Abstract
Ossabaw swine have a “thrifty genotype” and consumption of excess calories induces many classical components of the metabolic syndrome, including obesity, insulin resistance, impaired glucose tolerance, dyslipidemia, hyperleptinemia, and hypertension. Earlier studies indicate that the metabolic syndrome is associated with diminished cardiac function; however, to what degree this impairment is associated with alterations in myocardial β1- and β2-adrenoceptor (AR) expression has not been fully elucidated. Accordingly, the present study was designed to investigate the effects of the metabolic syndrome on cardiac β1- and β2-AR expression. Studies were conducted on left ventricular tissue samples obtained from control lean and chronically (50 weeks) high-fat-fed obese animals. Chronic feeding significantly increased fasting plasma insulin, total cholesterol, triglycerides, blood glucose, systolic and diastolic blood pressure, and heart rate. Real-time polymerase chain reaction revealed no significant alterations in cardiac β1- and β2-AR mRNA expression. In contrast, Western blot analysis revealed a significant decrease in ventricular β1- and β2-AR protein expression. This is the first report in a novel large animal model that induction of metabolic syndrome is accompanied by a significant reduction in cardiac β1- and β2-AR protein expression that could contribute to impaired cardiac function.
Keywords: hypertension, metabolic syndrome, β-AR, impaired cardiac function
Introduction
With the increasing burden of childhood obesity, the prevalence of metabolic syndrome in children still varies widely.1 However, using Adult Treatment Panel (ATP) III and World Health Organization criteria, a school-based study on metabolic syndrome has reported prevalence at 4.2% and 8.4%, respectively.1 Importantly, pediatric metabolic syndrome needs to be tracked very carefully from childhood to adulthood to prevent cardiometabolic risk.2 Metabolic syndrome is known to be associated with higher cardiovascular mortality and morbidity, which is referred to as “cardiometabolic risk” in the clinics.3 Despite genetic and environmental factors, the key elements of metabolic syndrome are obesity, impaired glucose tolerance, insulin resistance, increased low-density lipoprotein:high-density lipoprotein ratio, increased fasting and postprandial triglyceride levels, and hypertension.4 Specifically, centrally distributed adiposity in obesity strongly correlates with adult cardiometabolic risk.3
This multifaceted syndrome can result in a variety of cardiac and hemodynamic alterations that ultimately result in development of congestive heart failure.2,3 The mechanisms responsible for these disturbances are mainly multifactorial but could be related to a hyperdynamic circulatory state, which is characterized by increased blood pressure, total blood volume, cardiac output, and tissue metabolic demand.5–7 Basal catecholamine level generally increases, a finding supported by many studies.6,8,9 Increased sympathetic system activity could stimulate G-protein-coupled β-adrenoceptors (ARs), elevating intracellular cyclic adenosine monophosphate (cAMP), thereby activating protein kinase A (PKA) or calcium calmoduline kinase II (CaMKII).10–12 The duration of the disease is the key factor that attenuates β-AR-mediated cardiac responses and underlying mechanisms.
Determining the effects of metabolic syndrome on cardiac β-AR expression seems to be one of the novel therapeutic strategies against obesity-induced cardiometabolic risk. The author of this study determined hemodynamic parameters such as systolic, diastolic, and mean arterial blood pressures as well as heart rate. Real-time polymerase chain reaction (PCR) and Western blot analysis were used to assess mRNA and protein expressions in the left ventricles obtained from control and chronically (50 weeks) high-fat-fed animals. For that purpose, I selected obese Ossabaw swine, which are one of the novel large animals and the most similar model to human metabolic syndrome.
Methods
High-fat diet
This investigation was approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Ossabaw pigs were fed either a normal diet (Teklad, ∼13% calories from fat; n ∼ 7) or a high-fat diet (∼60% of calories from fat; n = 9). The high-fat diet was administered in the morning and afternoon for ∼50 weeks. The pigs were maintained on this diet throughout the experimental protocol.
