Abstract
Background:
DLBS1442, a proprietary and standardized semipolar bioactive extract of the fruit Phaleria macrocarpa, is preclinically proven to have anti-inflammatory properties. The current clinical study evaluated the efficacy and safety of DLBS1442 in alleviating symptoms of premenstrual syndrome and primary dysmenorrhea.
Methods:
This was an open study over four menstrual cycles (with two control cycles, followed by two treatment cycles). Women with premenstrual syndrome and/or primary dysmenorrhea, 18–40 years of age, and with a regular menstrual cycle were included in the study. In the treatment cycles, DLBS1442 extract was given as a 100 mg capsule two or three times daily (for those with mild and moderate-to-severe baseline abdominal pain, respectively), for an average of six days, ie, three days before until the end of the first three days of the menstrual period. Throughout all four study cycles, daily self-assessment of symptoms related to premenstrual syndrome was made by each subject using a visual analog scale (VAS). Data were descriptively analyzed and profiled in curves of VAS score versus time point evaluation starting from day 5 before menstruation to day 5 of menstruation.
Results:
Twenty-three subjects of mean age 27.35 ± 5.73 years were evaluable for the intention to treat analysis. Most subjects experienced the primary efficacy variable (abdominal pain), peaking on days 1–2 of the menstrual phase, with a mean VAS score of 36.8 ± 24.3 mm and 30.0 ± 29.6 mm, respectively, during control cycles. DLBS1442 markedly reduced VAS scores by 13.76 ± 28.27 mm (37.46%) and 13.28 ± 29.06 mm (44.28%), respectively. Other symptoms of premenstrual syndrome were also markedly alleviated by DLBS1442. Some mild adverse events were observed and resolved by the end of the study.
Conclusion:
This preliminary study indicates the effectiveness of DLBS1442 in alleviating primary dysmenorrhea and abdominal pain, as well as other symptoms related to premenstrual syndrome. DLBS1442 was safe and well tolerated in women with premenstrual syndrome and/or dysmenorrhea.
Keywords: Phaleria macrocarpa, DLBS1442, premenstrual syndrome, dysmenorrhea
Introduction
Premenstrual syndrome and dysmenorrhea affect a large proportion of women of child-bearing age. Premenstrual syndrome is characterized by consistent recurrence of emotional and physical symptoms during the luteal phase of the menstrual cycle. It has been reported that about 85% of women of reproductive age experience pre-menstrual symptoms.1 About 3%–8% of women meet the criteria of more disabling and severe symptoms, manifesting as premenstrual syndrome and premenstrual dysphoric disorder, in which emotional (mood-related) symptoms predominate.2 In both premenstrual syndrome and premenstrual dysphoric disorder, symptoms abate rapidly at the onset of menses or shortly afterwards. Because no tests can confirm premenstrual syndrome or premenstrual dysphoric disorder, the diagnosis is made on the basis of a patient-completed daily symptom calendar and the exclusion of other medical disorders.3 Treatment is necessary for women with premenstrual syndrome and premenstrual dysphoric disorder because of the degree of incapacity and distress experienced, which often negatively affects quality of life.1,3
Most women with premenstrual syndrome also have to cope with abdominal cramping, which is closely associated with primary dysmenorrhea. Primary dysmenorrhea is defined as cramping pain in the lower abdomen occurring just before or during menstruation, in the absence of other pelvic disease, such as endometriosis. Prevalence rates are as high as 90%. Initial presentation of primary dysmenorrhea typically occurs in adolescence.4 Women with primary dysmenorrhea have higher levels than nondysmenorrhoeic women of endometrial prostaglandins F2-α and E2 and leukotrienes, resulting in increased uterine smooth muscle tone and stronger, more frequent uterine contractions, as well as modification of local blood flow, which taken together are responsible for the painful abdominal cramps.4,5
Lifestyle changes, including reducing stress and having a balanced healthy diet, including caffeine restriction, are often recommended to prevent premenstrual symptoms from worsening.1,6 However, to date, pharmacotherapy remains the most reliable and effective treatment for abdominal pain related to premenstrual syndrome or primary dysmenorrhea. Nonsteroidal anti-inflammatory drugs are the mainstay of treatment, with the addition of the oral contraceptive pill when necessary.4,7
In search of a more natural treatment for premenstrual syndrome and dysmenorrhea with a potentially better safety profile than a synthetic drug substance, we developed DLBS1442, a proprietary and standardized semipolar bioactive extract of the fruit Phaleria macrocarpa. This fruit contains tannin, terpenoids, flavonoids, alkaloids, and saponins, some of which have been proven preclinically to have antioxidant, anti-inflammatory, antiproliferative, and anticancer properties.8,9 Fresh P. macrocarpa fruit has a tannin content four times higher than older fruit. As a traditional medicine, P. macrocarpa has been used to treat various acute illnesses, including rheumatism, due to its analgesic, antipyretic, and anti-inflammatory activities. It is often also used in combination with other herbs to treat diabetes, hypertension, and liver disorders.10
Our previous report indicated that DLBS1442 has an anti-inflammatory effect, exerted via selective inhibition of cyclooxygenase (COX)-2, an inducible prostaglandin synthase.11 Abdominal cramping related to premenstrual syndrome resulting from uterine vasoconstriction, anoxia, and contractions, is mediated by prostaglandins. Therefore, symptomatic relief may be obtained from the use of agents that inhibit prostaglandin synthesis and have anti-inflammatory properties, so hypothetically DLBS1442 could reduce menstruation-related abdominal cramp. We conducted the present preliminary clinical study to explore this hypothesis, and evaluated the anti-inflammatory effect of DLBS1442, indicated by abdominal pain relief, in women with premenstrual syndrome and/or primary dysmenorrhea. Other symptoms commonly observed in premenstrual syndrome were also evaluated as secondary endpoints. Along with efficacy, the study also evaluated the safety of DLBS1442 in human subjects.
