Abstract
Thyroid hormone receptor β (TRβ also listed as THRB on the MGI Database)-selective agonists activate brown adipose tissue (BAT) thermogenesis, while only minimally affecting cardiac activity or lean body mass. Here, we tested the hypothesis that daily administration of the TRβ agonist GC-24 prevents the metabolic alterations associated with a hypercaloric diet. Rats were placed on a high-fat diet and after a month exhibited increased body weight (BW) and adiposity, fasting hyperglycemia and glucose intolerance, increased plasma levels of triglycerides, cholesterol, nonesterified fatty acids and interleukin-6. While GC-24 administration to these animals did not affect food ingestion or modified the progression of BW gain, it did increase energy expenditure, eliminating the increase in adiposity without causing cardiac hypertrophy. Fasting hyperglycemia remained unchanged, but treatment with GC-24 improved glucose tolerance by increasing insulin sensitivity, and also normalized plasma triglyceride levels. Plasma cholesterol levels were only partially normalized and liver cholesterol content remained high in the GC-24-treated animals. Gene expression in liver, skeletal muscle, and white adipose tissue was only minimally affected by treatment with GC-24, with the main target being BAT. In conclusion, during high-fat feeding treatment with the TRβ-selective agonist, GC-24 only partially improves metabolic control probably as a result of accelerating the resting metabolic rate.
Introduction
Thyroid hormone is a highly metabolic molecule. When given to animals and humans, it rapidly increases energy expenditure (Klitgaard et al. 1952), while lowering serum cholesterol (Hansson et al. 1983) and triglycerides (Abrams et al. 1981) levels. However, side effects resulting from the pleomorphic actions of thyroid hormone, such as cardiac arrhythmia (Klein & Ojamaa 2001), bone loss (Ross 1994, Murphy & Williams 2004, Galliford et al. 2005), nervousness, and anxiety (Placidi et al. 1998), to name a few, prevent it from widespread clinical use. Ideally, one would want to harness the beneficial metabolic effects of thyroid hormone mediated at the liver, adipose tissue, and skeletal muscle, while sparing the myocardium, bone, brain, and other tissues.
Our current understanding of thyroid hormone action allows for the development of such strategies. Tri-iodothyronine (T3) effects are mediated by thyroid hormone receptors (TRs), which are ligand-dependent transcription factors that regulate the expression of different sets of genes involved not only in metabolic control, but also in development and growth (Yen 2001). The fact that the TR encoding genes are differentially expressed in various tissues indicates that the T3 effects can be TR isoform specific. Also, studies in patients with syndrome of resistance to T3, and studies in mice with targeted disruption of TRα, TRβ, (listed as THRA and THRB on the MGI Database) or both, have illustrated selective functions of TRs, and some actions that are preferentially triggered by a specific TR isoform (Hsu & Brent 1998, Brent 2000, Bassett et al. 2007). As an example, studies using knockout and knockin mouse models have shown that TRβ is involved in mediating the T3 effects on liver metabolism such as reducing plasma cholesterol and triglycerides (Sadow et al. 2003, Fugier et al. 2006, Shin et al. 2006).
Thus, it makes perfect logic to use thyroid hormone analogs capable of tissue specificity either by selective uptake and/or by selective binding to the two TR isoforms (Chiellini et al. 1998, Ocasio & Scanlan 2006). Activation of TRβ with a selective thyroid hormone analog (GC-1 compound) in rats results in the induction of UCP1 gene, while only minimally mediating synergism between thyroid hormone and the sympathetic nervous system (Ribeiro et al. 2001). In fact, the use of GC-1 or other TRβ-selective agonists in rodents and primates has recently been shown to increase energy expenditure, decrease fat mass and plasma levels of cholesterol (Grover et al. 2004), while sparing the heart (Trost et al. 2000) and the skeletal system (Freitas et al. 2003). Also, in the ob/ob mouse model, the administration of different TRβ agonists improves glucose homeostasis (Bryzgalova et al. 2008). It is still not clear, however, how much of the effects of these molecules is due to TR selectivity as opposed to liver-specific uptake (Trost et al. 2000).
In the present study, we tested the hypothesis that chronic TRβ activation minimizes the metabolic consequences of feeding a high-fat diet. To such end, we used GC-24, a second generation TRβ-selective molecule, which binds to TRβ with 40-fold higher affinity than TRα (Borngraeber et al. 2003, Miyabara et al. 2005). Our results indicate that the use of GC-24 prevents much of the metabolic alterations associated with feeding a high-fat diet, while not affecting the overall body and cardiac weights. Notably, changes in gene expression triggered by GC-24 were primarily detected in the brown adipose tissue (BAT), while only minimally affecting liver, skeletal muscle, and white adipose tissue.
Materials and Methods
Drugs and reagents
All drugs and reagents were purchased from Sigma Chemical Co. unless otherwise specified. GC-24 was kindly provided by Dr Thomas Scanlan.
Animals and treatments
Male Wistar rats weighing 150–200 g, were purchased from University of Sao Paulo Medical School (FMUSP, Sao Paulo, Brazil) and maintained on a 12 h light: 12 h darkness cycle at 25°C with food and water ad libitum.