Analytical procedures
Plasma supernatant was collected and stored at −80°C.
Isolation and quantitation of total RNA
Left ventricles obtained from control and high-fat-fed Ossabaw swine. They were placed in liquid N2 and stored at −80°C. Total RNA was extracted according to manufacturer’s instructions (SV Total RNA Isolation System, Promega Corp, Fitchburg, WI). At the end of the isolation, RNA samples were dissolved in nuclease-free water (pH 7.5). The total RNA quantity and quality were determined using the Experion™ Semi-automated Electrophoresis System (Bio-Rad Laboratories, Inc, Hercules, CA). In the Experion priming station, the microfluidic-based LabChip sample wells (RNA StdSens Chips, Experion, Bio-Rad) were filled with a polymer-sieving matrix containing a fluorescent dye and 1 μL of denatured total RNA. The concentration of total RNA was determined by using the ratio of the sample RNA area to the Experion RNA ladder area. RNA samples with distinct 18S and 28S ribosomal RNA fragments in the electropherogram were used to assess RNA quality.
Preparation of first-strand cDNA via reverse transcriptase reactions
RNA samples were used as templates for synthesis of first-strand cDNAs as described previously. Briefly, 1 μL of oligo(dT)15 primer (Promega) was added to equivalent amounts of total RNA obtained from left ventricles isolated from control (n = 5) and chronically high-fat-fed (n = 5) pigs. The mixtures were then placed into a thermocycler (My Cycler™, Bio-Rad) and held at 70°C for 5 minutes. The samples were then transferred into an ice bath for 5 minutes to permit selective binding of the oligo(dT)15 to the poly(A) tail of the mRNA. First-strand cDNA was then synthesized with an ImProm-II Reverse Transcriptase kit (Promega).8,17
Amplification of cDNA
Real-time PCR reactions were performed for β1-, β2-ARs, and β-actin in triplicate with a custom-designed SYBR® Green mix (2.4 μL of 25 mM MgCl2, 5 μL of 1:10,000 dilution SYBR Green I, Molecular Probes, Inc, Eugene, OR) and 5 μL of 1 nM fluorescein calibration dye (1 mM/L in dimethyl sulfoxide, Bio-Rad) in 50 μL of total reaction using Taq DNA polymerase (Promega).8,17 Primers were designed using the Genomatix Software Suite program (Müchen, Germany) based on sequences published in the National Center for Biotechnology Information GenBank database.18 Gene-specific primers were used: for β1-ARs, sense 5′-GACCGAAAGCAGGTGAACTC-3′ and antisense 5′-CTCCCATCCCTTCCCTAGTC-3′ (242 bp product; accession number AF042454); for β2-ARs, sense 5′-GCCATCGACTGCTATCACAA-3′ and antisense 5′-GGTTTGGGGAGTGGAATCTT-3′ (193 bp product; accession number U53185); for β-actin, primers were designed based on published sequences in the GenBank database (sense 5′-ACGTGGACATCAGGAAGGAC-3′ and antisense 5′-ACATCTGCTGGAAGGTGGAC-3′; accession number U07786).
Amplification was carried out with iCycler iQ multicolor real-time PCR detection system (Bio-Rad) as follows: 45-second denaturation (94°C) followed by 45-second annealing and 1-minute extension (72°C), repeated for a total of 40 cycles. β-actin was amplified in each set of PCR reactions and served as an internal reference during quantitation to correct for operator and/or experimental variations. The data were analyzed in triplicate using the 2−ΔΔCt equation.8,17,19
| (1) |
The mean threshold cycle (Ct) values for both the target (β1- and β2-ARs) and internal control (β-actin) genes were determined in each sample.
