Abstract
Invasive fungal disease is a significant cause of morbidity and mortality in the neonate. The current study aims to assess the 1, 3-βD-Glucan (BG) assay in a prospective analysis in neonates with suspected fungaemia. A multicentre, prospective cohort study was conducted in Johannesburg, South Africa. The study included 72 neonates with clinically suspected late onset sepsis who were at high risk of fungaemia. A BG assay was performed on each patient and correlated with a sepsis classification based on the full blood count, C-reactive protein and blood culture results as no fungaemia, possible fungaemia, probable fungaemia or definite fungaemia. Sensitivity and specificity of the BG assay at levels of 60 pg/mL are 73.2% and 71.0% respectively and at levels of 80 pg/mL are 70.7% and 77.4% respectively. Positive and negative predictive values at 60 pg/mL are 76.9% and 66.7% respectively and at 80 pg/mL are 80.6% and 66.7% respectively. The area under the receiver operating curve is 0.753. The BG assay is a useful adjunct to the diagnosis of invasive fungal disease in neonates. It does, however, need to be considered in the context of the clinical picture and supplementary laboratory investigations.
Key words: neonate; invasive fungal disease; 1,3-βD-Glucan assay.
Introduction
Neonatal mortality contributes approximately 36% to under-5 deaths globally.1 In South Africa, the neonatal mortality rate is 21/1000 live births with 21% of these deaths being due to severe infections.2 The impact of neonatal sepsis is clearly a significant one. Fungaemia is becoming increasingly important as a cause and is associated with substantial morbidity and mortality in the preterm infant.3,4
Candida species rapidly colonize the skin and mucous membranes of critically ill neonates which can progress to invasive infection. This is particularly so in critically ill neonates who are at increased risk of fungal infection due to immature immune systems, increased permeability of skin and mucous membranes, parenteral nutrition, broad-spectrum antibiotics, central venous catheters, postnatal steroids and mechanical ventilation.4 This high-risk population could benefit greatly from early diagnosis. The diagnosis of sepsis in neonates, including invasive fungal disease, is difficult as the clinical presentation is often subtle and signs are often nonspecific.5 There is no single diagnostic test available to reliably confirm or refute sepsis on presentation. Currently diagnosis is based on a combination of clinical features and laboratory investigations such as the full blood count (FBC), C reactive protein (CRP), erythrocyte sedimentation rate (ESR) and procalcitonin (PCT), amongst others.5,6 Definitive diagnosis depends on a positive blood culture in keeping with clinical features and other laboratory investigations. Blood cultures are often negative in neonates even in the presence of sepsis.7 This is even more problematic with fungal infections as blood cultures are only positive in approximately 50% of cases of invasive candidiasis and less than 10% of invasive aspergillosis.8,9
1, 3-β-D-Glucan (BG) is a component of the outer wall of a number of fungi including Candida species, Aspergillus species and Pneumocystis jiroveci. The antigen is released into the bloodstream during invasive infection due to such fungi and can be detected by the Fungitell™ assay.10 This assay uses enzymes from the Limulus polyphemus amoebocyte lysate and removes bacterial endotoxin-sensitive factor C from the lysate to form a reagent. BG from patient serum binds to Factor G in the reagent and activates the horseshoe crab coagulation cascade allowing quantitative assay. Values >80 pg/mL are considered positive, 60–80 pg/mL are considered equivocal (repeat testing recommended) and values less than 60 pg/mL are considered negative.10 The sensitivity and specificity of the BG assay for invasive fungal infections is reported as 69.9% and 87.1% respectively.10 Studies to date are based on adult haematological, immunocompromised, cancer and surgical patients. Little information is available with regard to neonates. New diagnostic markers are needed to improve the early diagnosis of fungaemia in newborns. The current study comprised a prospective analysis to determine the usefulness of the BG assay for detection of fungal antigen in clinically suspected neonatal invasive fungal disease at three academic Hospitals in Johannesburg.
Objectives
To determine the 1, 3-β-D-Glucan assay in a prospective diagnostic analysis in neonates with suspected fungaemia. To correlate possible, probable or definite fungaemia with BG assay results and determine the sensitivity and specificity of the test in neonates.
Materials and Methods
Study design
A multicentre, prospective cohort study was conducted including 72 neonates admitted to the neonatal units at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), Rahima Moosa Mother and Child Hospital and Chris Hani Baragwanath Hospital in Johannesburg.
