Abstract
L-dopa therapy for Parkinson's disease leads to dyskinesias or abnormal involuntary movement (AIMs) for which there are few treatment options. Our previous data showed that nicotine administration reduced L-dopa-induced AIMs in parkinsonian monkeys and rats. To further understand how nicotine mediates its antidyskinetic action, we investigated the effect of nicotinic receptor (nAChR) agonists in unilateral 6-OHDA-lesioned rats with varying striatal damage. We first tested the drugs in L-dopa-treated rats with a near-complete striatal dopamine lesion (>99%), the standard rodent dyskinesia model. Varenicline, an agonist that interacts with multiple nAChRs, did not significantly reduce L-dopa-induced AIMs, while 5-iodo-A-85380 (A-85380), which acts selectively at α4β2* and α6β2* subtypes, reduced AIMs by 20%. By contrast, both varenicline and A-85380 reduced L-dopa-induced AIMs by 40–50% in rats with a partial striatal dopamine lesion. Neither drug worsened the antiparkinsonian action of L-dopa. The results show that selective nicotinic agonists reduce dyskinesias, and that they are optimally effective in animals with partial striatal dopamine damage. These findings suggest that presynaptic dopamine terminal α4β2* and α6β2* nAChRs are critical for nicotine’s antidyskinetic action. The current data have important implications for the use of nicotinic receptor-directed drugs for L-dopa-induced dyskinesias, a debilitating motor complication of dopamine replacement therapy for Parkinson’s disease.
Keywords: A-85380, dyskinesia, L-dopa, nicotine, nicotinic, varenicline
1. Introduction
Dyskinesias are a complication of L-dopa treatment that eventually develops in the majority of patients with Parkinson's disease (Jenner, 2008; Lang, 2009; Pezzoli and Zini, 2010). These abnormal involuntary movements (AIMs) can be quite debilitating and represent a major drawback to continued L-dopa therapy. A variety of drugs targeting different neurotransmitter systems in the basal ganglia have been reported to exert beneficial effects against AIMs in parkinsonian animal models, including the glutamate, adenosine, noradrenaline, 5-hydroxytryptamine, cannabinoid, and opioid systems (Fabbrini et al., 2007; Fox et al., 2008). However, management of L-dopa-induced dyskinesias continues to represent a serious therapeutic challenge in Parkinson's disease patients.
Recent results showed that nicotine administration improves L-dopa-induced dyskinesias in parkinsonian animal models (Quik et al., 2009). Nicotine given in the drinking water reduced L-dopa-induced dyskinesias in both L-dopa naïve and L-dopa primed monkeys (Quik et al., 2007). Similar findings were obtained in parkinsonian rodent models of L-dopa-induced dyskinesias. Nicotine given via several modes of administration (drinking water, minipump or injection) significantly improved L-dopa-induced AIMs in parkinsonian rats and mice (Bordia et al., 2008; Huang et al., 2009). These treatment modalities readily lend themselves to use in Parkinson's disease patients, for instance, as an oral formulation (pill or solution) or a slow release mode (nicotine patch). Importantly, nicotine did not modify the anti-parkinsonian effect of L-dopa in any parkinsonian animal model (Bordia et al., 2008; Bordia et al., 2010; Huang et al., 2009; Quik et al., 2007).
Although the mechanism whereby nicotine exerts its antidyskinetic effect remains to be elucidated, studies with the nAChR antagonist mecamylamine indicate that nicotine may exert its antidyskinetic effect via an interaction at nAChRs (Bordia et al., 2010). These receptors are pentameric ligand gated ion channels expressed throughout the body including the central and peripheral nervous system and the neuromuscular junction. Multiple nAChR subunits have been identified (α1–α10, β1–β4, γ, δ and ε) which co-assemble to form a diverse family of receptor subtypes (Albuquerque et al., 2009; Gotti et al., 2009; Millar and Gotti, 2009). Notably, the nAChRs throughout the body are distinct from one another. For instance, the α1, β1, γ, δ and ε are exclusively expressed at the neuromuscular junction. The primary nAChRs in the peripheral nervous system are α3β4* and α7 expressing receptors, while the α4β2*, α6β2* and α7 nAChRs predominate in the CNS. The asterisk indicates the possible presence of other subunits in the receptor complex. The α4β2* nAChR subtype may play a significant antidyskinetic role since it is widely distributed throughout the brain, and highly expressed in the striatum, a region critical in the pathology of Parkinson's disease (Gotti et al., 2009; Millar and Gotti, 2009; Quik et al., 2009). In addition, the α6β2* nAChR population may be important as it has a restricted distribution to catecholaminergic systems, and plays a prominent role in modulating dopaminergic function in the nigrostriatal and mesolimbic pathways (Exley et al., 2008; Meyer et al., 2008; Perez et al., 2009; Perez et al., 2008). Although not densely express in striatum, α7 receptors may also be involved since they are located in numerous brain regions linked to the striatum (Gotti et al., 2009).
To directly elucidate whether nicotine exerts its ability to reduce AIMs or dyskinetic-like movements by acting at nAChRs, we tested varenicline and A-85380 in a well characterized rat model of L-dopa-induced dyskinesias. The nAChR agonist varenicline was selected because it interacts with multiple nAChRs, while 5-iodo-A-85380 (A-85380) was chosen because it acts selectively at α4β2* and α6β2* subtypes. In addition, we investigated the effect of partial and near-complete striatal dopaminergic damage to determine the involvement of presynaptic as compared to postsynaptic nAChRs in nicotine’s antidyskinetic effect. The results show that nAChR agonists best reduce L-dopa-induced dyskinesias in rats with only partial striatal dopamine damage. These data suggest that α4β2* and/or α6β2* nAChRs localized to presynaptic dopaminergic terminals are important for the antidyskinetic effect of nicotine.