Western blot analysis of β1- and β2-AR proteins
Left ventricles from control (n = 5) and high-fat-fed (n = 5) Ossabaw swine were isolated and placed in liquid nitrogen and stored at −80°C. Ventricles were homogenized in 150 μL of buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100 [Sigma-Aldrich, Inc, St Louis, MI], 0.1% SDS [sodium dodecyl sulphate], 1 mM EDTA [ethylene diamine tetraacetic acid], 1 mM EGTA [ethylene glycol-bis (β-aminoethylether)-N,N,N′,N′-tetraacetic acid], 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 5:1000 phenylmethylsulfonyl fluoride [200 nM], 5:1000 Na3VO4 [200 nM], and 5:1000 NaF [200 nM]). The homogenates were centrifuged at 45,000 rpm for 30 minutes at 4°C. The supernatants were collected and used for analysis. Protein quantity and quality were determined using the Experion Semi-automated Electrophoresis System. Basically, 4 μL of denatured protein for each sample was loaded into a Pro 260 chip (Experion, Bio-Rad). Equivalent amounts of protein were separated by gel electrophoresis (10% Tris-HCl Criterion Precast Gel, Bio-Rad). Proteins were transferred onto nitrocellulose membrane (0.2 μm, Schleicher and Schuell, London, England, UK) by semidry electrob-lotting (Trans Blot SD, Bio-Rad) at 15 V for 1 hour. The nitrocellulose membrane was soaked in 10 mM Tris-HCl containing 5% nonfat dry milk (Bio-Rad) and 0.7% polyoxyethylene-sorbitan monolaurate (Tween® 20, Promega), pH = 7.2, overnight at 4°C to block nonspecific sites. The membranes were then incubated with the β1- and β2-AR purified polyclonal antibodies (1:300 dilution in Tris-buffered saline [TBS] with 5% nonfat dry milk and 0.1% Tween 20; Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at room temperature. β-actin receptor antiserum was used for internal control (1:3000 dilution in TBS with 5% nonfat dry milk and 0.1% Tween 20; Santa Cruz Biotechnology). Blots were washed and incubated with donkey anti-goat IgG-HRP secondary antibody (1:3000 dilution; Santa Cruz Biotechnology) for 1 hour at room temperature. Immunoreactivity was visualized with an enhanced chemiluminescence Western blotting detection kit (Amersham ECL™ GST Western Blotting Detection Kit, GE Healthcare, Little Chalfont, UK). Quantitative assessment of band densities was performed by scanning densitometry.
Statistical analyses
Data are expressed as mean ± standard error of the mean. Statistical testing was directed to detect overall treatment effects. An unpaired t-test was used to compare differences in bodyweight, plasma sample and hemodynamic data, and specific β1- and β2-AR gene and protein expression between the control and high-fat-fed animals.
Results
Phenotype of Ossabaw swine
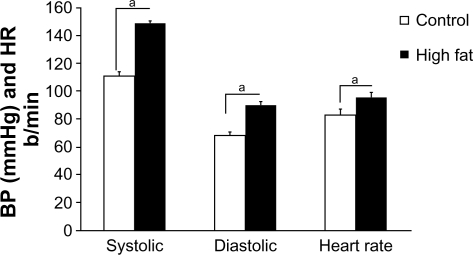
Chronic high-fat feeding caused many common features of the metabolic syndrome in Ossabaw swine: animals were obese, hyperinsulinemic, dyslipidemic, hypercholes-trolemic, and hypertensive (Figure 1 and Table 1). In contrast, control (normal-fed) animals were lean, normotensive, normoglycemic, and had normal lipid profiles.
Figure 1.
Systolic BP, diastolic BP, and heart rate from lean and chronic high-fat-diet Ossabaw swine. Systolic and diastolic BPs and heart rate were significantly elevated in the metabolic syndrome group.
Note: aP < 0.05 versus control.
Abbreviations: BP, blood pressure; HR, heart rate.
Table 1.
Chronic high-fat feeding significantly increased fasting blood glucose, fasting insulin level, total cholesterol, and triglyceride levels
| Control group (n = 7) | High-fat-diet group (n = 9) | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 79 ± 3 | 106 ± 9a |
| Fasting insulin level (μU/mL) | 4.8 ± 1.6 | 12.1 ± 4.6a |
| Total cholesterol level (mg/dL) | 57.0 ± 5.5 | 165.6 ± 8.3a |
| Total triglyceride level (mg/dL) | 24.7 ± 3.2 | 49.6 ± 2.9a |
Note:
P < 0.05 versus control.