In one set of experiments, animals were fed either chow (~1·8 kcal/g) or high-fat diet (~4·5 kcal/g), consisting of 42% carbohydrate, 24% proteins, and 23% fat. After 10 days on the high-fat diet, the animals were started on a treatment with T3 (30 ng/g body weight (BW) per day) or equimolar doses of GC-24 (17 ng/g BW per day) for 3 weeks. Administration was via daily i.p. injections. At the end of the experimental period, animals were studied for resting metabolic rate (RMR), glucose tolerance test (GTT), and insulin tolerance test (ITT). Animals were subsequently killed by exsanguinations under urethane anesthesia, and blood processed for plasma isolation and tissues samples were collected and immediately frozen for further analyses. Carcasses were frozen at −80 °C for further processing.
On a second set of experiments, animals fed chow diet were treated with T3 (15 ng/g BW per day) or equimolar doses of GC-24 (8·5 ng/g BW per day) for 45 days. Administration was via daily i.p. or s.c. injections. At the end of experimental period, animals were killed by exsanguinations under urethane anesthesia, and blood processed for plasma cholesterol determination.
Oxygen consumption
RMR was estimated by measuring oxygen consumption (VO2) in an open circuit respirometer system (O2-10, Sable System, Las Vegas, NV, USA) as previously described (Withers 1977, Curcio et al. 1999). The experiments were carried out over a period of 30 min in the afternoon (1400–1800 h) at room temperature (25 °C) in animals fed ad libitum. Animals were maintained in their normal experimental conditions until immediately before the measurements. The data were collected and analyzed by the Sable Systems software. The results are expressed as milliliters of O2/min per g BW.
Glucose tolerance test
Animals were fasted overnight, and glucose (2 g/kg) was administered by i.p. injection between 0900 and 1000 h. Blood samples were collected from the tail at various times after the glucose load, as indicated, and glycemia was immediately determined on a glucose analyzer (LifeScan, Inc., Milipitas, CA, USA).
Insulin tolerance test
Food was removed 6 h before the experiment, which was carried out between 1400 and 1500 h. Blood samples were collected from the tail at various times after insulin (0·5 U/kg) was administered by i.p. injection, as indicated, and glucose serum levels determined immediately using a glucose analyzer.
Blood chemistry
Plasma cholesterol and triglycerides were assessed by colorimetric method using a commercial kit (Roche Molecular Biochemicals). Plasma NEFA was assessed by colorimetric method using a commercial kit (WACO NEFA C kit; Wako Chemical Industries USA, Inc., Richmond, VA, USA). The plasma concentrations of tumour necrosis factor α (TNFα) and interleukin-6 (IL6) were measured by commercial ELISA kits (TNFα, IL-6, R&D System, Belgium), according to the manufacturer’s instructions (Yu et al. 2007). The protein concentration was determined by the method of Bradford (1976).
Liver chemistry
Lipids were extracted from the liver by disrupting ~200 mg frozen liver samples in 2 ml isopropyl alcohol with a Potter Elvehjem homogenizer (model MA 099; Marconi, Piracicaba, SP, Brazil). Homogenates were maintained at 37 °C for 30 min and then at 4 °C overnight. Total cholesterol and triacylglycerols were determined by enzymatic methods (Roche Diagnóstica) in a supernatant aliquot. Protein concentration was determined according to the method of Lowry et al. (1951) in liver samples (200 mg) previously homogenized in 4 ml of water.
mRNA analysis
Total RNA of liver, epididymal white fat and interscapular BAT was extracted using the Trizol (Life Technologies Inc.), according to the manufacturer’s instructions, and quantified by spectrophotometry. For the reverse transcriptase reaction, 0·8 μg of total RNA was used in the SuperScrit First-Strand Synthesis System for real-time (RT)-PCR (Invitrogen) on Robocycler thermocycler (Stratagene, La Jolla CA, USA). About 120 ng cDNA was used for amplification. Quantitative RT-PCR (RT-qPCR) was performed using IQ SYBR Green PCR kit (BioRad) on iCycler thermal cycler machine (Bio-Rad). Primers were designed with the help of Beacon Designer 3.0 (Premiere Biosoft Intl., Palo Alto, CA, USA), and the housekeeping gene cyclophilin A used as internal reference. Primer sequences are available upon request. The cycle conditions were: 5 min at 94 °C (Hot start); 30 s at 94 °C, 30 s at 58 °C, and 45 s at 72 °C for 50 cycles followed by the melting curve protocol to verify the specificity of amplicon generation. Gene expression was determined by ΔΔCt, and all values were expressed using cyclophilin A mRNA as an internal control (Christoffolete et al. 2004).
RIA
Total thyroxine (T4) and T3 serum levels were measured in 25 μl serum samples in duplicate using specific RIA (Coat-A-Count T3 Uptake Test Kit and Coat-A-Count T4 Uptake Test Kit; DPC, Los Angeles, CA, USA).
Fat mass measurement
Carcasses were thawed overnight at 4 °C, weighed, chopped in small pieces and then, using a motorized blender (Kinematica AG, Lucerne Switzerland), thoroughly homogenized in a volume of distilled water that equals the weight of the carcasses. Aliquots of homogenates were used for measurements of water, protein, and fat content. The water content was calculated as the weight variance after 24 h at 100 °C, as previously described (Bertin et al. 1998). Fat content was determined as previously described (Folch et al. 1957, Hartsook & Hershberger 1963, Azain et al. 1995), after lipid was extracted with a 2:1 chloroform:methanol solution. The lipid-containing chloroform layer was separated and dried to constant weight. The protein content was determined as described by Bradford (1976).
