Abstract
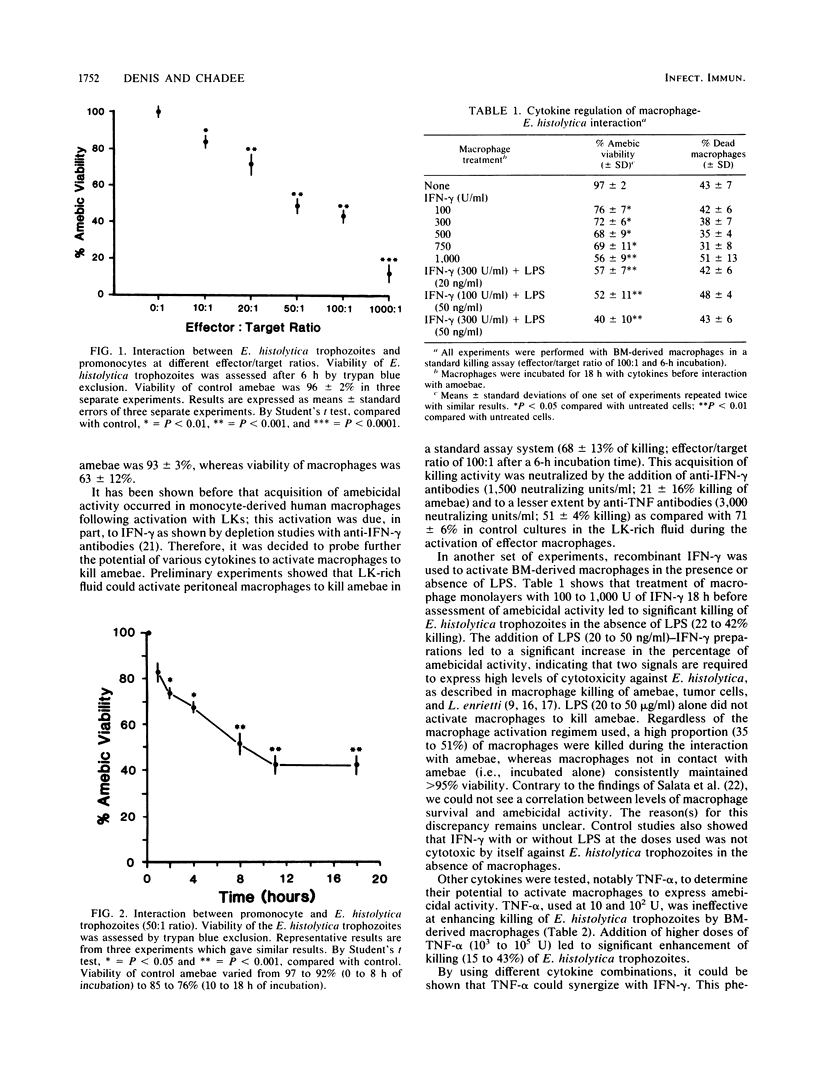
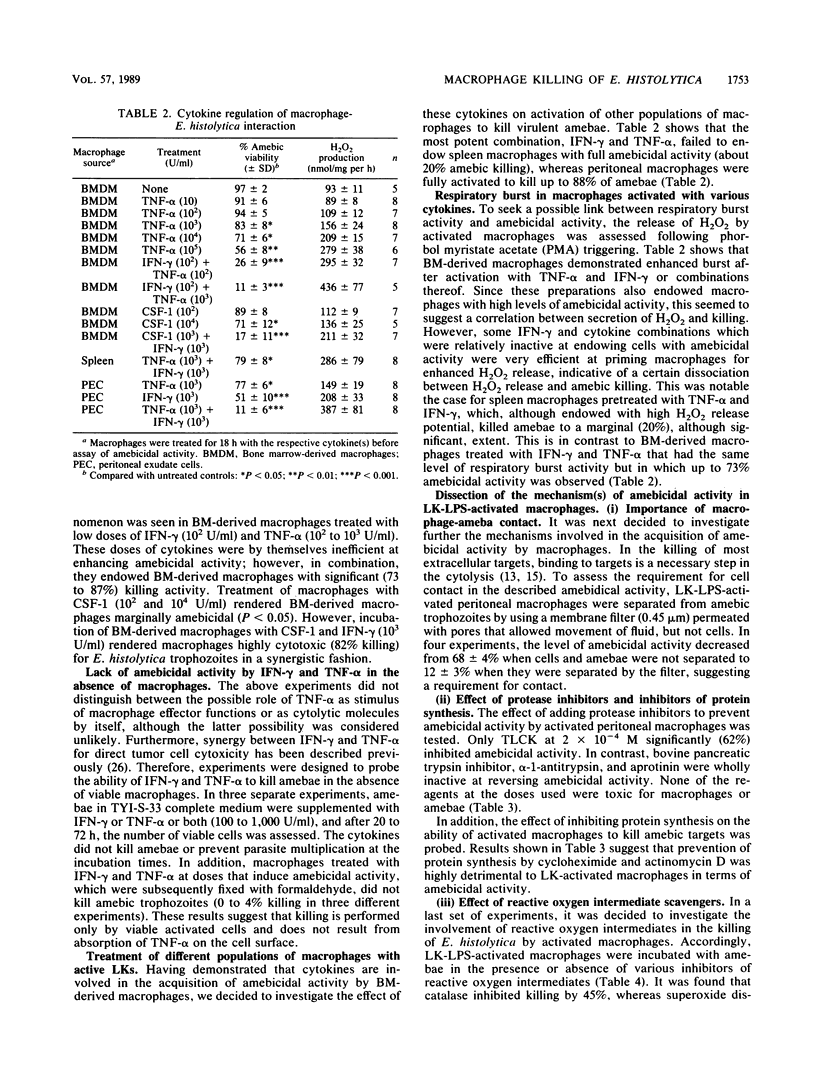
Macrophage-mediated effector mechanisms against the protozoan parasite Entamoeba histolytica were studied. Unstimulated macrophages were inefficient at killing E. histolytica trophozoites in vitro and were killed by the trophozoites. Conversely, immature cells of the mononuclear phagocyte lineage (promonocytes) were shown to display a strong spontaneous amebicidal activity. The acquisition of macrophage amebicidal activity following cytokine treatment was investigated. Gamma interferon, tumor necrosis factor alpha, and macrophage colony-stimulating factor 1, or combinations thereof, were shown to endow murine bone marrow-derived macrophages with significant amebicidal activity. Low doses of gamma interferon and tumor necrosis factor alpha and of gamma interferon and colony-stimulating factor 1 were shown to act synergistically in this phenomenon. This enhancement of amebicidal activity was shown to operate on bone marrow-derived macrophages, elicited peritoneal macrophages, and, to a much lesser extent, spleen macrophages. Although acquisition of amebicidal activity was associated with a strong respiratory burst, the addition of oxygen-free radical scavengers showed that the killing activity was approximately 45% H2O2 dependent. In addition, amebicidal activity by macrophages was shown to be contact dependent and was inhibited by 61% with the protease inhibitor tosyl lysyl chloromethyl ketone. Our results indicate that immunologic production of gamma interferon, tumor necrosis factor alpha, and colony-stimulating factor 1 could be important in the activation of macrophages for host defense against amebiasis and that promonocytes are strong effector cells against virulent amebae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Lohmann-Matthes M. L. In vitro natural cell-mediated cytotoxicity against Candida albicans: macrophage precursors as effector cells. J Immunol. 1985 Apr;134(4):2658–2665. [PubMed] [Google Scholar]
