinetics of immune response to MV encoding secretory form of NAP antigen
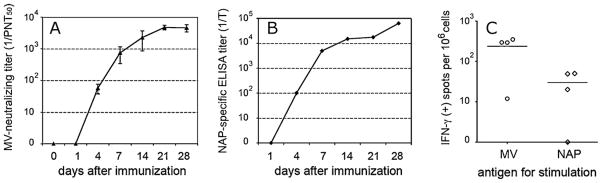
MV neutralizing antibodies in MV-lambda-NAP-immunized Ifnarko-CD46Ge mice appeared on day 4, reached measles-protective levels (PNT50 > 1:120) in all animals on day 7 and peaked on day 21 with a mean PNT50 of 1:4748 (Fig. 7A). Primary immune response to NAP transgene followed similar kinetics. MV-lambda-NAP induced detectable antibody response to the H. pylori antigen 4 days after a single i.p. injection of 106 PFU (Fig. 7B). ELISA titer gradually increased in all five Ifnarko-CD46Ge mice with a peak between 1:25,600 and 1:102,400 on day 28. Since IgM antibodies are the first to be produced in the early phase of primary immune response, a polyvalent secondary antibody against the different mouse immunoglobulin classes (IgA, IgM and IgG) was used in ELISA. This allowed detection of the NAP specific antibodies in the serum at earlier time points – days 4–7 post immunization. Spleen cells were harvested on day 35 and assayed for antigen-specific induction of IFN-γ expression. Cell-mediated immunity against NAP was detected in 3 of 4 animals, while response to MV was positive for all mice tested (Fig. 7C). Both in vitro and in vivo data support that the secretory NAP antigen was produce in a large amount by MV-lambda-NAP infected cells thus, stimulating a strong anti-NAP response in vaccinated animals.
Fig. 7.
Kinetics of immune response in genetically modified Ifnarko-CD46Ge transgenic mice immunized by a single injection of MV-lambda-NAP. Animals (n = 5) were immunized with 106 PFU of the virus administered by i.p. route. VN titer against measles (A) and antibody response to NAP antigen in ELISA (B) were determined on days 1, 4, 7, 14, 21 and 28 post-immunization. On day 35, mice were sacrificed and spleen cells were collected. Cell-mediated response to MV antigens and NAP was determined in four animals using IFN-γ ELISpot assay (C).
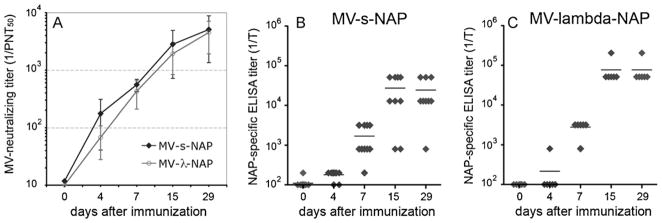
We also generated MV-s-NAP encoding a truncated variant of secretory NAP to avoid the potential development of immune reaction to lambda immunoglobulin antigens. To compare the immunogenicity of the two MV strains expressing secretory NAP forms, groups of Ifnarko-CD46Ge mice were vaccinated by a single i.p. injection of 5 × 105 PFU of MV-s-NAP or MV-lambda-NAP. Both strains induced protective anti-measles humoral response within the first week post vaccination (Fig. 8A). Neutralization titer continued to increase and reached 1:5,083 for MV-s-NAP group and 1:4523 for MV-lambda-NAP control group respectively. MV-s-NAP immunized animals developed anti-NAP antibodies with ELISA titers varying from 1:12,800 to 1:1:51200 (Fig. 8B). The titers for MV-lambda-NAP group were 1:51,200 or higher (Fig. 8C). All sera except one per group reacted positively with purified NAP in immunoblot at dilution of 1:500 (data not shown). The total concentration of NAP-specific antibodies was measured by antigen-mediated ELISA (using MAb 16F4 as a standard) to be 36 μg/ml for MV-s-NAP vaccinated and 167 μg/ml for MV-lambda-NAP vaccinated group.