Hemodynamic parameters
A high-fat diet of 50 weeks induced many common features of the prediabetic metabolic syndrome. Systolic, diastolic, and mean arterial blood pressures and heart rate were significantly elevated in high-fat-fed Ossabaw swine, compared with their lean controls (Figure 1).
mRNA and protein expression of cardiac β1- and β2-ARs in control and chronically high-fat-fed Ossabaw swine
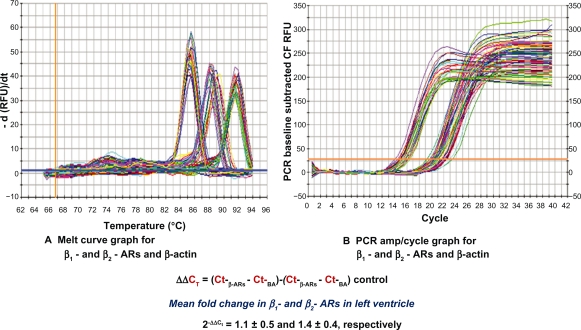
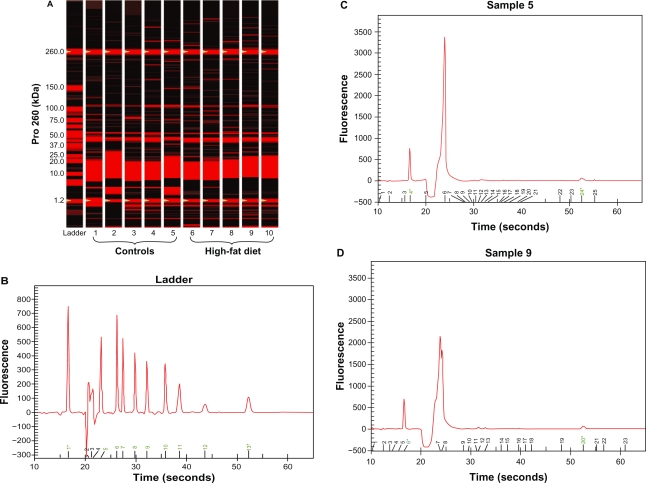
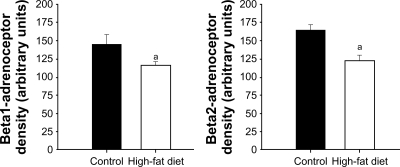
The quality of total RNA was determined by using distinct 18S and 28S ribosomal RNA fragments in the electropherogram. All samples used for analysis were of the same quality and quantity demonstrated in microfluidic-based LabChip sample wells. However, real-time PCR revealed no significant alterations in ventricular β1- and β2-AR mRNA expression in high-fat-fed Ossabaw swine compared with their controls (Figure 2). Pro260 chip–automated gel electrophoresis was used for determining protein quality and quantity (Figure 3). Western blot analysis demonstrated a significant decrease in ventricular β1- and β2-AR protein expression (19.86% and 22.6%, respectively) (Figure 4).
Figure 2.
Using real-time PCR, no significant changes in mRNA of β1- and β2-ARs were detected in left ventricle obtained from high-fat-fed Ossabaw swine when compared with controls. (A) Melt curve graph for SYBR®-β1- and β2-ARs and β-actin. (B) PCR amp/cycle graph for β1- and β2-ARs and β-actin.
Abbreviations: CF, curve fit; AR, adrenoceptor; PCR, polymerase chain reaction; RFU, relative fluorescence units; CT, mean cycle threshold value.
Figure 3.