Materials and methods
Identification of DLBS1442
DLBS1442 is a dried bioactive extract of P. macrocarpa fruit collected in Cikarang, West Java, Indonesia, and has been identified by Herbarium Bogoriense, Research Center of Biology, Indonesian Institute of Sciences. A quantity of ripe P. macrocarpa was powdered using a disk-mill machine, then macerated with methanol for 15 minutes, and percolated for 45 minutes at 50°C. Thereafter, it was evaporated and dried using a spray dryer, resulting in DLBS1442, a dry powder with a drug to extract ratio of 1:10.
The identification of DLBS1442 was done using thin layer gel 60 F254 with chloroform/methanol chromatography SiO2 in a ratio of 9:1 as the mobile phase. The eluent was allowed to move along the thin layer chromatography plate for a distance of 8 cm. The chromatogram was then observed under ultraviolet light at 254 nm and 366 nm. DLBS1442 was identified using a high-pressure liquid chromatography Waters (Watertown, MA) 1525 binary pump with a Waters 2487 dual λ absorbance as the detector, and a Waters SunFireTM C18 (4.6 mm × 250 mm, 5.0 μm) as the column. Absorbance was measured at 290 nm. The gradient elution used was methanol and acetic acid 0.01% with a flow rate of 1 mL/min, with a change in ratio at minutes 3, 15, 17, and 20. Thin layer chromatography and high-performance liquid chromatography showed various organic compounds, but the major compound, having an Rf of 0.6 and a retention time of 10.096 minutes, was believed to be benzophenone glycoside.
Clinical study
The clinical study was undertaken between November 2009 and May 2010, and was conducted in accordance with the principles of the Declaration of Helsinki12 and Good Clinical Practice.13 Written informed consent was obtained from each subject prior to participation in the study, which spanned four menstrual cycles. The first and second (control) cycles profiled the baseline severity of premenstrual symptoms, while the third and fourth (treatment) cycles evaluated changes in severity of premenstrual symptoms after treatment. Two consecutive cycles were included in each study period (control and treatment) to meet the minimum number of menstrual cycles needed to obtain the mean as well as consistency of baseline and post-treatment values. Severity of premenstrual symptoms was stratified into mild, moderate, and moderate-to-severe, using a visual analog scale (VAS) evaluation of physical and mood symptoms. For each symptom, a subject was judged to suffer only mildly from that symptom if the mean baseline VAS score was 0–50, moderately if 50–75, and moderately-to-severely if >75.
All volunteers met the inclusion criteria, ie: age 18–40 years, a regular menstrual cycle (22–35 days per cycle), a history of abdominal pain in almost every menstrual cycle since a few months or years after menarche (primary dysmenorrhea), and at least one affective and one somatic symptom in the 5 days before onset of menstruation in two consecutive cycles. The affective symptoms included depression, explosive anger, irritability, tension, unhappiness, anxiousness, feelings of isolation, and mood swings; somatic symptoms included abdominal pain, breast tenderness, bloating, headache, back pain, and swelling of arms and/or legs. Diagnosis of primary dysmenorrhea was confirmed by the investigator (a gynecologist), using physical examination, abdominal ultrasonography, and evaluation of surgical history, as well as a daily subjective self-assessment questionnaire.
Subjects were excluded if they were: suffering from amenorrhea, secondary dysmenorrhea, or endometriosis; had liver, kidney, or other disease, which, in the investigator’s opinion, could interfere with trial participation or evaluation (eg, anemia, anorexia/bulimia, diabetes mellitus, thyroid disease, mental disease); planning to become pregnant; already taking a drug/product regularly to relieve premenstrual syndrome, especially analgesic drugs (except for rescue therapy, ie, paracetamol); on contraception/hormonal or antidepressant therapy; substance abusers; or participating in any other clinical trial during the course of this study.