Histological examination
After careful dissection, tissues were immersed in buffered formol solution and fixed for 24 h. Paraffin-embedded tissues were sectioned and processed as described (Kerr et al. 1995). Analyses were performed after hematoxylin–eosin staining. The area of the white adipocytes was estimated by analyzing pictures taken at 100× magnification. Picture printouts were cut, and the area of at least 40 adipocytes per animal was estimated.
Statistical analysis
Results are expressed as the mean ± S.E.M. throughout the text, tables, and figures. Multiple comparisons were performed by one-way ANOVA, followed by Student–Newman–Keuls test.
Results
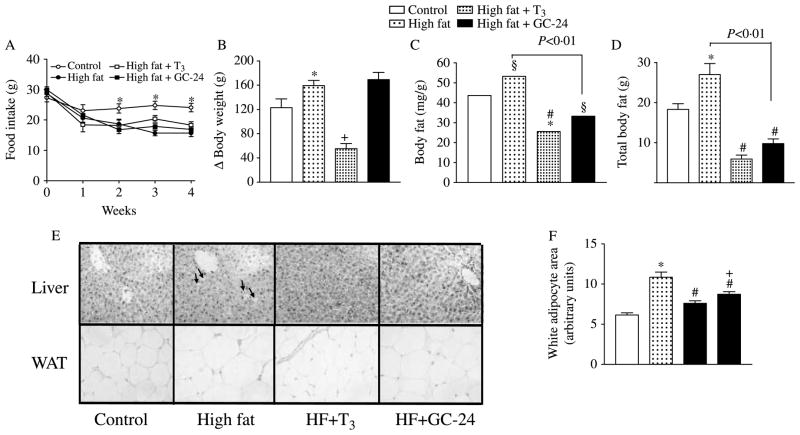
Feeding a high-fat diet resulted in ~30% compensatory reduction in food intake as early as the first experimental week (Fig. 1A). Despite this, at the end of the 4-week period, the animals on the high-fat diet gained significantly more BW (~30%; Fig. 1B), and accumulated more fat in absolute and relative terms (~22 and ~50%; Fig. 1C and D). Concomitant treatment with GC-24 (1·7 μg/100 g BW per day) did not affect serum T3 levels or the T3/T4 ratio in the serum (Table 1). At the same time, it did reduce body fat to levels below those found in animals fed chow diet (−~23%; Fig. 1C and D), but did not affect total body water nor prevent the accelerated increase in BW (Fig. 1B; Table 2). High-fat feeding caused visible fat deposition in liver, which was prevented by treatment with GC-24 (Fig. 1E). A similar, but much less dramatic, pattern was observed with the size of the fat cells (Fig. 1E and F). The estimated adipocyte area was doubled with the high-fat diet, while treatment with GC-24 only partially reduced it, to levels still above controls (~40%; Fig. 1E and F). Notably, this effect of GC-24 took place without changes in food ingestion (Fig. 1A) or cardiac weight (Table 1), a sensitive index of thyroid hormone effects in the heart. As a reference, other animals were placed on a high-fat diet and treated with equimolar amounts of T3 (3 μg/100 g BW per day), in a dosage equivalent to 10 times the daily T3 replacement dose. Such animals gained less BW (−~65%), had less total fat content (−~70%), less total body water (~15%; Table 2), while at the same time exhibited cardiac hypertrophy (~23%; Table 1).
Figure 1.
Effects of GC-24 on body weight and composition. Rats were placed on a high-fat diet for 4 weeks and treated with of T3 10× (30 ng/g BW per day) or GC-24 in equimolar dosage. (A) Food intake; *P<0·005 versus other groups; (B) total body weight gain; *P<0·001 versus control; +P<0·001 versus high fat; (C) body fat content; *P<0·05 versus control; +P<0·001 versus high fat; (D) total body fat; *P<0·001 versus control, #P<0·001 versus high fat; (E) sections of epididymal adipose tissue and liver stained by H–E; magnification is 200×; (F) estimated individual adipocyte area; 40 cells for each group were analyzed. All entries are mean ± S.E.M.
Table 1.
Effects of tri-iodothyronine (T3) (10×) or equimolar doses of GC-24 in animals placed on a high-fat diet. Values indicated as mean ± S.E.M.
| Control |
HF |
HF+T3 |
HF+GC-24 |
|
|---|---|---|---|---|
| Heart (mg/g) | 3·8±0·1 | 3·6±0·2 | 4·7±0·16* | 3·5±0·1 |
| Fasting glucose (mg/dl) | 65±7·8 | 86±5* | 90±5* | 97±7* |
| TG (mg/dl) | 67±6 | 130±19* | 80±15‡ | 70±6·9‡ |
| Cholesterol (mg/dl) | 82±8 | 125±21* | 65±5§ | 99±7·5|| |
| NEFA (mg/dl) | 6±0·3 | 12±0·5† | 14±0·3‡ | 14±1·6‡ |
| IL6 | 44·6±3·6 | 61±3·8*,¶ | 47±3·1§ | 59±2·7* |
| TNFα | 8·1±1·1 | 10±0·5* | 9±0·8‡ | 10±0·9|| |
| Liver cholesterol (mg/mg) | 1·7±0·2 | 2·5±0·7* | 1·7±0·05§ | 2·8±0·5 |
| T3 (ng/dl) | 62·3±7·8 | 62·9±2·6 | 52·4±1 | 66·8±11·9 |
| T4 (μg/dl) | 3·8±0·4 | 4·8±1·9 | <1 | 3·1±0·7 |
P<0·01 versus control;
P<0·001 versus control;
P<0·05 versus high fat (HF);
P<0·01 versus high fat;
P<0·05 versus control. High fat +T3 (HF+T3); high fat +GC-24 (HF+GC-24);
P<0·05.