2. Methods
2.1. 6-Hydroxydopamine (6-OHDA) lesioning
Male Sprague-Dawley rats (~250 g) were purchased from Charles River Laboratories, Gilroy, CA. They were group housed in a temperature- and humidity-controlled environment under a 12 h light/dark cycle with free access to food and water. After a 2–3 day acclimation period, they were unilaterally lesioned by intracranial administration of 6-OHDA (free base; Sigma-Aldrich Co., St. Louis, MO). Two injections of 2 µl each (3 µg free base/µl dissolved in 0.02% ascorbic acid, 0.9% saline) were stereotaxically administered into the right medial forebrain bundle under isofluorane anesthesia as previously described (Bordia et al., 2008; Bordia et al., 2010; Cenci and Lundblad, 2007). The coordinates of the two sites were as follows: (1) AP, −4.4; ML, 1.2; DV, 7.8; tooth bar at −2.4; (2) AP, −4.0; ML, 0.75; DV, 8.0; tooth bar at +3.4 relative to the bregma. Delivery of 6-OHDA into the target area was done over a 2 min period, with the needle retained at the injection site for a further 2 min before withdrawal. The rats were given buprenorphine (0.02 mg/kg s.c.) postoperatively. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Agonist treatment
Varenicline dihydrochloride (from F. I. Carroll, RTI International, Research Triangle Park, NC), iodo-A-85380 dihydrochloride (Tocris Bioscience, Ellisville, MO), nicotine base (Sigma-Aldrich Co., St. Louis, MO) or saline were administered twice daily via s.c. or i.p. injection at an 8-hr interval for 4 consecutive days. There was a 3 to 4 week washout period between the different drugs. The number of rats in the different treatment groups is indicated in the figure legends.
2.3. L-dopa treatment and AIMs measurement
Rats were injected with L-dopa methyl ester (6–8 mg/kg s.c.) plus benserazide (15 mg/kg s.c.) for 3 wk until they were stably dyskinetic as assessed using an established AIMs rating scale (Bordia et al., 2010; Cenci and Lundblad, 2007). They were then given L-dopa methyl ester (6–8 mg/kg s.c.) plus benserazide (15 mg/kg s.c.) once daily 10 min prior to the first dose of agonist treatment for 4 consecutive days. On the 4th day, three different AIMs subtypes were measured, including axial dystonia, abnormal orolingual movements and abnormal forelimb movements (Bordia et al., 2008; Bordia et al., 2010; Cenci and Lundblad, 2007). For each AIM subtype, rats were scored on a scale from 0 to 4. AIMs were rated by a blinded rater for 2 min once during the 20 min baseline period and for 2 min every 20 min for 3 h following L-dopa injection. The maximum possible score for each animal was 108 (max score per session = 12; number of sessions over 3 h = 9).
2.4. Limb use asymmetry test
The forelimb asymmetry (cylinder) test was used as a measure of parkinsonism (Bordia et al., 2008; Bordia et al., 2010; Schallert et al., 2000). Exploratory activity was evaluated by a blinded rater for a 5 min period immediately before and 60 min after L-dopa administration. Wall exploration was expressed in terms of the % use of the impaired forelimb (contralateral) compared to the total number of limb use movements.
2.5. Dopamine transporter autoradiography
Rats were killed by CO2 exposure, and the brains quickly removed and frozen in isopentane on dry ice. Eight µm sections were cut at −15°C in a cryostat, thaw mounted onto poly-L-lysine coated slides, dried, and stored at −80°C. [125I]RTI-121 (specific activity, 2200 Ci/mmol; PerkinElmer Life and Analytical Science, Boston, MA, USA) autoradiography was performed as previously described (Bordia et al., 2008; Bordia et al., 2010). Brain sections were preincubated 2 × 15 min in buffer (50 mM Tris-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl) and then incubated for 2 h with 50 pM [125I]RTI-121 in the same buffer also containing 0.025% BSA and 1 µM fluoxetine. Sections were washed 4 × 15 min in ice cold buffer, 1 × 10 s in ice cold doubly-distilled H2O, dried and exposed to Kodak MR film (Easterman Kodak Co., Rochester, NY, USA) for 2–3 days along with 3H-microscale standards (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Nonspecific binding was determined in the presence of the dopamine uptake inhibitor nomifensine (100 µM).
2.6. Data analyses
The ImageQuant program from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK) was used to determine optical density values from autoradiographic films. To evaluate specific binding, nonspecific binding was subtracted from total tissue binding. A calibration curve of radioactivity (nCi/mg wet weight tissue) versus optical density was generated using the 3H-microscale standards. The radioactivity in nCi/mg was then converted to fmol/mg tissue. The 3H standards were calibrated for 125I autoradiography using the corrections described, including exposure time, section thickness and concentration of radioactivity (Artymyshyn et al., 1990). Care was taken to ensure that the optical density readings of the samples were within the linear range of the film.
2.7. Statistical analyses
GraphPad Prism® (GraphPad Software, Inc, San Diego, CA) was used for all data analyses. Differences in ratings between treatment groups were determined using a Mann Whitney test or analysis of variance (ANOVA) followed by the appropriate post hoc test. A level of 0.05 was considered significant. Values are the mean ± S.E.M. of the indicated number of rats.
3. Results
3.1. The nonselective nAChR agonist varenicline decreased L-dopa-induced AIMs with a partial but not with a near-complete striatal dopamine lesion
As a first approach to test whether nAChR agonists reduced L-dopa-induced AIMs, we used varenicline because this drug interacts with a variety of subtypes, including α3β4*, α4β2* and α7* nAChRs (Coe et al., 2005; Gonzales et al., 2006; Jorenby et al., 2006; Mihalak et al., 2006; Rollema et al., 2007a; Rollema et al., 2007b). In addition, our recent unpublished data indicate that varenicline inhibits 125I-conotoxinMII binding, which labels α6β2* nAChRs (Quik et al, in preparation).