- Baccarini M., Hockertz S., Kiderlen A. F., Lohmann-Matthes M. L. Extracellular killing of Leishmania promastigotes and amastigotes by macrophage precursors derived from bone marrow cultures. J Exp Med. 1988 Apr 1;167(4):1486–1492. doi: 10.1084/jem.167.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E., Moreau F. In vitro and in vivo interaction between trophozoites of Entamoeba histolytica and gerbil lymphoid cells. Infect Immun. 1985 Sep;49(3):828–832. doi: 10.1128/iai.49.3.828-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Chadee K. In vitro and in vivo studies of macrophage functions in amebiasis. Infect Immun. 1988 Dec;56(12):3126–3131. doi: 10.1128/iai.56.12.3126-3131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Bout D. T. In vitro killing of Entamoeba histolytica trophozoites by interferon-gamma-activated mouse macrophages. Immunobiology. 1988 Mar;176(4-5):341–353. doi: 10.1016/s0171-2985(88)80018-4. [DOI] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E., Hartmann D. P. Protection against amebic liver abscess in hamsters by means of immunization with amebic antigen and some of its fractions. Am J Trop Med Hyg. 1980 Sep;29(5):779–784. doi: 10.4269/ajtmh.1980.29.779. [DOI] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E. Macrophage requirement for host defence against experimental hepatic amoebiasis in the hamster. Parasite Immunol. 1982 Jul;4(4):219–225. doi: 10.1111/j.1365-3024.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- James S. L., Glaven J. A. Effects of inhibitors of tumoricidal activity upon schistosomulum killing by activated macrophages. Infect Immun. 1987 Dec;55(12):3174–3180. doi: 10.1128/iai.55.12.3174-3180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. T., Warren M. K. CSF-1-induced resistance to viral infection in murine macrophages. J Immunol. 1987 May 1;138(9):3019–3022. [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. Interaction of Bacillus Calmette--Guérin-activated macrophages and neoplastic cells in vitro. I. Conditions of binding and its selectivity. Cell Immunol. 1980 Aug 15;54(1):11–25. doi: 10.1016/0008-8749(80)90185-9. [DOI] [PubMed] [Google Scholar]