Protein gel electrophoresis (chip). (A) Pro260 chip automated gel electrophoresis. Five controls and five high-fat-diet left ventricle protein gel electrophoresis obtained from Ossabaw swine heart. Basically, 4 μL of denatured protein for each sample was loaded into a Pro260 Experion chip (Bio-Rad). (B) Experion Pro260 Ladder electropherogram. The Pro260 ladder contains nine proteins ranging from 10–260 kDa as well as 1.2 kDa lower marker. (C and D) Sample electropherograms obtained from control (C) and high-fat-fed (D) left ventricle protein extractions. Left ventricles were homogenized in radio immunoprecipitation assay lysis buffer (which included phenylmethylsulfonyl fluoride and Na orthovanadate solutions as well as protease inhibitor cocktail). The homogenates were centrifuged at 45,000 rpm for 30 minutes at 4°C. The supernatants were collected and used for analysis.
Figure 4.
Western blot analysis of β1- and β2-ARs obtained from control and high-fat-fed Ossabaw swine left ventricles. Western blot analyses revealed a significant decrease in ventricular β1- and β2-ARs: 19.86% and 22.6%, respectively. Signal intensities were normalized to concomitant β-actin.
Note: aP < 0.05 versus control.
Abbreviation: AR, adrenoceptor.
Discussion
The purpose of the present investigation was to examine the hypothesis that metabolic syndrome could alter expression of cardiac β1,2-AR subtypes. Syndrome X, the deadly quartet, insulin resistance syndrome, and metabolic syndrome have been described previously and have many features of obesity and insulin deficiency in humans. We selected high-fat-fed Ossabaw swine as a cardiometabolic risk model because it is a very well established research model on the cardiovascular system. Ossabaw swine have applicability to human cardiometabolic syndrome and have a “thrifty genotype.” If they are fed with a high-fat diet for a long time, they develop metabolic syndrome.
Chronic high-fat feeding caused many common features of the metabolic syndrome in Ossabaw swine, ie, obesity, hyperinsulinemia, dyslipidemia, hypercholestrolemia, and hypertension, compared with the lean control group (Table 1 and Figure 1). We found that their systolic, diastolic, and mean arterial blood pressures and heart rate were significantly elevated compared with their lean controls (Figure 1). This hemodynamic picture indicates that they have a hyperdynamic circulatory state, which demonstrates increased sympathetic system activity. These findings were very consistent with our previous studies, where acute high-fat feeding triggered positive-feedback mechanisms to correct hemodynamic imbalances.8,17,18 These cardiovascular risk clusters could directly attenuate main cardiovascular function.
Increased sympathetic system activity could cause downregulation of cardiac β-ARs and could change their secondary and tertiary structures. A persistent hypercat-echolaminergic state, which is seen in high cardiometabolic risk subjects, triggers reversible or irreversible protein modifications in cell surface receptors. Both β1 and β2 subtypes have been shown to functionally coexist in the human heart.13–16 The hazardous effects of elevated catecholamine levels are found to be mediated primarily by β1-ARs contrary to β2-ARs stimulation, which may be adaptive in some cases.13,15,16 β1- and β2-ARs each couple to Gs; however, a growing body of recent evidence suggests that β2-ARs also couple to inhibitory protein Gi.10,12 In spite of these differences, the author and colleagues have previously demonstrated that both β1- and β2-AR expression and β1- and β2-AR-mediated inotropic and chronotropic responses were decreased in chronic diabetic hearts.13–16 There are a number of explanations for decreased β-AR expression in metabolic syndrome. Systemic hemodynamic alterations, key protein modifications, and intrinsic functional changes are major factors in attenuating β-AR-dependent cardiac inotropic and chronotropic responses.
This study found that chronic high-fat feeding resulted in decreased ventricular β1- and β2-AR protein expression, as expected. Real-time PCR revealed no significant alterations in ventricular β1- and β2-AR mRNA. These data directly demonstrate that downregulation of ventricular β1- and β2-ARs is possibly related to constant sympathetic system stimulation. Increased sympathetic system activity stimulates β-ARs and elevates intracellular cAMP, thereby activating PKA and/or CaMKII.10 Recent evidence demonstrates that constant β-ARs stimulation switches the signaling pathway from PKA to CaMKII predominance in more chronic stages.12 Activation of the CaMKII pathway instead of PKA as well as PKA activity and underlying mechanisms were not determined; further experimentation is necessary.