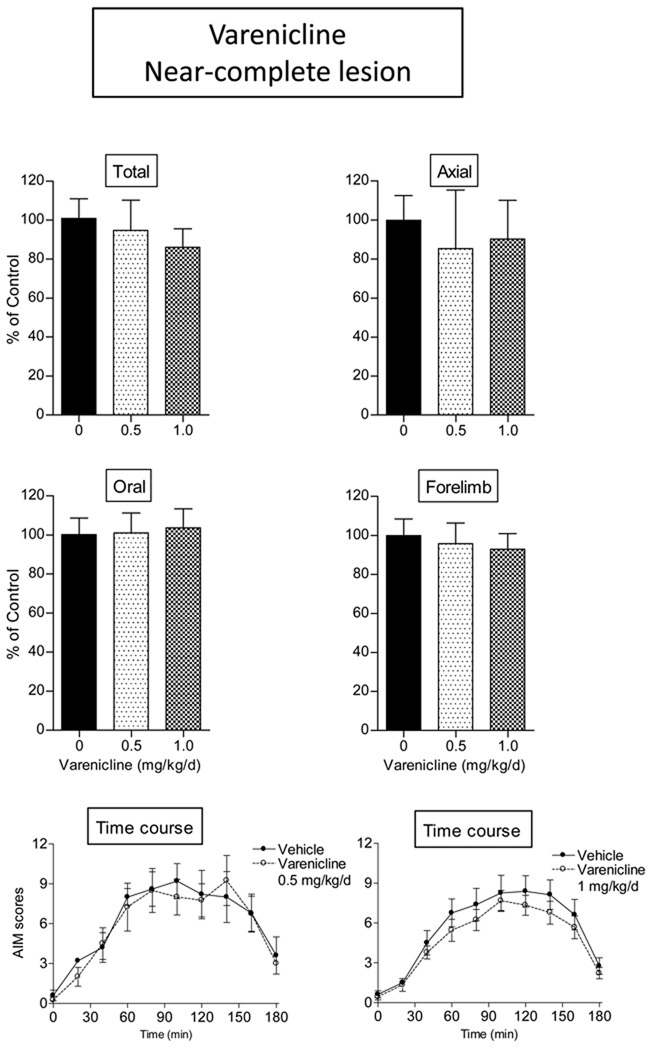
We first evaluated the antidyskinetic effect of varenicline in rats with a near-complete striatal dopamine lesion, a rat model commonly used to test effects of drugs on L-dopa-induced AIMs (Cenci and Lundblad, 2007). As shown in Table 1, there was a >99% loss in striatal dopamine transporter values (11.2 ± 0.6 and 0.1 ± 0.1 fmol/mg tissue on the unlesioned and lesioned side, respectively) in this set of rats. Two doses of varenicline (0.5 and 1.0 mg/kg/day s.c.) were tested since previous studies had showed that they most effectively modulated other behavioral changes in rodents (LeSage et al., 2009; Rollema et al., 2009). However, the results in Fig.1 demonstrated that varenicline did not decrease total L-dopa-induced AIMs, or axial, oral or forelimb subtypes in rats with near-complete striatal damage. The time course of the effect of the 0.5 and 1.0 mg/kg varenicline (Fig. 1 bottom panels) shows that there was no difference in AIMs scores at any time with near-complete striatal dopamine damage. Maximal AIM scores in rats with near-complete striatal damage ranged from 8 to 9 at the 90 min time point.
Table 1.
Striatal dopamine transporter values in rats with partial and near-complete striatal damage 125I-RTI-121 autoradiography was done to quantify levels of the dopamine transporter as described in Materials and Methods. Each value represents the mean ± S.E.M of the indicated number of rats.
| Lesion | # rats |
Intact side | Lesioned side |
|---|---|---|---|
| (fmol/mg tissue) | |||
| Near-complete | 10 | 11.2 ± 0.6 | 0.1 ± 0.1*** |
| Partial | 13 | 15.5 ± 0.3 | 5.1 ± 1.3* |
Significance of difference from the intact side: *p < 0.05,
p < 0.001.
Data were analyzed by a t-test.
Fig. 1.
The nonselective nAChR agonist varenicline did not reduce L-dopa-induced AIMs in rats with a near-complete striatal dopamine lesion. Lesioned rats were given varenicline (s.c.) twice daily at an 8-hr interval for 4 consecutive days. L-dopa methyl ester (8 mg/kg) plus benserazide (15 mg/kg) was given s.c. once daily 10 min after the first dose of varenicline. After 4 days of treatment, the rats were evaluated for axial, oral, and forelimb AIMs, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. Controls, n = 12; varenicline-treated (0.5 mg/kg), n = 4; varenicline-treated (1.0 mg/kg), n = 9.
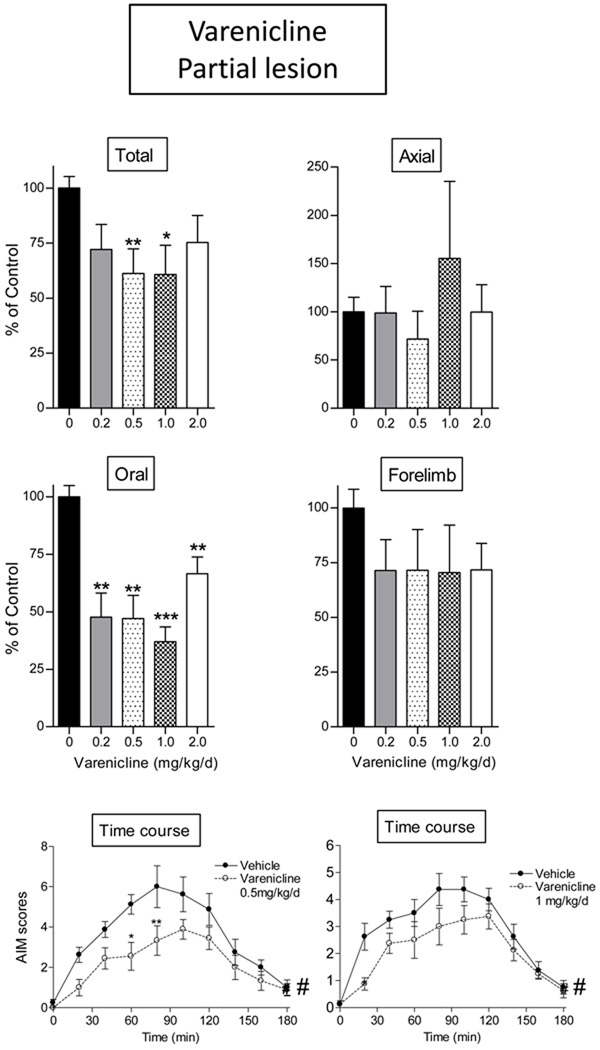
We next tested varenicline for its ability to reduce L-dopa-induced AIMs in rats with a partial striatal dopamine lesion. Our rationale for evaluating effects in rats with a partial striatal lesion was based on a study which had shown that drug-induced improvements in parkinsonian were observable in rats with partial but not near complete striatal damage (Carta et al., 2007). Striatal dopamine transporter values in this group of rats were reduced from 15.5 ± 0.3 to 5.1 ± 1.3 fmol/mg tissue (Table 1). Varenicline (0.5 and 1.0 mg/kg/day s.c.) significantly decreased total AIMs to a much greater extent in the partially lesioned animals as compared to the rats with a near-complete lesion, with a 42% decline in L-dopa-induced AIMs (p < 0.01 and p < 0.05, respectively, Fig. 2). The decline appeared to be predominantly due to a reduction in oral AIMs (p < 0.01), with a trend for improvement in forelimb AIMs. Varenicline caused a worsening of axial AIMS at the 1 mg/kg/d dose. Although not significant, this may represent a possible contraindication for that drug.