No trial medication was given during the control cycles. Each eligible subject was given a subject identification number and instructed to avoid using other medication, supplements, and/or herbal medicines to alleviate premenstrual and menstrual symptoms during the study period. If the subjects could not endure their period pain, they were allowed to use rescue pain relief, ie, paracetamol 500 mg, as needed. The number of paracetamol tablets used by each subject was recorded. In the treatment cycles, DLBS1442 was given as one 100 mg capsule either three times daily (for those with moderate-to-severe baseline abdominal pain) or two times daily (for those with mild baseline abdominal pain), for an average of six days, ie, the last three days of the luteal phase until the first three days of the menstrual phase. Because the study subjects had regular menstrual cycles, they were expected to be able to predict their next menstrual days, and so were instructed to take the investigational product within the scheduled six days. The dose of 100 mg was calculated based on the result of our preclinical studies of DLBS1442.11 The most effective and safe dose providing an anti-inflammatory effect was then converted into a daily dose for human subjects.
Throughout the four study cycles, a daily self-assessment was made by each subject for the following 20 premenstrual symptoms: abdominal pain, breast tenderness, headache, back pain, myalgia, nausea, weight gain, overeating, insomnia, fatigue, lack of concentration, irritability, tenseness, unhappy feelings, mood swings, anxiousness, depression, anger, tearfulness, and social conflict. Subjects recorded scores for each item using a VAS, ranging from 0 mm (no symptoms) to 100 mm (unbearable symptoms), in a symptom diary. VAS is a validated tool for rapid and simple assessment of the effect size of pain-related symptoms in community practice.14
The primary efficacy endpoint was reduction of VAS for abdominal pain from baseline to study end. Secondary efficacy endpoints were reductions in VAS from baseline to study end for each of the remaining 19 symptoms. The data were analyzed descriptively and profiled in a curve of VAS versus time point evaluation starting from day 5 before menstruation (luteal phase) to day 5 of menstruation (menstrual phase). The analysis was based on the intention-to-treat population, which included all women with complete baseline values and at least one treatment cycle value, who had taken at least one dose of the investigational product.
The safety analysis included all exposed patients who had received at least one dose of the investigational product. The safety of DLBS1442 was evaluated descriptively for all adverse events occurring during the study. Because this was a preliminary clinical study, a sample size calculation was not required. Nonetheless, we recruited 20–30 healthy subjects who had abdominal pain during their menstrual period.
Results
Subject characteristics
A total of 27 eligible subjects who had given their written informed consent were enrolled in the study. Of these, only 23 met the criteria for the intention-to-treat efficacy analysis, having records for both control cycles and at least one treatment cycle. The other four subjects were not included in the analysis because of insufficient data (no available record of a treatment cycle). The subject demographics are summarized in Table 1. Their age range was 20–40 years. Age at menarche was 11–16 years, and symptoms of premenstrual syndrome were first experienced at the age of 11–21 years. Menstrual cycle length was 21–32 days, with a menstrual bleeding duration of 3–7 days.
Table 1.
Demographic data of study population
| Variables (n = 23) | Mean (SD) |
|---|---|
| Age at presentation (years) | 27.35 (5.73) |
| Age of menarche (years) | 12.70 (1.46) |
| Age when PMS first experienced (years) | 15.91 (2.78) |
| Length of menstrual cycle (days) | 28.17 (2.96) |
| Length of menstrual period (days) | 3.91 (1.12) |
| Body weight (kg) | 55.56 (9.11) |
Abbreviations: SD, standard deviation; PMS, premenstrual syndrome.
Efficacy
Primary efficacy endpoint
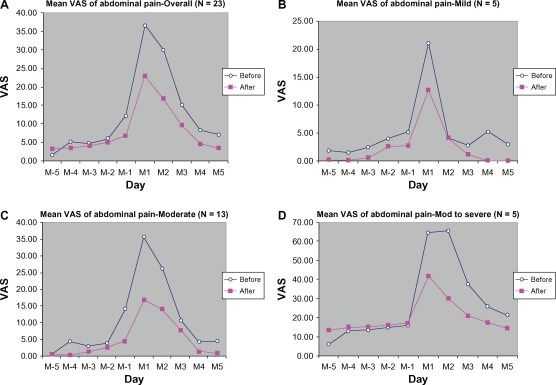
Abdominal pain was markedly reduced during the treatment period compared with the control period (Figure 1). A marked reduction was observed at one day before menstruation (M-1), and at day 1 (M1) and day 2 of the menstrual period (M2), with a VAS reduction of 5.52 ± 11.56 mm (45.50%), 13.76 ± 28.27 mm (37.46%), and 13.28 ± 29.06 mm (44.28%), respectively, from baseline.
Figure 1.
Mean VAS of abdominal pain from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Abbreviation: VAS, visual analog scale.
The reductions were even more marked when we stratified the subjects according to severity of baseline abdominal pain. The peak VAS score for abdominal pain was reduced from baseline by 8.40 ± 20.19 mm (39.80%), 19.08 ± 19.28 mm (53.28%), and 35.70 ± 52.68 mm (54.42%), respectively, for the mild, moderate, and moderate-to-severe groups. Most of the subjects had mild abdominal pain (n = 13), with some having moderate (n = 5) or severe (n = 5) abdominal pain.