Fig. 8.
Comparison of antibody response kinetics in Ifnarko-CD46Ge transgenic mice immunized i.p. by a single injection of 5 × 105 PFU of MV-s-NAP (10 mice) or MV-lambda-NAP (6 mice). VN titers against measles (A) and antibody response to the purified NAP antigen in ELISA (B and C) were determined on days 0, 4, 7, 15 and 29 post-immunization. The data for MV-s-NAP group (A, B) were calculated by testing 9 or 10 serum samples per time point. For the MV-lambda-NAP group the samples are 6 per time point. Although MV-s-NAP induced higher titer of neutralizing antibodies against measles at earlier time point (day 4), no statistically significant differences between the groups were observed. At day 7 all immunized mice developed titer >1:120 PNT50, considered protective against measles in humans.
4. Discussion
Attenuated MV is one of the components of the measles-mumps-rubella (MMR) prophylactic vaccine in humans [42]. MV can tolerate insertion of additional transgenes, including recombinant vaccine proteins without significant risk of recombination or loss of immunogenicity [29]. Here we show that MV Edmonston strain derivatives encoding a single H. pylori protective antigen are able to induce strong immune response in MV permissive animals. Because of the polar transcription gradient in paramyxoviruses [43], NAP was introduced in the MV genome upstream of nucleoprotein gene in order to achieve a high protein expression level. To promote extracellular secretion of the antigen, the NAP was cloned into the human light immunoglobulin gene replacing a large part of variable domain. The presence of the constant domain allowed precise quantification of recombinant protein by lambda chain specific ELISA. Incorporation of the NAP transgenes into MV genome did not affect the in vitro replication of the vector. Mammalian cells infected with MV strains encoding secretory NAP forms expressed large amounts of the chimeric NAP in the culture supernatant. Capture ELISA analysis suggested that MV-s-NAP-infected cells secreted multimeric forms of the antigen similarly to the native H. pylori NAP or prokaryotic expression system derived recombinant NAP [44]. Biological activity of s-NAP was confirmed by induction of IL-8 expression in monocytic cells.
We used a MV replication permissive rodent model to evaluate the immunogenicity of MV encoding NAP antigen as a potential vaccine candidate against H. pylori. Cloning of the foreign genes before N can increase the in vivo attenuation of paramyxoviruses [45]. The balance between viral growth kinetics and optimal transgene expression depends on the insert characteristics and can require additional vector engineering [46]. However, our studies showed that NAP transgene insertion upstream of the N gene did not affect MV replication in vivo. A single immunization of genetically modified mice with MV vectored to express secretory NAP antigen induced long-term humoral response and cell-mediated immune memory against measles. MV neutralizing titers were above the levels considered protective after vaccination in humans [38]. A high expression level combined with extracellular antigen secretion can explain the strong anti-NAP antibody response in mice immunized with MV-lambda-NAP and MV-s-NAP. Furthermore, delivery of the antigen by this live vector system contributed to the development of NAP-specific cell-mediated immunity.
A recently completed clinical trial has examined the immunogenicity of intramuscularly administered formulated CagA-VacA-NAP vaccine with aluminium hydroxide as adjuvant [26]. Serum antibody response to H. pylori antigens was induced in 86% of the immunized individuals. Cell-mediated response was detected against both CagA and VacA antigens. However, NAP induced undetectable or very weak cellular immunity and the authors suggested that the intrinsic characteristics of NAP molecule could be the reason for the poor T-cell response [26]. Animal studies suggest that both humoral and T-cell immune mechanisms are involved in protection against H. pylori infection [23,47]. Although the pathogen is considered to be non-invasive, recent reports confirm the presence of intracellular H. pylori capable of expressing virulence factors within the gastric mucosa cells in patients [48]. These data indicate the potential importance of vaccine-induced cellular immunity for elimination of Helicobacter infection. Our experiments demonstrated that in contrast to recombinant protein vaccine, immunization with a live replication competent vaccine expressing secretory NAP can induce not only robust antibody production but also distinctive cell-mediated response against both H. pylori antigen and MV vector.