Conclusion
This is the first report in a novel large animal model where induction of metabolic syndrome is accompanied by a significant reduction in cardiac β1- and β2-AR protein expression that could contribute to impaired cardiac function.
Acknowledgments
The author would like to thank Ridvan and Binnaz Ege (founder of Ufuk University School of Medicine) for their support, and also thanks Michael Sturek, Johnathan D Tune, and Mouhamad Alloosh (Department of Cellular and Integrative Physiology, Indiana University School of Medicine, Indianapolis, IN) for their contributions. This work was supported by grants of the National Institutes of Health: HL67804 JD Tune; and HL062552 and RR013223 M Sturek. The author would also like to thank Yang Gao for his technical assistance and contribution. All contributors gave their permission for this manuscript to be published independently from their own work.
Footnotes
Author’s contributions
U Deniz Dincer MD, PhD designed the experiment and analyzed the data discussed in this paper, and wrote the manuscript. This work was completed at the Indiana University School of Medicine, Department of Cellular and Integrative Physiology, IUPUI, Indianapolis, IN (Dr JD Tune’s laboratory).
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 2.Alpert MA. Management of obesity cardiomyopathy. Am J Med Sci. 2001;321:237–241. doi: 10.1097/00000441-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56:35–45. [PubMed] [Google Scholar]
- 5.Carroll JF, Jones AE, Hester RL, Reinhart GA, Cockrell K, Mizelle HL. Reduced cardiac contractile responsiveness to isoproterenol in obese rabbits. Hypertension. 1997;30:1376–1381. doi: 10.1161/01.hyp.30.6.1376. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Brands MW, Zappe DH, et al. Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension. 1995;25:994–1002. doi: 10.1161/01.hyp.25.5.994. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, Brands MW, Zappe DH, Alonso GM. Insulin resistance, hyperinsulinemia, and hypertension: causes, consequences, or merely correlations? Proc Soc Exp Biol Med. 1995;208:317–329. doi: 10.3181/00379727-208-43862b. [DOI] [PubMed] [Google Scholar]
- 8.Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation. 2006;13:587–595. doi: 10.1080/10739680600885228. [DOI] [PubMed] [Google Scholar]
- 9.Grassi G, Dell’Oro R, Quarti-Trevano F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 10.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 11.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao RP, Zhu W, Zheng M, et al. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Dincer UD, Onay A, Ari N, Ozcelikay AT, Altan VM. The effects of diabetes on beta-adrenoceptor mediated responsiveness of human and rat atria. Diabetes Res Clin Pract. 1998;40:113–122. doi: 10.1016/s0168-8227(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 14.Dincer UD, Ozcelikay AT, Yilmaz ED. The effects of chronic L-name and L-arginine administration on beta-adrenergic responsiveness of STZ-diabetic rat atria. Pharmacol Res. 2000;41:565–570. doi: 10.1006/phrs.1999.0623. [DOI] [PubMed] [Google Scholar]
- 15.Dincer UD, Bidasee KR, Guner S, Tay A, Ozcelikay AT, Altan VM. The effect of diabetes on expression of beta1-, beta2-, and beta3-adrenoreceptors in rat hearts. Diabetes. 2001;50:455–461. doi: 10.2337/diabetes.50.2.455. [DOI] [PubMed] [Google Scholar]
- 16.Dincer UD, Guner S, Tay A, et al. Decreased expression of beta1- and beta2-adrenoceptors in human diabetic atrial appendage. Cardiovasc Diabetol. 2003;2:6. doi: 10.1186/1475-2840-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol. 2006;41:108–114. doi: 10.1016/j.yjmcc.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol. 2003;98:416–423. doi: 10.1007/s00395-003-0418-7. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]