Fig. 2.
The nonselective nAChR agonist varenicline reduced L-dopa-induced AIMs in rats with a partial striatal dopamine lesion. Lesioned rats were given varenicline (s.c.) twice daily at an 8-hr interval for 4 consecutive days. L-dopa methyl ester (8 mg/kg) plus benserazide (15 mg/kg) was given s.c. once daily 10 min after the first dose of varenicline. After 4 days of treatment, the rats were evaluated for axial, oral, and forelimb AIMs, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. Controls, n = 8; varenicline-treated (0.2 mg/kg), n = 8; varenicline treated (0.5 mg/kg), n = 9; varenicline-treated (1.0 mg/kg), n = 8; varenicline treated (2.0 mg/kg), n = 9. Significance of difference from vehicle: *p < 0.05, **p < 0.01, ***p < 0.001. There was a significant main effect of nicotine with time (bottom panels, #p < 0.05). Data were analyzed by one-way ANOVA followed by Dunnett’s multiple comparison test (total, axial, oral, and forelimb AIMs) or one-way repeated ANOVA followed by a Bonferroni post hoc test (time course).
The overall AIM scores over the 3-hr rating period were significantly reduced with both 0.5 and 1.0 mg/kg/day varenicline treatment (F1,135 = 7.22 and F1,126 = 5.63, respectively, p < 0.05) analyzed by one-way repeated ANOVA. Bonferroni post hoc tests indicated that AIM scores at 60 and 80 min after L-dopa administration were significantly decreased with 0.5 mg/kg/day varenicline treatment (p < 0.05 and p < 0.01, respectively). Varenicline was also tested at doses of 0.2 and 2.0 mg/kg/day in rats with a partial striatal lesion (Fig. 2). There was significant decrease in oral AIMs (p < 0.001 and p < 0.01, respectively) with no effect on total, axial and forelimb AIMs. The time course of the effect of the 0.5 and 1.0 mg/kg varenicline (Fig. 2 bottom panels) in rats with partial striatal dopamine damage shows that there was a general decline in L-dopa-induced AIMs which appeared somewhat more prominent at the early time points. There was a significant main effect of nicotine with time (bottom panels, p < 0.05) using one-way repeated ANOVA followed by a Bonferroni post hoc test. Maximal AIM scores in rats with partial striatal damage ranged from 4 to 6 at the 90 min time point.
3.2. The β2* selective nAChR agonist A-85380 decreased L-dopa-induced AIMs most effectively in rats with a partial compared to near-complete striatal dopamine lesion
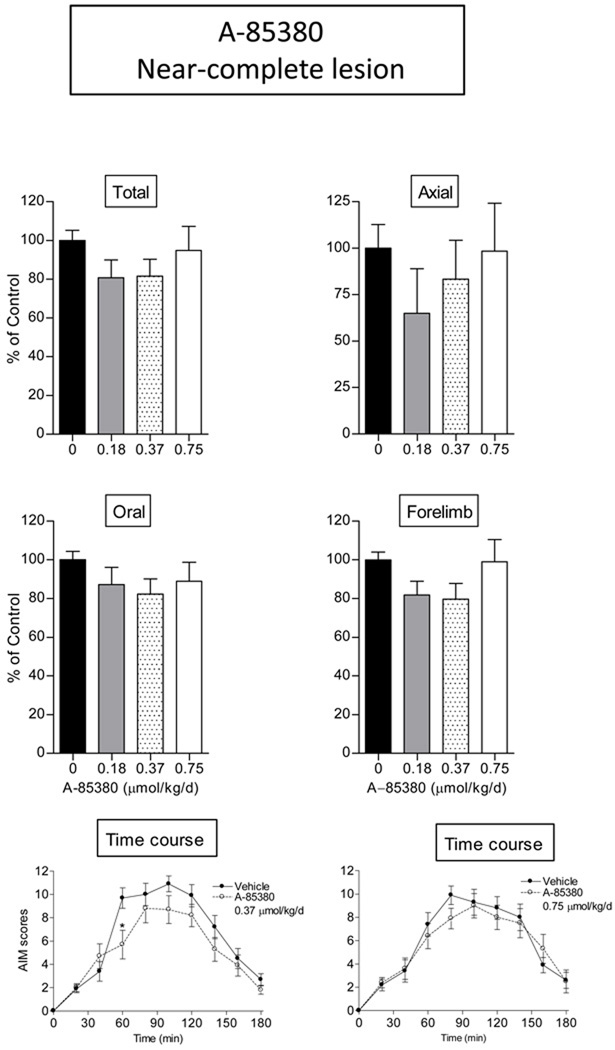
Our data with varenicline showed that a generally acting nAChR agonist reduced L-dopa-induced AIMs. Since the α4β2* and α6β2* receptor subtypes are the major nAChRs in the nigrostriatal pathway, we next tested the β2* nAChR agonist selective A-85380 for its ability to reduce L-dopa-induced AIMs in rats with a near-complete or a partial striatal dopamine lesion (Fig. 3 and 4). Various doses of A-85380 (0.18, 0.37 and 0.75 µmol/kg/day i.p.) were first tested in rats with near-complete striatal dopamine damage, the standard rat model for investigating drug effects on L-dopa-induced AIMs (Cenci and Lundblad, 2007). The time course data show that with such a lesion, there is a small but significant reduction in total AIMs 60 min after L-dopa injection with 0.37 µmol/kg/day A-85380 (p < 0.05), but not with other doses tested (Fig. 3, lower panels). No effects were observed on axial, oral and forelimb AIM subtypes.
Fig. 3.
Minimal decrease in L-dopa-induced AIMs in rats with a near-complete striatal dopamine lesion with the α4β2*/α6β2* selective nAChR agonist A-85380. Lesioned rats were given A-85380 (A85, i.p.) twice daily at an 8-hr interval for 4 consecutive days. L-dopa methyl ester (8 mg/kg s.c.) plus benserazide (15 mg/kg s.c.) was administered once daily 10 min after the first dose of A-85380. After 4 days of treatment, the rats were evaluated for axial, oral, and forelimb AIMs, with total AIMs representing the sum of these three components. Values are the mean ± S.E.M of 10 rats in each treatment group. Significance of difference from vehicle: *p < 0.05. Data were analyzed by one-way repeated ANOVA followed by a Bonferroni post hoc test (time course).