Table 2.
Effects of tri-iodothyronine (T3) (10×) or equimolar doses of GC-24 in animals placed on a high-fat diet. Values indicated as mean ± S.E.M.
| Control |
HF |
HF+T3 |
HF+GC-24 |
|
|---|---|---|---|---|
| Total water (ml/kg BW) | 274±13 | 260±23 | 236±7* | 274±19 |
| Lean body mass (%) | 85±2·2 | 79±9 | 91±3·7 | 88±2·4 |
P<0·05 versus HF and P<0·05 versus HG+GC-24.
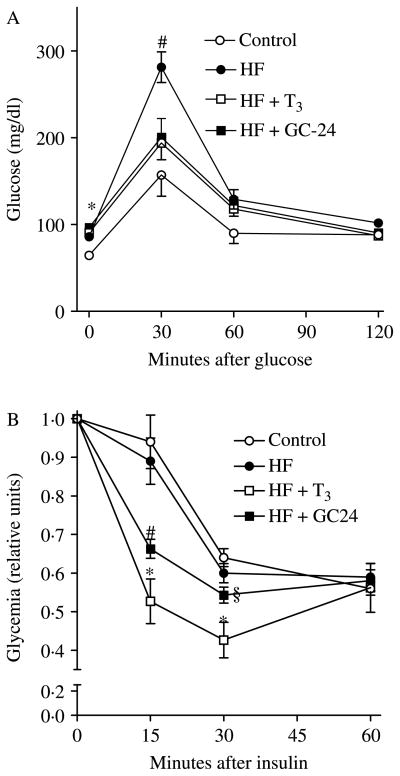
Rats placed on a high-fat diet developed the expected fasting hyperglycemia (86 vs 65 mg/dl) and glucose intolerance with a 30-min peak after glucose load reaching 281 mg/dl (Fig. 2A); at least in this setting, these animals did not exhibit resistance to insulin (Fig. 2B). Whereas the GC-24-treated animals still had significant fasting hyperglycemia (96·5 mg/dl), the maximum 30-min glucose peak was ~30% reduced (Fig. 2A). Insulin sensitivity was increased at early time points after insulin administration in GC-24-treated animals (Fig. 2B). Likewise, treatment with T3 mimicked the effects of GC-24 (Fig. 2A and B).
Figure 2.
Effects of GC-24 on GTT and ITT. Rats were placed on a high-fat diet for 4 weeks and treated with of T3 10× (30 ng/g BW per day) or GC-24 in equimolar dosage. (A) Glycemic levels (mg/dl). After fasting overnight, animals were injected with 2 g/kg of glucose, and plasma glucose levels were measured in 30 min intervals via tail bleeding; #P<0·01 versus all other groups; *P<0·01 versus other groups. (B) Glycemic levels (in arbitrary units). After fasting for 6 h, animals were injected with 0·5 U/kg of insulin and glucose blood levels were measured in 15 min intervals via tail bleeding; *P<0·001 versus control and high fat; #P<0·05 versus control and high fat; §P<0·001 versus high fat+T3. Entries are mean ± S.E.M.; n=5.
Animals placed on a high-fat diet exhibited significantly higher plasma levels of triglycerides, total cholesterol, NEFA, and IL6 (Table 1). At the same time, treatment with GC-24 normalized plasma triglycerides concentration but failed to prevent the increase in NEFA and IL-6 (Table 1). Plasma cholesterol concentration was only partially normalized by GC-24 (Table 1). In addition, liver total cholesterol levels, which were increased during high-fat feeding, were not at all affected by treatment with GC-24 (Table 1). Notably, treatment with T3 had very similar metabolic effects as GC-24, except that it did prevent the increase in plasma and liver cholesterol associated with the high-fat diet (Table 1).