Secondary efficacy endpoints
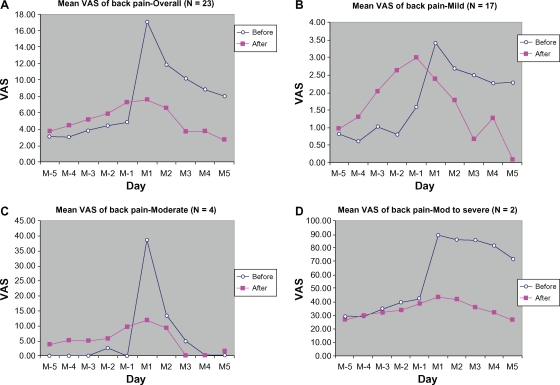
Back pain was also markedly relieved during treatment cycles as compared with control cycles (Figure 2). There were much lower VAS scores at day 1 and day 3 (M3) of the menstrual phase. The reductions from baseline of VAS scores for back pain were 9.41 ± 22.89 mm (55.23%), 5.26 ± 19.69 mm (44.49%), and 6.52 ± 22.20 mm (64.24%), respectively, for M1, M2, and M3. Stratified group analyses showed no difference between before and after treatment for the mildly symptomatic group (Figure 2A–2D). However, the groups with moderate and moderate-to-severe back pain showed marked symptom reduction. The baseline back pain score in the moderate group peaked at M-1 and decreased by 26.88 ± 9.24 mm (69.35%) after treatment; in the group with moderate-to-severe back pain, a decrease in VAS score of 47.20 ± 68.24 mm (56.99%) was recorded at M1. Most of the subjects had mild back pain (n = 17), and some had moderate (n = 4) or severe (n = 2) back pain.
Figure 2.
Mean VAS of back pain from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Abbreviation: VAS, visual analog scale.
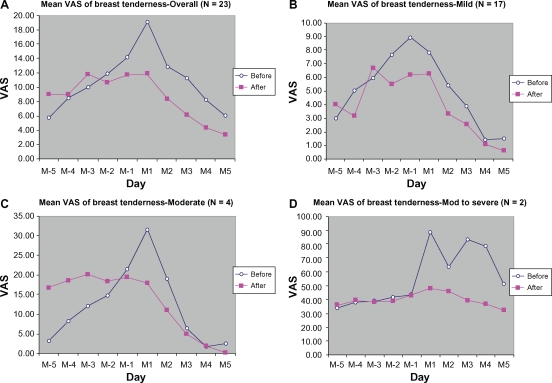
An obvious reduction in breast tenderness was observed in subjects with moderate and moderate-to-severe symptoms (Figure 3). In the moderately symptomatic group, the VAS peak score for breast tenderness (at M1) decreased from 31.63 ± 4.25 mm during the control cycle to 17.88 ± 9.42 mm in the active treatment cycle, representing a 43.48% reduction in breast tenderness as a result of treatment with DLBS1442. In the moderate-to-severe symptom group, the peak VAS score decreased from 89.00 ± 5.66 mm to 42.69 ± 67.88 mm (a 52.03% reduction from baseline).
Figure 3.
Mean VAS of breast tenderness from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Abbreviation: VAS, visual analog scale.
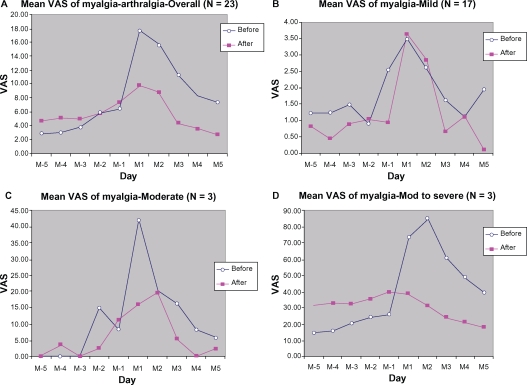
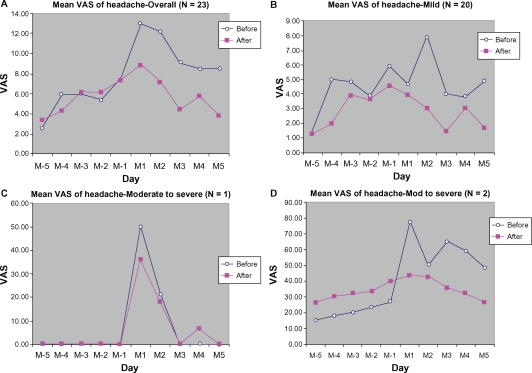
Overall, the data analysis for myalgia-arthralgia (Figure 4) as well as for headache (Figure 5) demonstrated lower VAS scores during the treatment cycle than during the control cycle. Stratified analysis based on symptom severity at baseline also showed symptomatic relief during the treatment cycle.
Figure 4.
Mean VAS of myalgia-athralgia from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Notes: *Marked changes, before versus after.
Abbreviation: VAS, visual analog scale.
Figure 5.
Mean VAS of headache from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Abbreviation: VAS, visual analog scale.