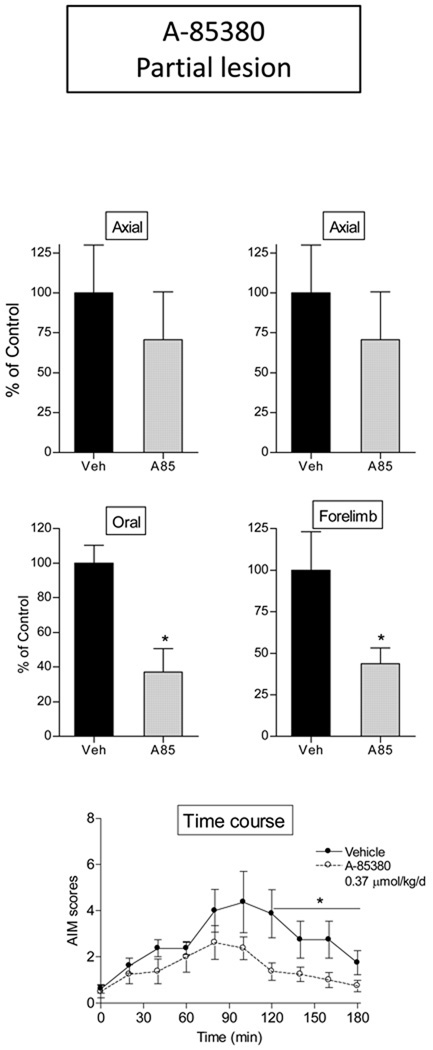
Fig. 4.
The α4β2*/α6β2* selective nAChR agonist A-85380 decreased L-dopa-induced AIMs more effectively in rats with a partial striatal dopamine lesion. Lesioned rats were given A-85380 (A85, i.p.) twice daily at an 8-hr interval for 4 consecutive days. L-dopa methyl ester (8 mg/kg s.c.) plus benserazide (15 mg/kg s.c.) was administered once daily 10 min after the first dose of A-85380. After 4 days of treatment, the rats were evaluated for axial, oral, and forelimb AIMs, with total AIMs representing the sum of these three components. Values are the mean ± S.E.M of 8 rats in each treatment group. Significance of difference from vehicle: *p < 0.05. Data were analyzed by a Mann-Whitney test (total, axial, oral, and forelimb AIMs) or one-way repeated ANOVA followed by a Bonferroni post hoc test (time course).
By contrast, in rats with a partial striatal dopamine lesion, A-85380 (0.37 µmol/kg/day) significantly decreased total AIMs (~50%, p < 0.05). This appeared to be due primarily to a reduction in oral and forelimb AIMs (Fig. 4). The time course of the effect of A-85380 on total AIM scores in rats with a partial striatal dopamine lesion is shown in Fig. 4 bottom. A-85380 treatment significantly decreased total AIM scores 120–180 min after L-dopa treatment analyzed by one-way repeated ANOVA (F1,42 = 4.81, p < 0.05). Maximal AIM scores in rats with partial striatal damage were ~4 at the 90 min time point.
These data with A85380 indicate that the striatal α4β2* and α6β2* nAChR subtypes are involved in the antidyskinetic effect of nicotine.
3.3. Nicotinic receptor agonists did not affect parkinsonism in lesioned rats
Experiments were subsequently done to evaluate the effect of the nAChR agonists varenicline and A-85380 on parkinsonism. For these studies we used the limb use asymmetry (cylinder) test because it is a sensitive measure of the degree of unilateral dopamine loss which does not require the use of dopaminergic drugs (Bordia et al., 2008; Cenci and Lundblad, 2007; Dekundy et al., 2007). As well, measurement of forelimb use more closely resembles the motor deficits observed with nigrostriatal damage as occurs in Parkinson's disease (Schallert et al., 2000). The values in the saline control groups were 50 ± 13 (n = 5) and 70 ± 7 (n = 8) percent total impaired forelimb use (Table 2). These values were not statistically significantly different (p = 0.17) and most likely represent inter-experiment variability. Varenicline and A-85380 treatment did not change the % impaired limb use 60 min after L-dopa treatment (Table 2), showing that these drugs do not worsen parkinsonism. These results are similar to our previous data with nicotine treatment, which also did not affect parkinsonism (Bordia et al., 2008). The effect of varenicline or A-85380 was not evaluated in rats with near-complete striatal damage since the drugs were less effective or ineffective in reducing L-dopa-induced AIMs with such a lesion.
Table 2.
The nicotinic receptor agonists A-85380 and varenicline did not worsen the antiparkinsonian effect of L-dopa
Rats with a partial loss of the striatal dopamine transporter were rated for forelimb use asymmetry (cylinder test) to determine whether A-85380 and varenicline affected the antiparkinsonian action of L-dopa. Impaired forelimb use was measured for 5 min 60 min after s.c. administration of L-dopa (8 mg/kg) plus benserazide (15 mg/kg). This time point was selected since the effect of L-dopa is maximal. The values represent the mean ± S.E.M of 5–9 rats per group.
| Treatment | # rats | Impaired forelimb use(% total) |
|---|---|---|
| A-85380 | 6 | 40 ± 7 |
| Saline | 5 | 50 ± 13 |
| Varenicline | 9 | 68 ± 7 |
| Saline | 8 | 70 ± 7 |
3.4 nAChR agonist treatment does not modify striatal dopamine transporter values
The questions arose whether nAChR agonist treatment might influence expression of L-dopa induced AIMs via alterations in dopamine transporter values. To address this, we measured transporter values in striatum of L-dopa-injected rats with a partial or near-complete striatal dopamine lesion treated with and without nAChR agonists. In rats with a near-complete lesion, striatal dopamine transporter values were 11.1 ± 0.9 (n = 8) fmol/mg tissue with chronic agonist treatment and 11.2 ± 0.8 (n = 8) fmol/mg tissue with vehicle treatment on the intact side. Dopamine transporter values were greatly reduced in the lesioned striatum of rats with a near-complete lesions, with values of 0.14 ± 0.06 (n = 8) fmol/mg tissue with agonist treatment and 0.06 ± 0.02 (n = 8) fmol/mg tissue with vehicle treatment. Thus, agonist treatment did not significantly affect dopamine transporter values in the lesioned or intact striatum in the near-complete lesion groups.