Recent simulated analyses on infection control, gastric cancer prevention and cost-effectiveness have supported the value of successful H. pylori vaccine prophylaxis for infants [49,50]. Our data suggest that MV is a suitable vector platform for expression and delivery of H. pylori protective antigens promoting both humoral and cell-mediated response without a negative impact on development of anti-measles immunity. MV encoding secretory NAP antigen as component of MMR vaccine could be an ideal candidate for infant H. pylori infection prophylaxis. Inhalation of aerosolized MV vaccine is very efficient in generation of protective anti-measles immunity [51]. Previous data show that combinations of mucosal and systemic immunizations can enhance long-term protection against Helicobacter infection [52,53]. Thus, mucosal administration of NAP-vectored MV can be used as an alternative route of primary vaccination, as well as, for secondary boosters in order to avoid neutralization of the vector by systemic anti-measles immunity following the intramuscular or subcutaneous route of immunization.
In conclusion, here we demonstrated that genetically modified attenuated MV strains engineered to express H. pylori protective antigens are able to induce significant humoral and cellular immune response against both MV and NAP transgene. We consider development of multivalent live vector system encoding also CagA and VacA antigenic determinants. Further experiments, including pre-clinical primate studies, will likely be required prior to clinical translation of MV-based vaccines in prevention of Helicobacter infection.
Acknowledgments
We wish to thank our colleagues from Mayo Clinic, Rochester MN: Dr. G. Poland (Director of the Mayo Clinic Vaccine Research Group, Mayo Clinic, Rochester MN 55905) for the help with the cell-mediated immunity analysis and critical reading of the manuscript, Dr. R. Cattaneo for p(+)MV-eGFP plasmid and 293-3-46 rescue cells, Dr. D. Jelinek for KAS-6/1 MM line and Dr. A. Penheiter for the helpful discussion and suggestions about recombinant NAP purification. We also wish to thank Bacteriology laboratory, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester MN for H. pylori strain cultures, Toxicology and Pharmacology Laboratory in the Mayo Clinic Comprehensive Cancer Center Gene and Virus Therapy Shared Resource, Mayo Clinic, Rochester MN for providing Ifnarko and Ifnarko-CD46Ge transgenic mice and Dr. D. McCormick and B. Madden, Mayo Proteomics Research Center, Mayo Clinic, Rochester MN for the mass-spectrometry analysis.
This work was supported by the Atwater grand, P50CA108961 and Paul Leibson Memorial Fund.