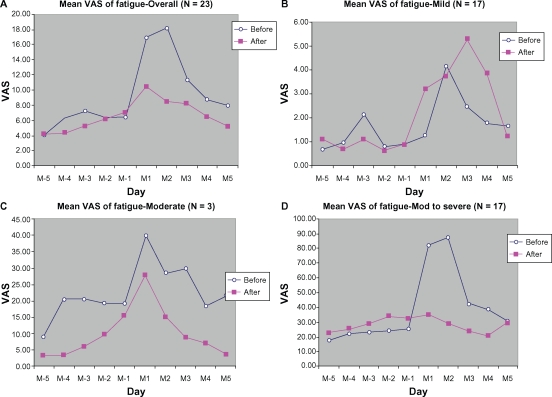
The overall VAS score for fatigue during the menstrual phase was slightly lower during the treatment cycle than in the control cycle. Stratified analysis according to symptom severity at baseline also demonstrated better scores during the treatment cycle (Figure 6).
Figure 6.
Mean VAS of Fatigue from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5): (A) overall, (B) mild, (C) moderate, (D) moderate-to-severe.
Abbreviation: VAS, visual analog scale.
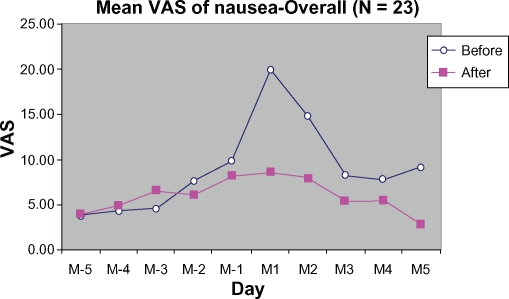
There were markedly lower VAS scores for nausea at M1 during the treatment cycle than for the control cycle. The nausea score was reduced from 20.07 ± 25.55 mm to 8.59 ± 20.37 mm, representing a reduction of 57.20%. No stratification was done for nausea (Figure 7).
Figure 7.
Mean VAS of nausea symptoms from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
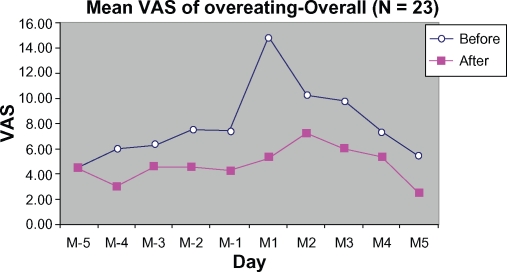
DLBS1442 also markedly lowered VAS scores as observed in the first two days of the premenstrual phase and the first day of the menstrual phase (M-1, M-2, and M1, respectively). The peak score for the overeating symptom was reduced at M1 by 64.46% (Figure 8) compared with the control cycle. No stratification was done for this symptom.
Figure 8.
Mean VAS of overeating from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
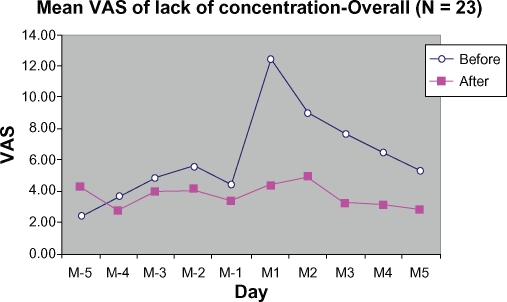
As compared with the control cycle, scores for lack of concentration during the treatment cycle were greatly reduced on day 1 and day 2 during the menstrual phase (M1 and M2), by 64.97% and 45.03%, respectively (Figure 9).
Figure 9.
Mean VAS of lack-of-concentration from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
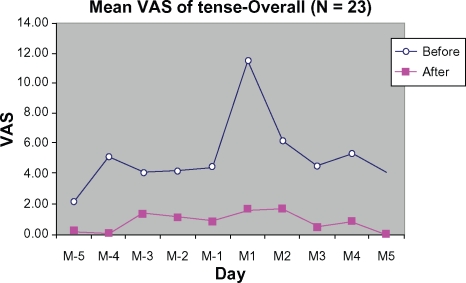
Figure 10 demonstrates improvement in relief of tension during the treatment cycle compared with the control cycle. The peak score for tension (at M1) during the treatment cycle was reduced by 9.78 ± 26.37 mm (85.55%) from baseline (control).
Figure 10.
Mean VAS of tenseness from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
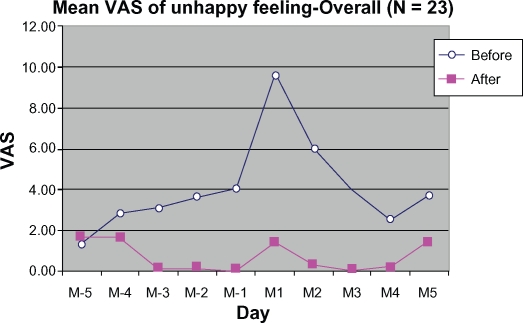
Figure 11 shows improvement in relief of unhappy feelings during the treatment cycle as compared with the control cycle. Significant reduction was observed at M-1, M2, and M3 by 4.07 ± 14.25 mm (100%), 5.74 ± 24.53 mm (94.97%), and 4.00 ± 15.52 mm (100%), respectively.