Patient selection
Patients with clinically suspected late onset sepsis, defined as onset of sepsis after 72 hours of life, were identified and classified as no or high risk for invasive fungal disease. Patients were regarded as high risk for invasive fungal disease and were eligible for inclusion in the study if they had 3 or more of the following criteria: i) Low birth weight (<2500 g); ii) Duration of hospitalisation > 3 weeks; iii) Prolonged invasive (intermittent positive pressure) ventilation or non-invasive (continuous positive pressure) ventilation (>1 week); iv) Systemic antibiotic exposure >72 hours, including poor clinical response to first or second line antibiotic therapy; v) Postoperative patients or patients with abdominal wall defects; vi) Central venous or arterial catheterisation >72 hours; vii) Received total parenteral nutrition (TPN); viii) Splenomegaly; ix) Persistent severe thrombocytopenia (platelet count <100,000/mm3 despite second line antibiotic treatment).
Patients on systemic antifungal therapy prior to investigation for new onset sepsis were excluded. Patients fulfilling the above criteria were investigated for sepsis as per unit protocol. This included blood investigations (FBC with platelet count, CRP and blood culture) as well as urine cultures, lumbar puncture and radiological investigations where indicated.
Laboratory investigations
The blood investigations included an FBC with platelet count, CRP and blood culture collected in a FAN blood culture bottle (as per laboratory standard operative procedures). In addition to these investigations, a sample of blood was drawn for a 1, 3-β-D-Glucan assay. The assay was performed using the Fungitell™ assay kit (Fungitell® Cape Cod) and was performed in the Microbiology Laboratory at CMJAH according to manufacturer instructions. The assay was performed in duplicate on each sample and the average of the results obtained was determined. In the case of extremely discrepant results, the results have been described as uninterpretable. Further investigations such as lumbar puncture and radiological investigations were performed at the discretion of the attending physician. Informed consent was obtained from the primary caregiver before inclusion in the study. This study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (approval number M090211).
Diagnostic criteria for invasive fungal disease
Based on the above investigations patients were categorised as one of the following: i) no fungaemia: a high index of suspicion for fungaemia including 3 or more risk factors as described above; blood culture negative; white cell count (WCC), platelet count (<100 000/mm3) and C-reactive protein (CRP) within normal limits; no evidence of colonisation with a fungal organism; OR definite bacterial sepsis; ii) possible fungaemia: a high index of suspicion for fungaemia including 3 or more risk factors as described above; blood cultures negative; no evidence of colonisation; two or more of the following present: abnormal WCC, low platelet count or elevated CRP (>10); iii) probable fungaemia: a high index of suspicion for fungaemia including 3 or more risk factors as described above PLUS evidence of colonisation with a fungal organism in the form of positive stool or non-sterile urine sample (collected by urine bag) for yeasts; blood cultures negative; and one or more of the following are present: abnormal WCC, low platelet count (<100 000/mm3) or elevated CRP (>10); iv) definite fungaemia: positive culture for a fungal organism from a normally sterile site (including blood or tissue culture). The BG results were correlated with the classification as described above, no fungaemia, possible fungaemia, probable fungaemia or definite fungaemia.
Statistical analysis
The sample comprised 72 neonates. Continuous variables (including birth weight and gestational age) were not normally distributed and have therefore been described as median and range. Categorical variables have been described using proportions. Sensitivity and specificity of the 1,3-β-D-Glucan assay have been determined and the positive and negative predictive values of the test have been calculated. The categories possible, probable and definite fungaemia were combined and considered diagnostic for fungaemia for the purpose of statistical analysis. The category no infection or confirmed bacterial infection were analysed as no fungaemia. Receiver operating curves have been used to further analyse the results.
Results
The initial sample included 79 neonates of which 72 formed part of the analysis. Of the seven potential candidates removed from the study, two caregivers refused consent, two samples were rejected at laboratory level (one was contaminated and the other was haemolysed), two samples had a laboratory error and one result was uninterpretable. The sample characterisitics are summarized in Table 1.
Table 1. Sample characteristics (n=72).
| Variable | Number (%) |
|---|---|
| Male | 45 (62.5%) |
| Female | 27 (37.5%) |
| Birth weight (g) | 1340 (720–4600)1 |
| Gestational age (weeks) | 31 (26–40)1 |
| Surgical intervention2 | 34 (47.2%) |
| IPPV3 | 61 (84.7%) |
| CPAP4 | 5 (6.9%) |
| HFOV5 | 3 (4.2%) |
| TPN6 | 49 (68.1%) |
| Central venous / arterial catheter | 30 (41.7%) |
| Previous antibiotic exposure | 72 (100%) |
| Empiric antifungal therapy | 18 (25%) |
Median and range;
Surgical diagnoses included NEC III, gastroschisis, omphalocoele, intestinal atresia/stenosis, intestinal perforation, imperforate anus, tracheosophageal fistula, colonic duplication cyst, congenital diaphragmatic hernia;
Intermittent positive pressure ventilation;
Continuous positive airway pressure;
High frequency oscillatory ventilation;
Total parenteral nutrition.
