Abstract
Background:
Agenesis of the inferior vena cava (IVC) as a cause of recurrent deep vein thrombosis (DVT) is uncommon.
Case:
A 33-year-old male with no family history of thrombophilia, who had experienced multiple recurrent episodes of DVT over a 15-year period of unknown cause, was admitted into our hospital because of cellulitis in the right leg. Computer tomography with contrast of the abdomen showed an absence of IVC.
Conclusion:
Congenital absence of the IVC could be a rare risk factor for idiopathic DVT, especially in young individuals.
Keywords: deep vein thrombosis, agenesis, inferior vena cava
Background
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism, has an incidence of 1 to 3 per 1000 individuals per year in Western populations.1 Congenital anomalies of the inferior vena cava (IVC) are uncommon, and have been associated with the development of venous thrombosis of the lower limbs.2 Congenital anomalies of the IVC has been reported as a risk factor for DVT, especially in individuals <30 years old, and a concomitant thrombophilic disorder has been found in such individuals.3 We report a case of recurrent DVT in a 33-year-old man with agenesis of the IVC. The patient had experienced recurring episodes of idiopathic DVT in the right leg for 15 years.
Case
A 33-year-old man was admitted to the Internal Medicine Department, Holy Family Hospital, Nazareth, Israel, because of cellulitis in the right leg. One week prior to his admission, he complained about pain and increased local heat in the left ankle and thumb of the right leg. The patient had no history of previous trauma, surgery, insect bites, dysuria, or joint symptoms, and no family history of thrombophilia. He reported that he had (a) rheumatic fever without any complications when he was 19 years old, which was treated with penicillin, (b) been hospitalized when he was 23 years old because of infected skin ulcers on the right calf, for which he was treated by parenteral antibiotics, and (c) recurrent episodes of idiopathic DVT for the last 15 years. He also reported that he had not been treated with warfarin, but he had been on prophylactic enoxaparin therapy for DVT some years ago which has since been stopped and that he had been recently treated with allopurinol and colchicine for a presumed diagnosis of gout. He had been investigated several times for a primary hypercoagulability state, and the results were negative.
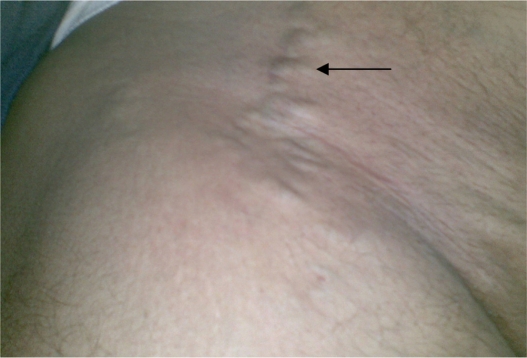
On examination, the most outstanding clinical findings were swelling of ankles, mild edema, redness, and increased temperature of the right ankle and calf with trophic skin changes (skin discoloration with ulcers), and superficial varicose veins in the lower abdomen (Figure 1). The clinical laboratory findings (erythrocyte sedimentation rate, leukocyte and platelet counts, and plasma hemoglobulin, plasma protein C, plasma protein S, fibrinogen, and antithrombin III levels), the results of the kidney and liver function tests, and resistance to activated protein C were all normal. Polymorphisms of the genes that encode for methylenetetrahydrofolate reductase were not detected, and the factor V Leiden and prothrombin mutations G20210A were absent. The results of the clinical immunological studies for complement C3 and C4 and rheumatoid factor were negative, and no circulating titers for antinuclear antibody, antineutrophil cytoplasmic antibody, and cardiolipin antibody were found. Cultures from the infected skin ulcers of the right leg were positive for methicillin-resistant Staphylococcus aureus (MRSA).
Figure 1.
Superficial varicose veins of the right lower abdomen.
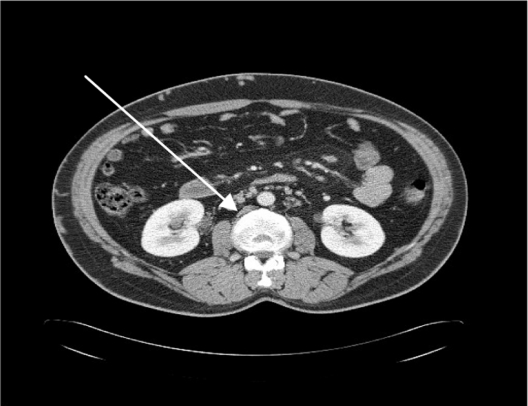
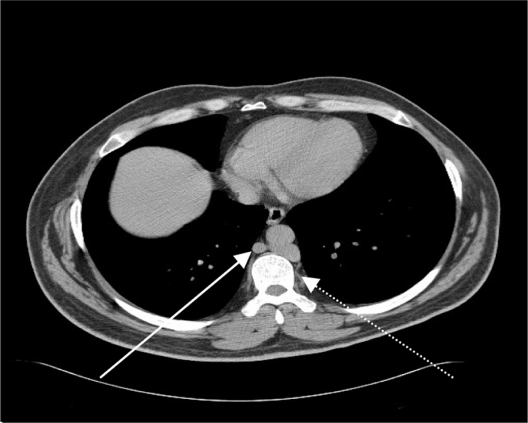
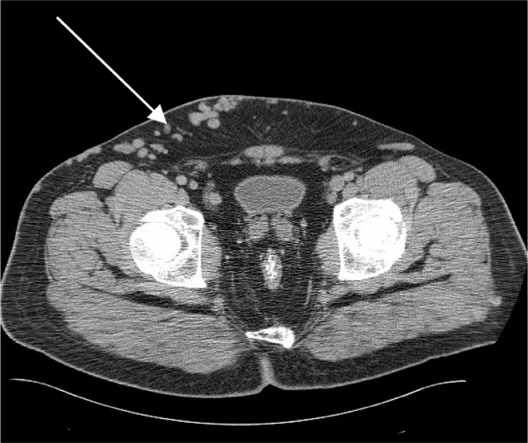
Ultrasound imaging of the leg veins showed a previous DVT in the right common femoral vein, and dilated superficial inguinal veins. Computer tomography with contrast of the abdomen showed agenesis of the infrarenal segment of the IVC (Figure 2) with dilated azygos and hemiazygos veins (Figure 3). There were also varicose veins in the abdominal wall and right groin, which were associated with dilated superficial and collateral veins (Figure 4). Transthoracic echocardiography of the patient’s heart revealed mild atrial enlargement and good systolic function of the left ventricle, and no pathological valvular flows. The patient was diagnosed as having agenesis of the infrarenal segment of the IVC and DVT of the right leg without concomitant risk factors for VTE. Since we attributed the agenesis of the IVC to be the underlying cause of the recurrent episodes of the DVTs, the patient was started on anticoagulant therapy (subcutaneous enoxaparin 160 mg/day) for DVT, antibiotic therapy (intravenous vancomycin 1.5 g/day for MRSA skin infection), and referred to a vascular surgeon specialist but the patient refused. At follow-up in the Internal Medicine Clinic, 3 months after his discharge, he reported that he was continuing with warfarin. The most outstanding clinical findings were swelling of left ankle, redness, with trophic skin changes, and a mild improvement of the skin ulcers. Despite several phone calls for follow-up, the patient rejects further treatment.