Figure 11.
Mean VAS of unhappy feeling 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
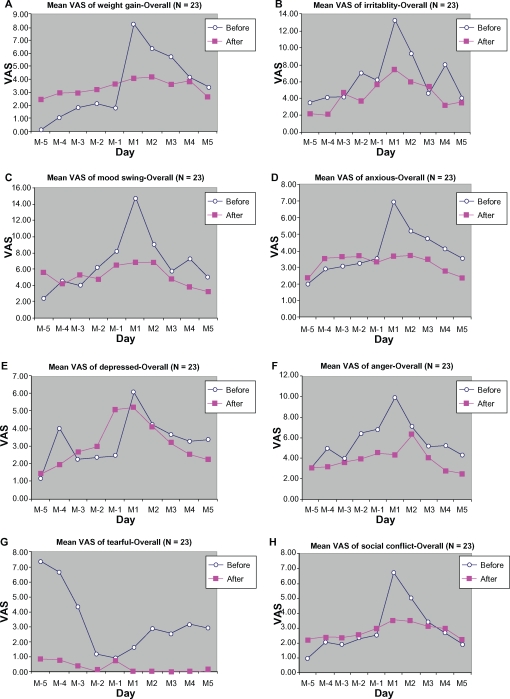
Other symptoms (weight gain, irritability, mood swings, anxiousness, depression, anger, tearfulness, and social conflict) were slightly improved by treatment with DLBS1442 (Figure 12). All symptoms, both somatic and affective, abated in all subjects during the follicular phase of menstruation, and were even absent in some subjects.
Figure 12.
Mean VAS of weight gain (A), irritability (B), mood swings (C), anxiousness (D), depression (E), anger (F), tearfulness (G), and social conflict (H), from 5 days before (M-5 to M-1) to 5 days during menstruation (M1 to M5).
Abbreviation: VAS, visual analog scale.
Overall, the administration of DLBS1442 for three days before and for three days during menstruation relieved abdominal pain, other pain-related symptoms, and some physiological symptoms experienced by subjects with pre-menstrual syndrome and/or dysmenorrhea.
Safety
Few adverse events were reported, and none were clinically important. Patient reports of adverse events are listed in Table 2. The most common events possibly related to DLBS1442 were diarrhea and dyspepsia.
Table 2.
Reported adverse events
| Description | AE (n) | Respondents with AE (n) |
% AE numbers |
|
|---|---|---|---|---|
| Based on total respondents with AE | Based on total number of AEs | |||
| Constipation | 1 | 1 | 12.5 | 3.7 |
| Diarrhea | 6 | 4 | 75.0 | 22.2 |
| Pharyngitis | 2 | 2 | 25.0 | 7.4 |
| Fever | 2 | 2 | 25.0 | 7.4 |
| Arthritis | 1 | 1 | 12.5 | 3.7 |
| Dizziness | 2 | 2 | 25.0 | 7.4 |
| Dyspepsia | 4 | 3 | 50.0 | 14.8 |
| Influenza | 3 | 3 | 37.5 | 11.1 |
| Common cold | 1 | 1 | 12.5 | 3.7 |
| Cough | 2 | 2 | 25.0 | 7.4 |
| Typhoid | 2 | 2 | 25.0 | 7.4 |
| Mild urticaria | 1 | 1 | 12.5 | 3.7 |
| Total events = 27 | Total respondents = 8 | |||
Abbreviation: AE, adverse event.
Discussion
Abdominal pain experienced by the study subjects during the menstrual phase (day 1 and day 2 of menstrual period) in the DLBS1442 treatment cycles was much alleviated compared with control (baseline) cycles. It is plausible that palliation of symptoms was primarily due to the anti-inflammatory properties of DLBS1442. Our previous study has shown that P. macrocarpa downregulates the phosphoinositide 3-kinase/AKT pathway which further downregulates the eicosanoid pathway.9,15 VAS reductions for abdominal pain on day 1 and day 2 of the menstrual period were 13.76 ± 28.27 mm (37.46%) and 13.28 ± 29.06 mm (44.28%), respectively. Such a magnitude of reduction has significant practical importance. Regardless of baseline pain severity, the difference in VAS pain score (on a 100 mm scale) should be 9–14 mm at a minimum to be considered clinically significant.16–18 It has also been reported that the minimal clinically significant difference in VAS pain score does not differ with gender, age, or cause of pain.17
Subgroup analysis in the mild, moderate, and moderate-to-severe groups (each with peak VAS abdominal pain scores of <25 mm, 25–50 mm, and >50 mm, respectively) demonstrated that in cycles during which the subjects were treated with DLBS1442, the peak scores were much lower than those of baseline cycles. The reductions in peak score for the subgroups with mild, moderate, and severe abdominal pain were 8.40 ± 20.19 mm (39.80% lower from baseline), 19.08 ± 19.28 mm (53.28%), and 35.70 ± 52.68 mm (54.42%), respectively. Given the minimal clinically significant difference in VAS pain score being 9–14 mm, the reductions in the moderately severe and severe groups were clinically significant, but not so in the mild group. This is not surprising, because the more severe the baseline pain, the greater the magnitude of pain reduction.