Correlation of sepsis category with blood culture results, evidence of colonisation and 1,3-βD-Glucan assay level are presented in Table 2. Fungal culture from a normally sterile site was positive in 10 cases (nine blood samples and one tissue sample). Four of the samples cultured Candida albicans, four cultured Candida parapsilosis, one cultured Sacchyromyces cerevisiae and one blood culture was reported as yeast but not identified. The lowest BG assay in cases with a positive fungal culture was 109 pg/mL with seven fungal culture positive cases having a BG assay >500 pg/mL. In the categories possible fungaemia and probable fungaemia, in which cultures from a normally sterile site remained negative and invasive fungal disease would previously not have been diagnosed, there were an additional 19 BG assay levels >80 pg/mL.
Table 2. Correlation of sepsis category with blood culture results and 1,3-βD-Glucan assay level (n=72).
| Sepsis category1 | n | Positive fungal culture2 | Positive Bacterial culture2 | Evidence of colonisation3 | 1,3-βD-Glucan assay level | ||
|---|---|---|---|---|---|---|---|
| <60 | 61–80 | >80 | |||||
| 1 | 31 | 0 | 13 | 7 | 22 | 2 | 7 |
| 2 | 22 | 0 | 0 | 2 | 7 | 1 | 14 |
| 3 | 9 | 0 | 2 | 9 | 4 | 0 | 5 |
| 4 | 10 | 10 | 1 | 3 | 0 | 0 | 10 |
Sepsis categories as described under methods;
Positive cuture from a normally sterile site (including blood or tissue culture);
Stool or nonsterile urine sample (urine bag specimen) positive for yeasts.
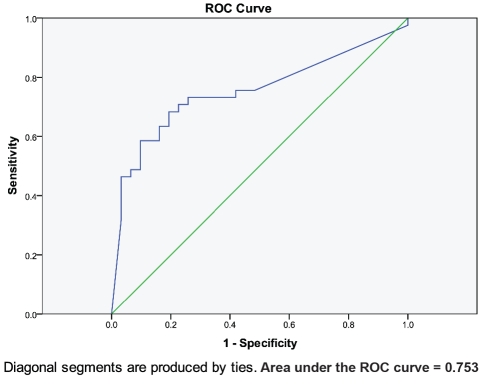
The sensitivity and specificity of the BG assay at levels of 60 pg/mL are 73.2% and 71.0% respectively and at levels of 80 pg/mL are 70.7% and 77.4% respectively. The positive and negative predictive values at 60 pg/mL are 76.9% and 66.7% respectively and at 80 pg/mL are 80.6% and 66.7% respectively. In addition, the area under the ROC curve is 0.753. Sensitivity, specificity and positive and negative predictive values calculated according to varying BG assay levels are presented in Table 3. ROC statistics and curve are presented in Figure 1.
Table 3. Sensitivity, specificity and positive and negative predictive values at varying 1,3-βD-Glucan assay levels (n=72).
| Positive if 1,3-βD-Glucan greater than or equal to | Sensitivity | Specificity | PPV1 | NPV2 |
|---|---|---|---|---|
| 60 | 0.732 | 0.710 | 0.769 | 0.667 |
| 80 | 0.707 | 0.774 | 0.806 | 0.667 |
| 150 | 0.585 | 0.839 | 0.828 | 0.605 |
| 200 | 0.512 | 0.903 | 0.875 | 0.583 |
| 500 | 0.317 | 0.968 | 0.923 | 0.517 |
Figure 1.
Receiver operating characteristic curve for the 1,3-βD-Glucan assay.
Discussion
The current study shows the 1,3-βD-Glucan assay to be a useful adjunct in the diagnosis of fungaemia in neonates. Existing recommendations for positivity and negativity of the assay appear to be appropriate for the neonatal period. Despite reasonable sensitivity and specificity of the 1,3-βD-Glucan assay, there remain a significant number of false positive and false negative results.