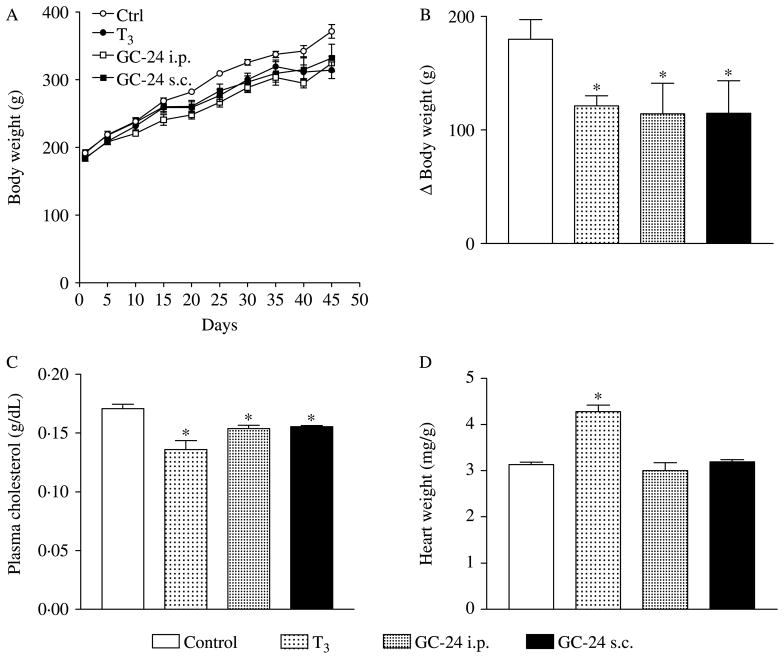
Because TRβ-selective agonists were shown to reduce cholesterol levels in a number of different settings (Trost et al. 2000, Grover et al. 2003, 2004, 2005), we tested whether the failure to do so in the present study was explained by the fact that the animals were placed on a high-fat diet. Route of administration (s.c. versus i.p.) was also evaluated because of the possibility that the metabolic effects of T3 and/or TRβ-selective agonists depend on a first-pass effect on the liver after i.p. administration. Thus, rats kept on a chow diet were treated with GC-24 (0·85 μg/100 g BW per day) for 45 days via i.p. or s.c. daily injections. Remarkably, regardless of the administration route, GC-24 administration significantly decreased BW gain (−~37%; Fig. 3A and B) and plasma cholesterol levels (−~10%; Fig. 3C), without affecting heart weight (Fig. 3D). Again, treatment with equimolar amounts of T3 (i.p.) produced similar effects, while increasing cardiac weight (~36%; Fig. 3A–D).
Figure 3.
Effects of GC-24 on body weight, cholesterol levels, and heart weight. Rats were placed on a chow diet and treated with of T3 5× (15 ng/g BW per day) or GC-24 in equimolar dose via i.p. or s.c. daily injections. (A) Total body weight. (B) Body weight gain; *P<0·01 versus control. (C) Plasma cholesterol levels; *P<0·001 versus control and +P<0·05 versus control. (D) Heart weight; *P<0·001 versus other groups. Entries are mean ± S.E.M.; n=6.
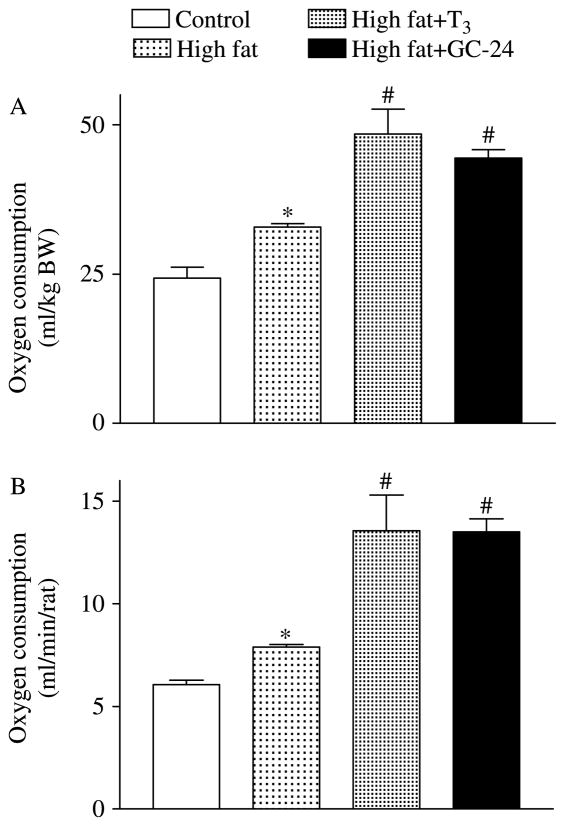
RMR was significantly increased during high-fat feeding, ~25% (Fig. 4A). This increase was observed even when oxygen consumption was corrected by total body mass (Fig. 4B). Notably, a further pronounced increase in RMR was observed in the GC-24-treated animals, which almost doubled the rates observed in the control animals (Fig. 4A and B). Likewise, T3 treatment also resulted in a substantial elevation in the RMR (Fig. 4A and B).
Figure 4.
Effects of GC-24 on resting metabolic rate (RMR). Rats were placed on a high-fat diet for 4 weeks and treated with of T3 10× (30 ng/g BW per day) or GC-24 in equimolar dosage. (A) Normalized RMR (ml/min per kg BW); *P<0·001 versus control; #P<0·01 versus high fat. (B) Total RMR (ml/min per rat); *P<0·001 versus control; #P<0·01 versus figh fat. Entries are mean ± S.E.M.; n=6.