Abdominal pain in premenstrual syndrome and primary dysmenorrhea is caused by increased tone and contractions in the musculature of the uterus, which is brought about by increased release of endometrial prostaglandins.4 Nonsteroidal anti-inflammatory drugs have long been used as the treatment of choice for primary dysmenorrhea due to their mode of action as prostaglandin synthesis inhibitors. The most studied nonsteroidal anti-inflammatory drugs in women with premenstrual syndrome are mefenamic acid and naproxen sodium.19 DLBS1442 similarly inhibits the transformation of arachidonic acid into prostaglandins by suppressing COX-2 activity,11 and hence may also alleviate pain related to premenstrual syndrome, especially abdominal pain, as demonstrated in our study. In addition, DLBS1442 seemed to relieve the mastalgia accompanying breast tenderness (Figure 3), a feature that nonsteroidal anti-inflammatory drugs do not appear to have, even though they may alleviate a wide range of symptoms of premenstrual syndrome.7
In addition to palliation of abdominal pain, other pains related to premenstrual syndrome were also relieved by DLBS1442, including back pain (9.41 ± 22.89 mm [55.23%] reduction in peak score) and fatigue (9.65 ± 26.72 mm [53.30%] reduction in peak score). While headache and myalgia/arthralgia were also consistently alleviated, the reductions were not of clinical importance because they were less than 9 mm. Such a slight reduction was again dependent on the baseline severity of symptoms, which in our study was only mild (13.04 ± 23.44 mm and 17.63 ± 22.62 mm for headache and myalgia/arthralgia, respectively). However, subgroup analyses for the mild, moderate, and moderate-to-severe groups demonstrated that the peak score reduction for each of the pain-related symptoms (back pain, fatigue, headache, and myalgia/arthralgia) was greater and more of clinical importance in the moderate and moderate-to-severe pain groups (14–83 mm VAS reduction from baseline) than in the mild group (<4 mm VAS reduction from baseline).
Abdominal pain, back pain, myalgia/arthralgia, headache, and fatigue are all symptoms related to pain and inflammation that can be reduced by prostaglandin suppression. Alleviation of these symptoms also indicates the anti-inflammatory activity of DLBS1442. In addition, there is emerging evidence suggesting that hormonal therapies may help to relieve menstrual-related migraine.20 Our previous in vitro study of DLBS1442 demonstrated its effect on the estrogen receptor,21 which is likely to account for the activity of the extract in relieving headache related to premenstrual syndrome.
Premenstrual nausea that usually accompanies abdominal cramping was also ameliorated by DLBS1442. The nausea VAS score peaking at menstrual day 1 was reduced by 11.48 ± 23.80 mm from baseline (20.07 ± 25.55 mm). However, such a reduction was not clinically significant because the minimal clinically significant difference in nausea should be 15 mm on a VAS.22
Breast tenderness is usually associated with an imbalance in female hormones, ie, estrogen, progesterone, and prolactin. Monthly changes in levels of those hormones may cause fluid retention in the breast, experienced as a sensation of breast fullness and pain.6 DLBS1442 ameliorated this symptom, and particularly so in a subgroup of moderately severe cases. In the latter group, DLBS1442 reduced the peak VAS score by up to 44 mm from baseline. Consistent with this finding, other symptoms which were also associated with this hormonal imbalance, including overeating, unhappy feelings, and tension, were also relieved by DLBS1442. However, because these symptoms were only very mild at baseline, the reduction was not of significance. Although these results have yet to be confirmed by more thorough preclinical and clinical studies, they may serve an early indication of the effect of DLBS1442 on hormonal imbalance and on the estrogen receptor, and are consistent with the results of a previous in vitro study of this extract.21
Other symptoms, like weight gain, insomnia, irritability, unhappy feelings, mood swings, anxiousness, depression, anger, tearfulness, and social conflict, were also ameliorated by DLBS1442 but not to a significant extent. The low VAS scores for these symptoms at baseline (control cycles) are likely to account for the only slight difference between control and treatment cycles.
The safety profile of DLBS1442 was investigated based on adverse event reporting. Overall, there were few adverse events reported and most were mild in severity. There were four reports of dyspepsia after taking DLBS1442. These events were possibly related to the mechanism of action of DLBS1442, ie, inhibition of COX. DLBS1442 is selective for COX-2, yet slightly affects COX-1, as reported by a previous preclinical study.11 Thus, adverse events related to peptic ulcer should be addressed specifically in a larger trial.
We realize that our study was preliminary and needs a larger randomized controlled trial for the results to be confirmed. However, the consistency of improvement found in most of the observed symptoms may be regarded as a promising indication of the benefits of DLBS1442 in treating women with premenstrual syndrome and/or primary dysmenorrhea.