References
- 1.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863–73. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med. 2009;265(4):421–38. doi: 10.1111/j.1365-2796.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 3.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenck RW, Jr, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5(8):705–13. doi: 10.1016/s1286-4579(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 5.Romano M, Iovene MR, Russo MI, Rocco A, Salerno R, Cozzolino D, et al. Failure of first-line eradication treatment significantly increases prevalence of antimicrobial-resistant Helicobacter pylori clinical isolates. J Clin Pathol. 2008;61(10):1112–5. doi: 10.1136/jcp.2008.060392. [DOI] [PubMed] [Google Scholar]
- 6.D’Elios MM, Montecucco C, de Bernard M. VacA and HP-NAP, Ying and Yang of Helicobacter pylori-associated gastric inflammation. Clin Chim Acta. 2007;381(1):32–8. doi: 10.1016/j.cca.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo C, Machado JC, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2005;10(Suppl 1):14–20. doi: 10.1111/j.1523-5378.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G, et al. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol. 1999;34(2):238–46. doi: 10.1046/j.1365-2958.1999.01584.x. [DOI] [PubMed] [Google Scholar]
- 9.Zanotti G, Papinutto E, Dundon W, Battistutta R, Seveso M, Giudice G, et al. Structure of the neutrophil-activating protein from Helicobacter pylori. J Mol Biol. 2002;323(1):125–30. doi: 10.1016/s0022-2836(02)00879-3. [DOI] [PubMed] [Google Scholar]
- 10.Brisslert M, Enarsson K, Lundin S, Karlsson A, Kusters JG, Svennerholm AM, et al. Helicobacter pylori induce neutrophil transendothelial migration: role of the bacterial HP-NAP. FEMS Microbiol Lett. 2005;249(1):95–103. doi: 10.1016/j.femsle.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N, et al. The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo. J Immunol. 2007;178(3):1312–20. doi: 10.4049/jimmunol.178.3.1312. [DOI] [PubMed] [Google Scholar]
- 12.Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32(3):671–6. doi: 10.1002/1521-4141(200203)32:3<671::aid-immu671>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Montecucco C, de Bernard M. Molecular and cellular mechanisms of action of the vacuolating cytotoxin (VacA) and neutrophil-activating protein (HP-NAP) virulence factors of Helicobacter pylori. Microbes Infect. 2003;5(8):715–21. doi: 10.1016/s1286-4579(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 14.Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, et al. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol. 2003;33(4):840–9. doi: 10.1002/eji.200323726. [DOI] [PubMed] [Google Scholar]
- 15.Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191(9):1467–76. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CA, Liu YC, Du SY, Lin CW, Fu HW. Helicobacter pylori neutrophil-activating protein promotes myeloperoxidase release from human neutrophils. Biochem Biophys Res Commun. 2008;377(1):52–6. doi: 10.1016/j.bbrc.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 17.Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116(4):1092–101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D’Elios MM, et al. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10(11):2355–63. doi: 10.1111/j.1462-5822.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 19.Del Prete G, Chiumiento L, Amedei A, Piazza M, D’Elios MM, Codolo G, et al. Immunosuppression of TH2 responses in Trichinella spiralis infection by Helicobacter pylori neutrophil-activating protein. J Allergy Clin Immunol. 2008;122(5):908–13. doi: 10.1016/j.jaci.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 20.D’Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol Med Microbiol. 2007;50(2):157–64. doi: 10.1111/j.1574-695X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 21.D’Elios MM, Codolo G, Amedei A, Mazzi P, Berton G, Zanotti G, et al. Helicobacter pylori, asthma and allergy. FEMS Immunol Med Microbiol. 2009;56(1):1–8. doi: 10.1111/j.1574-695X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 22.Kabir S. The current status of Helicobacter pylori vaccines: a review. Helicobacter. 2007;12(2):89–102. doi: 10.1111/j.1523-5378.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 23.Del Giudice G, Malfertheiner P, Rappuoli R. Development of vaccines against Helicobacter pylori. Expert Rev Vaccines. 2009;8(8):1037–49. doi: 10.1586/erv.09.62. [DOI] [PubMed] [Google Scholar]
- 24.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133(1):288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Rossi G, Ruggiero P, Peppoloni S, Pancotto L, Fortuna D, Lauretti L, et al. Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect Immun. 2004;72(6):3252–9. doi: 10.1128/IAI.72.6.3252-3259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malfertheiner P, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135(3):787–95. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 27.Griffin D. Measles virus. In: Knipe DM, Howley PM, editors. Field’s virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1401–41. [Google Scholar]