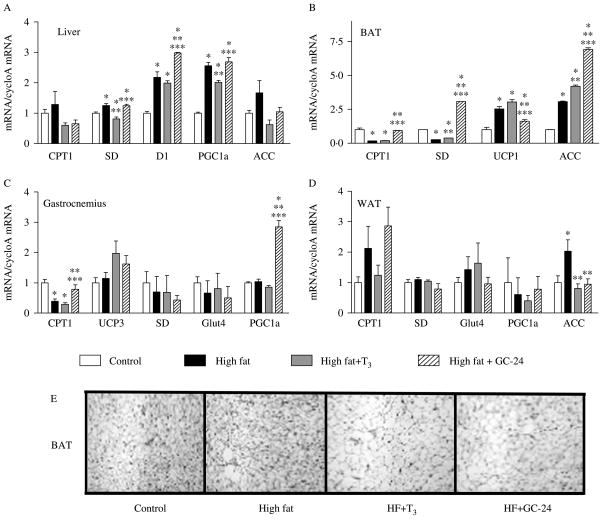
To gain insight into the mechanism by which GC-24 triggers such a wide array of metabolic effects, we used RT-qPCR for measuring the expression of multiple key metabolic genes and found that GC-24 acts predominantly in the BAT (Fig. 5A). While feeding a high-fat diet per se promoted marked changes in BAT gene expression (Fig. 5A), treatment with GC-24 stimulated even further the expression of several genes, such as ACC-1 (2·3-fold), CPTI (5·6-fold), and SD (11-fold; Fig. 5B). The effects of T3 in the BAT were less pronounced and restricted to ACC (1·4-fold; Fig. 5B). These changes in gene expression correlated with BAT activation as seen in Fig. 5E. Notably, liver, gastrocnemius, and white adipose tissue of animals fed a high-fat diet responded poorly to treatment with GC-24 or T3. There was a significant stimulation of gastrocnemius PGC-1α (threefold) and CPT1 (twofold) expression, with no major changes in white adipose tissue (Fig. 5). In the liver, only the type 1 iodothyronine deiodinase gene (Dio1), a known T3-resposive gene, was further stimulated by GC-24 (1·4-fold; Fig. 5A).
Figure 5.
GC-24 increases the expression of key metabolic genes and BAT histology. Gene expression profile of animals placed on a high-fat diet for 4 weeks and treated with of T3 10× (30 ng/g BW per day) or GC-24 in equimolar dosage, in liver (A), BAT (B), gastrocnemius (C), and white adipocyte tissue (D). Sections of brown adipose tissue stained by H–E; magnification is 200×. Tissue mRNA levels were measured using real-time qPCR technique, and data is expressed relative to control group. Where *P<0·05 versus control; **P<0·005 versus high fat; ***P<0·05 versus high fat treated with T3. Entries are mean ± S.E.M.; n=3.
Discussion
Recent studies indicate that the administration of TRβ-selective agonists has beneficial metabolic effects, e.g. increase in energy expenditure, while lowering serum cholesterol and triglycerides (Trost et al. 2000, Grover et al. 2004, Johansson et al. 2005, Villicev et al. 2007). In the present study, we expanded these findings and have shown that the administration of GC-24, a novel and even more selective TRβ agonist, prevented some of the metabolic abnormalities associated with feeding a high-fat diet, namely increase in fat mass (Fig. 1C and D), glucose intolerance (Fig. 2A), and hypertriglyceridemia (Table 1), while it did improve sensitivity to insulin (Fig. 2B). At the same time, other parameters that were elevated by high-fat feeding were only partially or not affected at all by treatment with GC-24, such as IL6, NEFA, and cholesterol levels (Table 1).
It is remarkable that in rats fed a high-fat diet, a substantial improvement in key metabolic parameters, i.e. plasma TG, body fat, glucose tolerance, and insulin sensitivity, is achieved with GC-24 treatment without affecting cardiac weight. One consideration is that GC-24 activates a number of well-known T3-responsive pathways, e.g. lipolysis, β-oxidation, which combined would improve the overall metabolic status of these animals. For example, it has been shown that T3 administration improves glucose tolerance in ob/ob mice via TRβ activation (Bryzgalova et al. 2008), while it up regulates GLUT4 expression (Casla et al. 1990, Torrance et al. 1997). At the same time, a more comprehensive explanation could simply be that GC-24 markedly accelerates the RMR by mimicking T3 actions (Fig. 4). An accelerated RMR would balance the increased energy intake, limit fat accumulation, and improve glucose homeostasis. This second hypothesis is supported by previous findings that a major contribution of TRβ-mediating effects in through energy expenditure (Grover et al. 2004, 2005, Villicev et al. 2007).
Given the predominance of TRβ in the liver (Schwartz et al. 1992) and the substantial effects of TRβ-selective agonists as cholesterol-lowering agents, it is assumed that the bulk of the effects of these molecules take place in the liver (Grover et al. 2004, Erion et al. 2007). However, our analysis of gene expression in liver, skeletal muscle, white fat and BAT indicates that the latter was predominantly affected. In the BAT, there was a significant increase in Cpt1, Sd, and Acc1 gene expression by GC-24, indicating the activation of this tissue (Fig. 5). In turn, this would explain the increase in metabolic rate by GC-24.
At the same time, a number of other metabolic parameters were not restored or affected by GC-24, namely increased IL6 and NEFA plasma concentrations, fasting hyperglycemia as well as hypercholesterolemia and liver cholesterol content. Persistently elevated IL6 and NEFA indicate that despite improvements, these animals remain metabolically challenged due to the elevated fat intake. Fasting hyperglycemia remains despite increased insulin sensitivity most likely as a result of higher hepatic glucose production via stimulation of PEPCk gene expression (Loose et al. 1985, Klieverik et al. 2008).