- Mauël J., Buchmüller-Rouiller Y. Effect of lipopolysaccharide on intracellular killing of Leishmania enriettii and correlation with macrophage oxidative metabolism. Eur J Immunol. 1987 Feb;17(2):203–208. doi: 10.1002/eji.1830170209. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Salata R. A., Martinez-Palomo A., Murray H. W., Conales L., Trevino N., Segovia E., Murphy C. F., Ravdin J. I. Patients treated for amebic liver abscess develop cell-mediated immune responses effective in vitro against Entamoeba histolytica. J Immunol. 1986 Apr 1;136(7):2633–2639. [PubMed] [Google Scholar]
- Salata R. A., Murray H. W., Rubin B. Y., Ravdin J. I. The role of gamma interferon in the generation of human macrophages cytotoxic for Entamoeba histolytica trophozoites. Am J Trop Med Hyg. 1987 Jul;37(1):72–78. doi: 10.4269/ajtmh.1987.37.72. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Pearson R. D., Ravdin J. I. Interaction of human leukocytes and Entamoeba histolytica. Killing of virulent amebae by the activated macrophage. J Clin Invest. 1985 Aug;76(2):491–499. doi: 10.1172/JCI111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Ravdin J. I. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. II. Mitogenic activity for human lymphocytes. J Infect Dis. 1985 May;151(5):816–822. doi: 10.1093/infdis/151.5.816. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Ravdin J. I. Review of the human immune mechanisms directed against Entamoeba histolytica. Rev Infect Dis. 1986 Mar-Apr;8(2):261–272. doi: 10.1093/clinids/8.2.261. [DOI] [PubMed] [Google Scholar]
- Stern J. J., Graybill J. R., Drutz D. J. Murine amebiasis: the role of the macrophage in host defense. Am J Trop Med Hyg. 1984 May;33(3):372–380. doi: 10.4269/ajtmh.1984.33.372. [DOI] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl D. Immunology of Entamoeba histolytica in human and animal hosts. Rev Infect Dis. 1982 Nov-Dec;4(6):1154–1184. doi: 10.1093/clinids/4.6.1154. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Ampel N. M., Waheed A., Shadduck R. K. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol. 1985 Sep;135(3):2052–2056. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