A similar lack of effect of the nAChR agonists was obtained in the rats with the partial striatal dopamine lesions. Striatal dopamine transporter values were 15.6 ± 1.0 (n = 7) fmol/mg tissue with chronic agonist treatment and 15.4 ± 1.1 (n = 6) fmol/mg tissue with vehicle treatment on the intact side. They were greatly reduced in the lesioned striatum of rats with a partial lesion, with values of 6.4 ± 5.4 (n = 7) fmol/mg tissue with agonist treatment and 3.5 ± 3.9 (n = 6) fmol/mg tissue with vehicle treatment on the lesioned side. Thus, agonist treatment did not significantly affect dopamine transporter values in the lesioned or intact striatum in the rats with a partial lesion.
Altogether these data show that nAChR agonist exposure does not influence expression of the dopamine transporter in intact or lesioned striatum from rats with a partial or near-complete striatal lesion. These data are consistent with our previous study which showed that the nAChR agonist nicotine did not modify striatal dopamine transporter binding (Bordia et al., 2008).
4. Discussion
The present results are the first to show that nAChR subtype agonists reduce L-dopa-induced abnormal movements in a parkinsonian rat model. Varenicline and A-85380 both reduced L-dopa-induced AIMs, directly demonstrating a role for nAChRs against dyskinesias. The nAChRs most likely involved are the α4β2* and α6β2* subtypes based on findings that A-85380, which targets β2* nAChRs, reduces L-dopa-induced AIMs. In addition, the results show that the antidyskinetic effect of nAChR agonists is greatly reduced with a near-complete striatal dopamine lesion, suggesting that α4β2* and α6β2* nAChRs expressed on nigrostriatal dopaminergic terminals play a key role in the nAChR-mediated improvement in dyskinesias.
Previous work had shown that nicotine reduces L-dopa-induced AIMs in parkinsonian rats and monkeys (Bordia et al., 2008; Bordia et al., 2010; Quik et al., 2007). Declines were observed when nicotine was administered via several routes of administration, including drinking water, minipump and injection, attesting to the robustness of its effect on L-dopa-induced AIMs (Bordia et al., 2008; Bordia et al., 2010; Quik et al., 2007). An important question is whether nicotine reduces L-dopa-induced dyskinesias through a nAChR-mediated mechanism. Our previous data had shown that the nAChR antagonist mecamylamine decreased L-dopa-induced AIMs and that the combined effects of nicotine and mecamylamine were not additive (Bordia et al., 2010). These observations indirectly suggested that nicotine decreased dyskinesias via an interaction at nAChRs and not through non-receptor mediated mechanisms.
In the current study, we used a direct approach to determine role of nAChRs through the use of receptor agonists. We tested varenicline because it interacts at multiple CNS receptor subtypes, including α4β2*, α3β4* and α7* nAChRs (Coe et al., 2005; Gonzales et al., 2006; Jorenby et al., 2006; Mihalak et al., 2006; Rollema et al., 2007a; Rollema et al., 2007b; Ween et al., 2010), as well as α6β2* nAChRs (Quik et al unpublished observation). We also tested A-85380, an agonist that interacts specifically with β2* nAChRs (Gotti et al., 2009; Kulak et al., 2002; Millar and Gotti, 2009).
We first determined the effect of the agonists in L-dopa-treated rats with a near-complete unilateral striatal dopamine lesion (>99%). This severely lesioned model is the standard one used to evaluate the effect of drugs because it offers the advantage that robust dyskinesias develop in the greater majority of the animals (Cenci and Lundblad, 2007; Jenner, 2008; Schallert et al., 2000). Unexpectedly, however, varenicline did not reduce L-dopa-induced AIMs, while 5-iodo-A-85380 (A-85380) reduced AIMs by only 20% in this series of animals (Table 3).
Table 3.
Summary of effect of nAChR agonists on L-dopa induced AIMs in rats with a partial and near-complete striatal dopamine lesion. Nicotine and nAChR drugs more effectively reduce L-dopa-induced AIMs with partial nigrostriatal damage.
| Drug | Drug-induced decrease in L-dopa-induced AIMs | |
|---|---|---|
| Partial lesion | Near-complete lesion | |
| Varenicline | ~40% | ~10% |
| A-85380 | ~50% | ~20% |
| Nicotine | 50–70%b | 20–40%a |
From Bordia et al., 2010; unpublished observations.
From Bordia et al., 2008
These observations raised the question whether the minimal decline in dyskinesias with nAChR ligands in severely lesioned rats may be because most of the nAChRs on the presynaptic striatal dopaminergic terminals have been destroyed. To evaluate this possibility, we next administered the drugs to rats with only partial striatal dopamine damage. One drawback of partial lesions is that L-dopa-induced dyskinesias do not develop as readily as in rats with severe nigrostriatal damage. However, with the partial striatal dopamine loss in this study, adequate AIMs still developed. Under these condition, varenicline decreased L-dopa-induced AIMs by ~40% while, A-85380 lead to a ~50% decline. These data support the hypothesis that nAChRs on striatal dopaminergic terminals are critical for the antidyskinetic effect of nAChR drugs. Moreover, the similarity in action of the two drugs suggests that β2* nAChRs are sufficient to reduce L-dopa-induced AIMs.