Figure 2.
A computer tomography scan with contrast of the abdomen which shows agenesis of the infrarenal segment of the inferior vena cava.
Figure 3.
A computer tomography scan of the abdomen which shows dilated azygos and hemiazygos veins (dashed arrow).
Figure 4.
A computer tomography scan of the abdomen which shows the superficial varicose veins of the right lower abdomen.
Conclusion
The normal IVC is composed of 4 segments: hepatic, suprarenal, renal, and infrarenal. Since many transformations can occur during the formation of the IVC, anomalies in their final form may occur. Such anomalies occur in 0.3% of otherwise healthy individuals, and in 0.6% to 2% of patients with other cardiovascular anomalies.4 Ruggeri et al reported 10 years ago 4 cases of congenital absence of the IVC in 75 young patients with idiopathic DVT over a 5-year period, and estimated that 5% of young patients with DVT had an anomaly of the IVC.5 Venous thrombosis is caused by the presence of isolated or combined risk factors. Almost 150 years ago, the nineteenth century pathologist Rudolf Virchow described 3 critically important causes of venous thrombosis: venous damage, coagulation defect(s), and venous stasis.6 Individuals with a congenital anomaly of the IVC are typically asymptomatic, and the anomaly is usually detected incidentally during radiological or abdominal procedures. Congenital absence of the IVC is infrequently associated with thromboembolic events.5 Patients who suffer from congenital anomalies of the IVC usually develop a compensatory circulation through the azygos veins or collateral abdominal veins in order to keep the venous return near normal levels.7
Most reported cases of congenital anomalies of IVC cases have been linked to thrombophilia disorders.3,5,7 However, the true prevalence of thrombophilia in congenital anomalies of the IVC is unknown because the screening for thrombophilia in patients with an IVC anomaly was usually incomplete.3 Anticoagulants, but not thrombolytic therapy, are usually prescribed for venous thrombosis, but the duration of the anticoagulant therapy is not well established. Hence, anticoagulant therapy for an indefinite duration will probably be prescribed, unless vascular reconstructive surgery is done on the anomalous IVC. Such surgery has been rarely reported, and its long-term outcome is undetermined.8 Congenital anomalies of the IVC may cause recurrent DVT, especially in young individuals.
Footnotes
Disclosure
The authors disclose no conflicts of interest.
References
- 1.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–1173. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 2.Vucicevic Z, Degoricija Y, Alfirevic Z, et al. Inferior vena cava agenesia and a massive bilateral iliofemoral venous thrombosis. Angiology. 2008;59:510–513. doi: 10.1177/0003319707305350. [DOI] [PubMed] [Google Scholar]
- 3.Gayer G, Luboshitz J, Hertz NM, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol. 2003;180:729–732. doi: 10.2214/ajr.180.3.1800729. [DOI] [PubMed] [Google Scholar]
- 4.Timmers GJ, Falke TH, Rauwerda JA, et al. Deep vein thrombosis as a presenting symptom of congenital interruption of the inferior vena cava. Int J Clin Pract. 1999;53:75–76. [PubMed] [Google Scholar]
- 5.Ruggeri M, Tosetto A, Castaman G, et al. Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet. 2001;357:441. doi: 10.1016/S0140-6736(00)04010-1. [DOI] [PubMed] [Google Scholar]
- 6.Markel A. Origin and natural history of deep vein thrombosis of the legs. Semin Vasc Med. 2005;5:65–74. doi: 10.1055/s-2005-871743. [DOI] [PubMed] [Google Scholar]
- 7.Obernosterer A, Aschauer M, Schnedl W, et al. Anomalies of the inferior vena cava in patients with iliac venous thrombosis. Ann Intern Med. 2002;136:37–41. doi: 10.7326/0003-4819-136-1-200201010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty MJ, Calligaro KD, DeLaurentis DA. Congenitally absent inferior vena cava presenting in adulthohood with venouse stasis and ulceration: a surgically treated case. J Vasc Surg. 1996;23:141–146. doi: 10.1016/s0741-5214(05)80044-8. [DOI] [PubMed] [Google Scholar]