Conclusion
This preliminary study indicates that DLBS1442 is effective in alleviating primary dysmenorrhea as well as abdominal pain and other symptoms related to premenstrual syndrome. The extract was also safe and well tolerated in female subjects with premenstrual syndrome and/or dysmenorrhea.
Acknowledgments
We sincerely thank all subjects who participated in this study.
Footnotes
Disclosure
This study was financially supported by PT Dexa Medica. The authors report no conflicts of interest in this work.
References
- 1.Dickerson LM, Mazyck PJ, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67(8):1743–1752. [PubMed] [Google Scholar]
- 2.Rendas-Baum R, Yang M, Gricar J, Wallenstein GV. Cost-effectiveness analysis of treatments for premenstrual dysphoric disorder. Appl Health Econ Health Policy. 2010;8(2):129–140. doi: 10.2165/11532210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Frackiewicz EJ, Shiovitz TM. Evaluation and management of premenstrual syndrome and premenstrual dysphoric disorder. J Am Pharm Assoc (Wash) 2001;41(3):437–447. doi: 10.1016/s1086-5802(16)31257-8. [DOI] [PubMed] [Google Scholar]
- 4.Coco AS. Primary dysmenorrhea. Am Fam Physician. 1999;60(2):489–496. [PubMed] [Google Scholar]
- 5.Tonini G. Dysmenorrhea, endometriosis and premenstrual syndrome. Minerva Pediatr. 2002;54(6):525–538. [PubMed] [Google Scholar]
- 6.Yonkers KA, O’Brien PM. Premenstrual syndrome. Lancet. 2008;371(9619):1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre G, Pinsonneault O, Antao V, et al. Primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2005;27(169):1119–1120. doi: 10.1016/s1701-2163(16)30395-4. [DOI] [PubMed] [Google Scholar]
- 8.Faried A, Kurnia D, Faried LS, et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30(3):605–613. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- 9.Tjandrawinata RR, Arifin PF, Tandrasasmita OM, Rahmi D, Aripin A. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. extract confers anti-proliferative and pro-apoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8(3):187–201. [PubMed] [Google Scholar]
- 10.Indoherbalfarma Crown god, Phaleria macrocarpa, as medicine. Available at: http://indoherbalfarma.co.cc/crown-god-phaleria-macrocarpa-as-medicine.html. Accessed June 25, 2010.
- 11.Arifin PF, Aripin AA, Tjandrawinata RR. DLBS1442: A Phaleria macrocarpa (Scheff.) Boerl. extract which confers anti-inflammatory effect. Medicinus. 2011 In press; [PubMed] [Google Scholar]
- 12.World Medical Association Declaration of Helsinki Recommendations guiding physicians in biomedical research involving human patients. Amended by the 52nd WMA General Assembly; Edinburgh, Scotland. October 2000; Available from: http://www.flebologia.unisi.it/lineeguida/declarationingl.htm. Accessed May 14, 2011. [Google Scholar]
- 13.International Conference on Harmonisation (ICH) Topic E 6 Harmonised Tripartite Guideline for Good Clinical Practice. Step. Consolidated Guideline 1 May 1996. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/ich/efficac/e6-eng.php. Accessed May 14, 2011.
- 14.Casper RF, Powell AM. Premenstrual syndrome: Documentation by a linear analog scale compared with two descriptive scales. Am J Obstet Gynecol. 1986;155(4):862–867. doi: 10.1016/s0002-9378(86)80040-0. [DOI] [PubMed] [Google Scholar]
- 15.Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria macrocarpa compound extract, via downregulation of PI3-kinase/AKT pathway. Cancer Biol Ther. 2010;10(8):814–823. doi: 10.4161/cbt.10.8.13085. [DOI] [PubMed] [Google Scholar]
- 16.Todd KH. Clinical versus statistical significance in the assessment of pain relief. Ann Emerg Med. 1996;27(4):439–441. doi: 10.1016/s0196-0644(96)70226-3. [DOI] [PubMed] [Google Scholar]
- 17.Kelly AM. Does the clinically significant difference in VAS pain score differ with age, gender or cause of pain? Acad Emerg Med. 1998;5(11):1086–1090. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moline ML, Zendell SM. Evaluating and managing premenstrual syndrome. Medscape Womens Health. 2000;5(2):1–16. [PubMed] [Google Scholar]
- 20.Calhoun AH, Hutchinson S. Hormonal therapies for menstrual migraine. Curr Pain Headache Rep. 2009;13(5):381–385. doi: 10.1007/s11916-009-0062-5. [DOI] [PubMed] [Google Scholar]
- 21.Arifin PF, Tjandrawinata RR. Role of DLBS1442 in decreasing the expression of estrogen receptor-alpha. Medicinus. 2011 In press; [Google Scholar]
- 22.Hendey GW, Donner NF, Fuller K. Clinically significant changes in nausea as measured on a visual analog scale. Ann Emerg Med. 2005;45(1):77–81. doi: 10.1016/j.annemergmed.2004.07.446. [DOI] [PubMed] [Google Scholar]