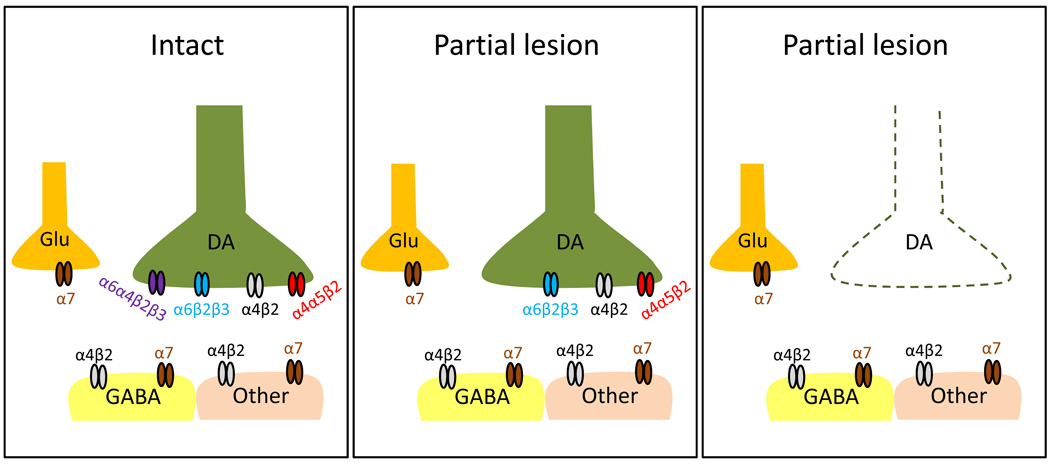
The presynaptic receptors most likely involved include the α6β2* and α4β2* subtypes (Fig. 5) (Perez et al., 2010; Quik et al., 2003). α6β2* nAChRs are exclusively expressed on nigrostriatal dopaminergic terminals, and may be composed of α6α4β2β3 and α6β2β3 subtypes (Bordia et al., 2007; Quik et al., 2001). The α6α4β2β3 subtype is lost with only <50% striatal lesion, while the α6β2β3 subtype persists and decreases in proportion to dopaminergic losses. These findings suggest that α6β2β3 subtype would be present in the partially lesioned striatum to mediate an antidyskinetic action.
Fig. 5.
Speculative schematic illustrating the striatal nAChR subtypes that may be involved in the nAChR-mediated antidyskinetic effect. In the intact striatum (left), α6β2* nAChRs are exclusively expressed on the nigrostriatal dopaminergic terminals (DA) and are composed of α6α4β2β3 and α6β2β3 subtypes (Bordia et al., 2007). α4β2* nAChRs (~20%) are also expressed on dopaminergic terminals and include both the α4β2 and α4α5β2 populations (Grady et al., 2009). The remaining 80% of α4β2* nAChRs are present on GABAergic neurons (GABA) as well as other striatal neuronal or non-neuronal cells (Other) and are most likely composed of only the α4β2 subunits (Gotti et al., 2009; Grady et al., 2009). In addition, α7 nAChRs are located on striatal glutamatergic (Glu) terminals (Kaiser and Wonnacott, 2000). In rats with a partial striatal dopamine lesion (middle), the α6α4β2β3 subtype is lost (Bordia et al., 2007). This suggests that α6β2β3 subtypes, as well as α4β2 and α4α5β2 nAChRs may mediate antidyskinetic effects. Since the majority of presynaptic nAChRs are lost in rats with a near-complete striatal lesion (right), postsynaptic α4β2 and possibly α7 nAChRs may contribute to the nAChR-mediated antidyskinetic effect in such rats. A potential role for nAChRs on dopaminergic neurons in the substantia nigra or in other brain areas remains to be determined.
α4β2* nAChR subtypes expressed on dopaminergic terminals include the α4β2 and α4α5β2 nAChRs (Fig. 5). It is not yet known whether these populations are differentially decreased with varying nigrostriatal damage. Thus, one or both of these subtypes may contribute to the antidyskinetic effect of nicotinic drugs. It should be noted that only ~20% of striatal α4β2* nAChRs are located on presynaptic dopamine terminals. The remaining 80% are present on other neuronal and possibly non-neuronal cells such as astrocytes (Delbro et al., 2009). These would not be affected by nigrostriatal damage unless trans-synaptic influences modulate their expression. Since A-85380 administration still resulted in a small decline in L-dopa-induced AIMs in rats with a near-complete lesion, it would suggest that α4β2* nAChRs located on other striatal neurons (or possibly nonneuronal cells) also contribute to the antidyskinetic effect of nicotine.
Although the majority of studies in the literature point to the striatum as the site of action of nAChR agonists on behaviors linked to dopaminergic activity, the exact locus of agonist action with systemic drug administration is uncertain at the present time. The substantia nigra, which contains the dopamine cells bodies and dendrites, may also mediate the consequences of these drugs on AIMs since nAChR activation regulates firing of nigral dopamine cells, as well as dopamine release in the striatum. In addition, actions at nAChRs in other brain regions may also critical for the decline in L-dopa-induced AIMs. This possibility is based on work showing that L-dopa-induced AIMs are influenced by multiple neurotransmitter systems throughout the brain including the glutamatergic, GABAergic, adrenergic, and various peptidergic systems which are localized in regions as diverse as the globus pallidus, thalamus, cortical areas, subthalamic nucleus, cerebellum, and other areas (Fox et al., 2008). In particular, recent work points a role for the serotonergic system in L-DOPA-induced dyskinesias (Carta et al., 2007). The relatively high levels of L-dopa also result in its uptake into striatal 5-HT terminals. Here it is converted to dopamine which is aberrantly released to result in dyskinesias. One point of note is that the loss of efficacy of nAChR agonists with near-complete dopamine lesions argues against a role for activation/desensitization of nAChR expression on serotonin axons in striatum or serotonin neurons in the raphe nuclei. On the other hand, the serotonergic system might contribute to the persistent AIMs seen with near-complete striatal dopamine loss. Additional support for an involvement of multiple brain areas in AIMs stems from evidence demonstrating beneficial effects of deep brain stimulation of the subthalamic nucleus in reducing dyskinesias (Limousin and Martinez-Torres, 2008).
An important question is the mechanism of action of nAChR drugs to reduce L-dopa-induced dyskinesias as such knowledge will assist in the development of targeted therapies to reduce their occurrence. Our current hypothesis is that long term nAChR desensitization plays a role. This idea is based on our previous findings that intermittent nicotine exposure via injection reduced L-dopa-induced AIMs to a similar extent as constant nicotine exposure via minipump, which would be expected to desensitize nAChR (Bordia et al., 2008; Bordia et al., 2010). In addition, the nAChR antagonist mecamylamine, which blocks nAChR-mediated activity, attenuated AIMs to a similar extent as nicotine (Bordia et al., 2010) and other nAChR agonists (present data). These combined observations suggest that nAChR agonists exert their effects via desensitization or inactivation of nAChRs. The above premise appears at odds with recent cyclic voltammetric data which show that acute nicotine exposure at desensitizing concentrations enhances burst-stimulated dopamine release in striatal slices (Exley et al., 2008; Perez et al., 2008). This might be predicted to further increase dopaminergic signaling in the striatum, and exacerbate AIMs, at least in the short term. However, the nAChR-mediated decline in L-dopa-induced AIMs is observed only after several days of nAChR drug treatment (Bordia et al., 2008; Bordia et al., 2010). Thus, other long term adaptive mechanisms most likely come into play to dampen dopaminergic tone.