The suboptimal effects of GC-24 on cholesterol metabolism are particularly notable. Thus, treatment with GC-24 only minimized, but not normalized, the increase in plasma cholesterol levels resulting from the high-fat feeding, while liver cholesterol content remained high (Table 1). Because TRβ-selective agonists were shown to be effective cholesterol-lowering agents (Trost et al. 2000, Grover et al. 2003, 2004, Johansson et al. 2005, Miyabara et al. 2005), one explanation is that GC-24/T3 signaling could be decreased in the liver of high-fat fed animals (Crunkhorn & Patti 2008). This is supported by the observation that in rats kept on chow diet, GC-24 did reduce plasma cholesterol (Fig. 3C). However, the liver expression of Dio1, Sd, and Pgc1α was all increased by GC-24 in high-fat fed animals, indicating that GC-24/T3 signaling seems to be preserved in these animals. In addition, in such animals, both plasma cholesterol and liver cholesterol concentrations were normalized by treatment with equimolar doses of T3 (Table 1). While it is not clear what the mechanism interfering with GC-24 actions in liver is, reduced effectiveness of this TRβ-selective agonist as a cholesterol-lowering agent when combined with a high-fat diet is an important finding, which could have substantial impact on their planned therapeutic utilization.
Most studies with TRβ-selective agonists so far involved either oral gavage or i.p. administration. Because both routes involve a first passage through the liver, it has been suggested that this anatomic aspect contributes to the liver selectivity of these compounds. Our data, however, indicate that this is not the case given that animals kept on chow diet while receiving s.c. injections of GC-24 had their plasma cholesterol levels lowered (−10×%) and gained less BW (−~37%; Fig. 3).
In conclusion, the present studies show that administration of a highly TRβ-selective agonist to rats during feeding with a high-fat diet prevented a number of metabolic alterations typical of this condition such as increase in fat mass (Fig. 1C and D), glucose intolerance (Fig. 2A), and hypertriglyceridemia (Table 1). The overall mechanism seems to be acceleration in the RMR, which takes place in BAT. However, GC-24 treatment did not restore hypercholesterolemia, increased hepatic cholesterol content, elevated NEFA and IL6 levels, indicating that these metabolic pathways are less sensitive to activation of TRβ signaling during feeding with a high-fat diet. These findings should have important repercussions to the potential usage of TRβ-selective agonists as cholesterol-lowering agents.
Acknowledgments
Funding
This work was supported by grants from MackPesquisa and FAPESP (05/56477-3 to M O R), NIH (DK65055 to A C B), and PIBIC (R N J).
Footnotes
Declaration of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research here reported.
References
- Abrams JJ, Grundy SM, Ginsberg H. Metabolism of plasma triglycerides in hypothyroidism and hyperthyroidism in man. Journal of Lipid Research. 1981;22:307–322. [PubMed] [Google Scholar]
- Azain MJ, Roberts TJ, Martin RJ, Kasser TR. Comparison of daily versus continuous administration of somatotropin on growth rate, feed intake, and body composition in intact female rats. Journal of Animal Science. 1995;73:1019–1029. doi: 10.2527/1995.7341019x. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Nordstrom K, Boyde A, Howell PG, Kelly S, Vennstrom B, Williams GR. Thyroid status during skeletal development determines adult bone structure and mineralization. Molecular Endocrinology. 2007;21:1893–1904. doi: 10.1210/me.2007-0157. [DOI] [PubMed] [Google Scholar]
- Bertin E, Ruiz JC, Mourot J, Peiniau P, Portha B. Evaluation of dual-energy X-ray absorptiometry for body-composition assessment in rats. Journal of Nutrition. 1998;128:1550–1554. doi: 10.1093/jn/128.9.1550. [DOI] [PubMed] [Google Scholar]
- Borngraeber S, Budny MJ, Chiellini G, Cunha-Lima ST, Togashi M, Webb P, Baxter JD, Scanlan TS, Fletterick RJ. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. PNAS. 2003;100:15358–15363. doi: 10.1073/pnas.2136689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent GA. Tissue-specific actions of thyroid hormone: insights from animal models. Reviews in Endocrine & Metabolic Disorders. 2000;1:27–33. doi: 10.1023/a:1010056202122. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. Journal of Steroid Biochemistry and Molecular Biology. 2008;111:262–267. doi: 10.1016/j.jsbmb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Casla A, Rovira A, Wells JA, Dohm GL. Increased glucose transporter (GLUT4) protein expression in hyperthyroidism. Biochemical and Biophysical Research Communications. 1990;171:182–188. doi: 10.1016/0006-291x(90)91374-2. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chemistry & Biology. 1998;5:299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- Crunkhorn S, Patti ME. Links between thyroid hormone action, oxidative metabolism, and diabetes risk? Thyroid. 2008;18:227–237. doi: 10.1089/thy.2007.0249. [DOI] [PubMed] [Google Scholar]
- Curcio C, Lopes AM, Ribeiro MO, Francoso OA, Jr, Carvalho SD, Lima FB, Bicudo JE, Bianco AC. Development of compensatory thermogenesis in response to overfeeding in hypothyroid rats. Endocrinology. 1999;140:3438–3443. doi: 10.1210/endo.140.8.6906. [DOI] [PubMed] [Google Scholar]
- Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, et al. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. PNAS. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Freitas FR, Moriscot AS, Jorgetti V, Soares AG, Passarelli M, Scanlan TS, Brent GA, Bianco AC, Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:E1135–E1141. doi: 10.1152/ajpendo.00506.2002. [DOI] [PubMed] [Google Scholar]