Potential causes of false positive reactivity include patients or specimens exposed to BG containing products (for example gauzes), patients undergoing haemodialysis with cellulose containing membranes, patients receiving intravenous immunoglobulin therapy and exposure to the antibiotic amoxicillin-clavulanic acid.11 Albumin, clotting factors and plasma protein concentrates are manufactured using filters containing high concentrations of BG and may lead to false positivity. In addition, the bacterial organisms Streptococcus pneumoniae and Alcaligenes faecalis are known to contain β-1,3-glucan in their cell walls and may also cause false reactivity.11 Other bacteria, specifically gram positive organisms and Pseudomonas aeruginosa, may also cause false positive reactivity.12,13
The early diagnosis and treatment of fungaemia, which has been shown to decrease mortality, is challenging.14 The blood culture as the gold standard of diagnosis is limited by delays of 18 to 72 hours to achieve positivity.8 In addition, only 50% of invasive candidiasis and less than 10% of invasive aspergillosis are blood culture positive. This curtails the potential survival benefit of earlier antifungal treatment. A reasonable clinical approach to the neonate with suspected fungaemia is to commence antifungal therapy based on a positive 1,3-βD-Glucan assay result (which can be obtained within 24 hours) whilst blood culture results are pending. In the case of a positive 1,3-βD-Glucan assay result and a negative blood culture result one needs to consider both the degree of positivity (for example1,3-βD-Glucan assay levels > 250 pg/mL have a specificity of 96.8%) and the presence of factors causing false positive reactivity. Results also need to be interpreted taking the infant’s clinical presentation into consideration.
One of the difficulties in the current study was the criteria used for diagnosing fungaemia. Use of the blood culture as gold standard would be ideal but the yield of positive blood cultures in neonatal sepsis is known to be low.7 This led to the use of alternative criteria for the diagnosis of fungaemia which, although unavoidable, may lead to inaccuracies in the analysis. A similar dilemma surrounding diagnostic criteria is demonstrated in a previous study by Odabasi et al.15 In this study sensitivity and specificity of the BG assay at a level of 60 pg/mL are reported as 100% and 90% respectively for proven or probable fungaemia and 70% and 96% respectively for proven, probable or possible fungaemia.
Limitations of the BG assay include the cost of the test (particularly important in a developing country such as South Africa) and the lack of species identification. A positive BG assay result indicates the presence of one of a number of possible fungi, including amongst others Candida species, the most common fungal isolate in neonates.3 It is, however, dependent upon the blood culture for identification and sensitivity of the causative organism. For this reason the BG assay may be of particular use in an epidemic or nosocomial outbreak setting.
Conclusions
The BG assay is a useful adjunct to the diagnosis of fungaemia in neonates with a predictive value of 0.753 on ROC analysis. BG varies in specificity, depending on the cut-off values used, due to a number of false positive results. The assay therefore needs to be considered in the context of the clinical picture and supplementary laboratory investigations. Ongoing research is required into further causes of false positive reactivity and the change in assay levels in response to treatment.
References
- 1.UNICEF. The State of the World's Children 2008: Child Survival. [Accessed 06/10, 2009];2008 Available from: http://www.unicef.org/publications/files/The_State_of_the_Worlds_Children_2008.pdf.
- 2.WHO. World Health Statistics 2006. [Accessed 31 January, 2009];2006 Available from: http://www.who.int/who-sis/en/.
- 3.Couto RC, Carvalho EA, Pedrosa TM, et al. A 10-year prospective surveillance of nosocomial infections in neonatal intensive care units. Am J Infect Control. 2007;35:183–9. doi: 10.1016/j.ajic.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman D, Boyle R, Hazen KC, et al. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–6. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 5.Arnon S, Litmanovitz I. Diagnostic tests in neonatal sepsis. Curr Opin Infect Dis. 2008;21:223–7. doi: 10.1097/QCO.0b013e3282fa15dd. [DOI] [PubMed] [Google Scholar]
- 6.Ballot DE, Perovic O, Galpin J, Cooper PA. Serum procalcitonin as an early marker of neonatal sepsis. S Afr Med J. 2004;94:851–4. [PubMed] [Google Scholar]
- 7.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17:638–80. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 9.Einsele H, Hebart H, Roller G, et al. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–60. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedzierska A, Kochan P, Pietrzyk A, Kedzierska J. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1-->3)-beta-D-glucan antigens. Eur J Clin Microbiol Infect Dis. 2007;26:755–66. doi: 10.1007/s10096-007-0373-6. [DOI] [PubMed] [Google Scholar]
- 11.Mennink-Kersten MA, Verweij PE. Non-culture-based diagnostics for opportunistic fungi. Infect Dis Clin North Am. 2006;20:711–27. doi: 10.1016/j.idc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Pickering JW, Sant HW, Bowles CA, et al. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–62. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mennink-Kersten MA, Ruegebrink D, Verweij PE. Pseudomonas aeruginosa as a cause of 1,3-beta-D-glucan assay reactivity. Clin Infect Dis. 2008;46:1930–1. doi: 10.1086/588563. [DOI] [PubMed] [Google Scholar]
- 14.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]