Our earlier work with nicotine suggests it is somewhat more effective in reducing L-dopa-induced AIMs than either varenicline or A-85380 (Table 3). This may be because (1) nicotine stimulates additional minor nAChR populations not activated by the agonists (Millar and Gotti, 2009). (2) Nicotine, varenicline and A-85380 may also exhibit differential sensitivities at high and low affinity nAChR subtypes (Nelson et al., 2003) and/or varying partial agonist profiles (Mihalak et al., 2006; Rollema et al., 2007a). (3) In addition, varying pharmacokinetics may play a role. The observation that the full nAChR agonists, nicotine and A-85380, the partial agonist varenicline, and the antagonist mecamylamine all reduce AIMs, suggests that desensitization may be involved in their mechanism of action.
In summary, the present data suggest that drugs directed to β2* nAChR subtypes may be useful in the treatment of the dyskinesias that develop with L-dopa-treatment in Parkinson's disease patients. Our data show that nAChR-directed ligands most effectively attenuate AIMs in an animal model with partial as opposed to near-complete striatal dopamine damage. These findings suggest that nAChR drugs would be effective in controlling L-dopa-induced dyskinesias in Parkinson's disease patients since there is ~80% striatal dopaminergic loss when motor symptoms arise (Bezard and Gross, 1998; Hornykiewicz and Kish, 1987; Zigmond et al., 1990).
Acknowledgements
This work was supported by grants from the Michael J Fox Foundation and the National Institutes of Health [NS42091, NS47162] to MQ, and by a fellowship from the California TRDRP [18FT-0058] to LH. The authors thank Y. Y. Lee for excellent technical assistance.
Abbreviations
- AIMs
abnormal involuntary movements
- ANOVA
analysis of variance
- nAChRs
nicotinic receptors
- CNS
central nervous system
- A-85380
5-iodo-A-85380
- 6-OHDA
6-hydroxydopamine
- [125I]RTI-121
[125I]-3β-(4-iodophenyl)tropane-2β-carboxylic acid isopropyl ester
- varenicline
7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3h](3)-benzazepine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymyshyn R, Smith A, Wolfe BB. The use of 3H standards in 125I autoradiography. J Neurosci Methods. 1990;32:185–192. doi: 10.1016/0165-0270(90)90139-7. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang LZ, Quik M. Continuous and intermittent nicotine treatment reduces L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson' disease striatum. Mol Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci. 2007;Chapter 9 doi: 10.1002/0471142301.ns0925s41. Unit 9 25. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O'Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Delbro D, Westerlund A, Bjorklund U, Hansson E. In inflammatory reactive astrocytes co-cultured with brain endothelial cells nicotine-evoked Ca(2+) transients are attenuated due to interleukin-1beta release and rearrangement of actin filaments. Neuroscience. 2009;159:770–779. doi: 10.1016/j.neuroscience.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. alpha6-Containing Nicotinic Acetylcholine Receptors Dominate the Nicotine Control of Dopamine Neurotransmission in Nucleus Accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22:1379–1389. doi: 10.1002/mds.21475. quiz 1523. [DOI] [PubMed] [Google Scholar]
- Fox SH, Chuang R, Brotchie JM. Parkinson's disease--opportunities for novel therapeutics to reduce the problems of levodopa therapy. Prog Brain Res. 2008;172:479–494. doi: 10.1016/S0079-6123(08)00923-0. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2009;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Huang LZ, Bordia T, Quik M. Nicotine treatment reduces L-dopa-induced dyskinetic-like movements in parkinsonian mice. Society for Neuroscience. 2009 Abstracts. [Google Scholar]
- Jenner P. Preventing and controlling dyskinesia in Parkinson's disease--a view of current knowledge and future opportunities. Mov Disord. 2008;23 Suppl 3:S585–S598. doi: 10.1002/mds.22022. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Sum J, Musachio JL, McIntosh JM, Quik M. 5-Iodo-A-85380 binds to alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors (nAChRs) as well as alpha4beta2* subtypes. J Neurochem. 2002;81:403–406. doi: 10.1046/j.1471-4159.2002.00868.x. [DOI] [PubMed] [Google Scholar]
- Lang AE. When and how should treatment be started in Parkinson disease? Neurology. 2009;72:S39–S43. doi: 10.1212/WNL.0b013e318198e177. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Martinez-Torres I. Deep brain stimulation for Parkinson's disease. Neurotherapeutics. 2008;5:309–319. doi: 10.1016/j.nurt.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EL, Yoshikami D, McIntosh JM. The neuronal nicotinic acetylcholine receptors alpha 4* and alpha 6* differentially modulate dopamine release in mouse striatal slices. J Neurochem. 2008;105:1761–1769. doi: 10.1111/j.1471-4159.2008.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Perez X, O'Leary K, Parameswaran N, McIntosh JM, Quik M. Prominent role of {alpha}3/{alpha}6{beta}2* nAChRs in regulating evoked dopamine release in primate putamen; effect of long-term nicotine treatment. Mol Pharmacol. 2009;75:938–946. doi: 10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Bordia T, Lee YY, McIntosh JM, Quik M. Both α6β2* and α4β2* nicotinic receptors modulate dopamine release with nigrostriatal damage. Soc. Neurosci. 2010 Abstr. [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Mol Pharmacol. 2008;74:844–853. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzoli G, Zini M. Levodopa in Parkinson's disease: from the past to the future. Expert Opin Pharmacother. 2010;11:627–635. doi: 10.1517/14656561003598919. [DOI] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of Neurology. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson's disease. Biochem Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Kulak JM, McIntosh JM. Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. J Neurosci. 2001;21:5494–5500. doi: 10.1523/JNEUROSCI.21-15-05494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, et al. Rationale, pharmacology and clinical efficacy of partial agonists of alpha(4)beta(2) nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, Picciotto MR. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Ween H, Thorin-Hagene K, Andersen E, Gronlien JH, Lee CH, Gopalakrishnan M, Malysz J. alpha3* and alpha7 nAChR-mediated Ca(2+) transient generation in IMR-32 neuroblastoma cells. Neurochem Int. 2010;57:269–277. doi: 10.1016/j.neuint.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]