- Fugier C, Tousaint JJ, Prieur X, Plateroti M, Samarut J, Delerive P. The lipoprotein lipase inhibitor ANGPTL3 is negatively regulated by thyroid hormone. Journal of Biological Chemistry. 2006;281:11553–11559. doi: 10.1074/jbc.M512554200. [DOI] [PubMed] [Google Scholar]
- Galliford TM, Murphy E, Williams AJ, Bassett JH, Williams GR. Effects of thyroid status on bone metabolism: a primary role for thyroid stimulating hormone or thyroid hormone? Minerva Endocrinologica. 2005;30:237–246. [PubMed] [Google Scholar]
- Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennstrom B, et al. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. PNAS. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellstrom K, Malm J. Development of the thyroid hormone receptor beta-subtype agonist KB-141: a strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovascular Drug Reviews. 2005;23:133–148. doi: 10.1111/j.1527-3466.2005.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Hansson P, Valdemarsson S, Nilsson-Ehle P. Experimental hyperthyroidism in man: effects on plasma lipoproteins, lipoprotein lipase and hepatic lipase. Hormone and Metabolic Research. 1983;15:449–452. doi: 10.1055/s-2007-1018751. [DOI] [PubMed] [Google Scholar]
- Hartsook EW, Hershberger TV. A simplified method for sampling small animal carcasses for analyses. Proceedings of the Society for Experimental Biology and Medicine. 1963;113:973–977. doi: 10.3181/00379727-113-28548. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Brent GA. Thyroid hormone receptor gene knockouts. Trends in Endocrinology and Metabolism. 1998;9:103–112. doi: 10.1016/s1043-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. PNAS. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Methods in Cell Biology. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. New England Journal of Medicine. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- Klieverik LP, Sauerwein HP, Ackermans MT, Boelen A, Kalsbeek A, Fliers E. Effects of thyrotoxicosis and selective hepatic autonomic denervation on hepatic glucose metabolism in rats. American Journal of Physiology. Endocrinology and Metabolism. 2008;294:E513–E520. doi: 10.1152/ajpendo.00659.2007. [DOI] [PubMed] [Google Scholar]
- Klitgaard HM, Dirks HB, Jr, Garlick WR, Barker SB. Protein-bound iodine in various tissues after injection of elemental iodine. Endocrinology. 1952;50:170–173. doi: 10.1210/endo-50-2-170. [DOI] [PubMed] [Google Scholar]
- Loose DS, Cameron DK, Short HP, Hanson RW. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1985;24:4509–4512. doi: 10.1021/bi00338a004. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Miyabara EH, Aoki MS, Soares AG, Saltao RM, Vilicev CM, Passarelli M, Scanlan TS, Gouveia CH, Moriscot AS. Thyroid hormone receptor-beta-selective agonist GC-24 spares skeletal muscle type I to II fiber shift. Cell Tissue Research. 2005;321:233–241. doi: 10.1007/s00441-005-1119-3. [DOI] [PubMed] [Google Scholar]
- Murphy E, Williams GR. The thyroid and the skeleton. Clinical Endocrinology. 2004;61:285–298. doi: 10.1111/j.1365-2265.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- Ocasio CA, Scanlan TS. Design and characterization of a thyroid hormone receptor α (TRα)-specific agonist. ACS Chemical Biology. 2006;1:585–593. doi: 10.1021/cb600311v. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Boldrini M, Patronelli A, Fiore E, Chiovato L, Perugi G, Marazziti D. Prevalence of psychiatric disorders in thyroid diseased patients. Neuropsychobiology. 1998;38:222–225. doi: 10.1159/000026545. [DOI] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone–sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. Journal of Clinical Investigation. 2001;108:97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DS. Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid. 1994;4:319–326. doi: 10.1089/thy.1994.4.319. [DOI] [PubMed] [Google Scholar]
- Sadow PM, Chassande O, Gauthier K, Samarut J, Xu J, O’Malley BW, Weiss RE. Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. American Journal of Physiology. Endocrinology and Metabolism. 2003;284:E36–E46. doi: 10.1152/ajpendo.00226.2002. [DOI] [PubMed] [Google Scholar]
- Schwartz HL, Strait KA, Ling NC, Oppenheimer JH. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. Journal of Biological Chemistry. 1992;267:11794–11799. [PubMed] [Google Scholar]
- Shin DJ, Plateroti M, Samarut J, Osborne TF. Two uniquely arranged thyroid hormone response elements in the far upstream 5′ flanking region confer direct thyroid hormone regulation to the murine cholesterol 7α hydroxylase gene. Nucleic Acids Research. 2006;34:3853–3861. doi: 10.1093/nar/gkl506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance CJ, Devente JE, Jones JP, Dohm GL. Effects of thyroid hormone on GLUT4 glucose transporter gene expression and NIDDM in rats. Endocrinology. 1997;138:1204–1214. doi: 10.1210/endo.138.3.4981. [DOI] [PubMed] [Google Scholar]
- Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, et al. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- Villicev CM, Freitas FR, Aoki MS, Taffarel C, Scanlan TS, Moriscot AS, Ribeiro MO, Bianco AC, Gouveia CH. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. Journal of Endocrinology. 2007;193:21–29. doi: 10.1677/joe.1.07066. [DOI] [PubMed] [Google Scholar]
- Withers PC. Metabolic, respiratory and haematological adjustments of the little pocket mouse to circadian torpor cycles. Respiration Physiology. 1977;31:295–307. doi: 10.1016/0034-5687(77)90073-1. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiological Reviews. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-kappaB and TLRs in rat intestine during CLP-induced sepsis. International Immunopharmacology. 2007;7:1076–1082. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]