- 28.Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J, et al. Attenuated measles virus as a vaccine vector. Vaccine. 2007;25(16):2974–83. doi: 10.1016/j.vaccine.2007.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol. 2007;81(19):10597–605. doi: 10.1128/JVI.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desprès P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191(2):207–14. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 31.Guerbois M, Moris A, Combredet C, Najburg V, Ruffié C, Février M, et al. Live attenuated measles vaccine expressing HIV-1 Gag virus like particles covered with gp160DeltaV1V2 is strongly immunogenic. Virology. 2009;388(1):191–203. doi: 10.1016/j.virol.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 32.Liniger M, Zuniga A, Morin TN, Combardiere B, Marty R, Wiegand M, et al. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine. 2009;27(25–26):3299–305. doi: 10.1016/j.vaccine.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iankov ID, Hillestad ML, Dietz AB, Russell SJ, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17(8):1395–403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, et al. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14(23):5773–84. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73(11):9568–75. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell AM. Laboratory techniques in biochemistry and molecular biology. Vol. 23. Amsterdam: Elsevier; 1991. Monoclonal antibody and immunosensor technology. [Google Scholar]
- 37.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 38.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol. 2008;15(7):1054–9. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72(2):891–9. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz CJ, Hourcade D, et al. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72(9):7420–7. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iankov ID, Pandey M, Harvey M, Griesmann GE, Federspiel MJ, Russell SJ. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol. 2006;80(17):8530–40. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tulchinsky TH, Ginsberg GM, Abed Y, Angeles MT, Akukwe C, Bonn J. Measles control in developing and developed countries: the case for a two-dose policy. Bull World Health Organ. 1993;71(1):93–103. [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb RA, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field’s virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1305–40. [Google Scholar]
- 44.Kottakis F, Papadopoulos G, Pappa EV, Cordopatis P, Pentas S, Choli-Papadopoulou T. Helicobacter pylori neutrophil-activating protein activates neutrophils by its C-terminal region even without dodecamer formation, which is a prerequisite for DNA protection—novel approaches against Helicobacter pylori inflammation. FEBS J. 2008;275(2):302–17. doi: 10.1111/j.1742-4658.2007.06201.x. [DOI] [PubMed] [Google Scholar]
- 45.von Messling V, Milosevic D, Cattaneo R. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc Natl Acad Sci U S A. 2004;101(39):14216–21. doi: 10.1073/pnas.0403597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reyes-Del Valle J, Hodge G, McChesney MB, Cattaneo R. Protective anti-hepatitis B responses in Rhesus monkeys primed with a vectored measles virus and boosted with a single hepatitis B surface antigen dose. J Virol. 2009;83(17):9013–7. doi: 10.1128/JVI.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor JM, Ziman ME, Canfield DR, Vajdy M, Solnick JV. Effects of a Th1-versus a Th2-biased immune response in protection against Helicobacter pylori challenge in mice. Microb Pathog. 2008;44(1):20–7. doi: 10.1016/j.micpath.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132(3):1009–23. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 49.Rupnow MF, Chang AH, Shachter RD, Owens DK, Parsonnet J. Cost-effectiveness of a potential prophylactic Helicobacter pylori vaccine in the United States. J Infect Dis. 2009;200(8):1311–7. doi: 10.1086/605845. [DOI] [PubMed] [Google Scholar]
- 50.Rupnow MF, Shachter RD, Owens DK, Parsonnet J. Quantifying the population impact of a prophylactic Helicobacter pylori vaccine. Vaccine. 2001;20(5–6):879–85. doi: 10.1016/s0264-410x(01)00401-7. [DOI] [PubMed] [Google Scholar]
- 51.Low N, Kraemer S, Schneider M, Restrepo AM. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine. 2008;26(3):383–98. doi: 10.1016/j.vaccine.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Taylor JM, Ziman ME, Fong J, Solnick JV, Vajdy M. Possible correlates of long-term protection against Helicobacter pylori following systemic or combinations of mucosal and systemic immunizations. Infect Immun. 2007;75(7):3462–9. doi: 10.1128/IAI.01470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vajdy M, Singh M, Ugozzoli M, Briones M, Soenawan E, Cuadra L, et al. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology. 2003;110(1):86–94. doi: 10.1046/j.1365-2567.2003.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]