Abstract
Acute lung injury (ALI) remains a significant source of morbidity and mortality in the critically ill patient population. Defined by a constellation of clinical criteria (acute onset of bilateral pulmonary infiltrates with hypoxemia without evidence of hydrostatic pulmonary edema), ALI has a high incidence (200,000 per year in the US) and overall mortality remains high. Pathogenesis of ALI is explained by injury to both the vascular endothelium and alveolar epithelium. Recent advances in the understanding of pathophysiology have identified several biologic markers that are associated with worse clinical outcomes. Phase III clinical trials by the NHLBI ARDS Network have resulted in improvement in survival and a reduction in the duration of mechanical ventilation with a lung-protective ventilation strategy and fluid conservative protocol. Potential areas of future treatments include nutritional strategies, statin therapy, and mesenchymal stem cells.
Key words: acute respiratory distress syndrome (ARDS), pulmonary edema, acute respiratory failure
Introduction
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) describe clinical syndromes of acute respiratory failure with substantial morbidity and mortality. Even in patients who survive ALI, there is evidence that their long-term quality of life is adversely affected.(1,2) Recent advances have been made in the understanding of the epidemiology, pathogenesis, and treatment of this disease. However, more progress is needed to further reduce mortality and morbidity from ALI and ARDS. Because this syndrome of acute respiratory failure is so common both in the United States and worldwide, it is fair to say that ALI/ARDS is an unmet medical need. In other words, novel therapies need to be developed to further improve clinical outcomes. This article provides an overview of the current definitions, pathogenesis, and treatment of acute lung injury.
Definition and Epidemiology
Ashbaugh and colleagues,(3) in 1967, were the first to use the phrase acute respiratory distress syndrome (ARDS) to describe a cohort of 12 critically ill patients with acute respiratory failure. In 1994, after decades of different definitions, the American-European Consensus Conference Committee recommended the adoption of a consensus definition for ALI/ARDS. This definition requires the acute onset of diffuse bilateral pulmonary infiltrates by chest radiograph; a PaO2/FiO2 ≤300 for ALI and ≤200 for ARDS; and a pulmonary artery wedge pressure (PAWP) ≤18 or no clinical evidence of left atrial hypertension.(4) The two primary advantages of this definition were the simplicity of clinical application and the ability to quantify the severity of lung injury. Although this definition has some limitations,(5–8) specifically in variability of chest radiograph interpretation, it has been widely adopted for both clinical and research purposes.
The incidence of ALI/ARDS has been difficult to assess due to nonuniform definitions, etiologic variations, geographical variation, inadequate documentation, and underrecognition of disease entity. In 2003, Goss and colleagues(9) used the NIH-funded ARDS network database to prospectively identify ALI patients from 1996–1999. They estimated an incidence of 64.2 cases per 100,000 person-years after adjusting for biases inherent within their study. More recently, Rubenfeld and colleagues(1) conducted a large prospective, population-based validated cohort study of ALI incidence in King County, Washington. The crude incidence was 78.9 per 100,000 person-years, with an age-adjusted incidence of 86.2 per 100,000 person-years. The strengths of this study were the prospective design, use of the consensus definition, and inclusion of a large number of patients from multiple intensive care units (ICUs) (21 hospitals) for 1 year. When this data was extrapolated to the United States as a whole, the investigators estimated that the incidence of ALI is approximately 200,000 patients with a mortality rate of 40%.(1)
Other factors such as age and associated clinical disorders may impact the incidence of ALI and ARDS. In the Rubenfeld cohort, similar to other studies,(10,11) the incidence of ALI increased with age from 16 per 100,000 person-years for those 15–19 years of age to 306 per 100,000 person-years for those 75–84 years of age.(1) Predisposing clinical factors include sepsis, pneumonia, aspiration, trauma, pancreatitis, blood transfusions, and smoke or toxic gas inhalation.(12) Severe sepsis and multiple transfusions are associated with the highest incidence of ARDS; the lowest rates occur in patients with trauma or drug overdoses.(1,13) For patients with multiple comorbidities, chronic alcohol abuse, or chronic lung disease, the risk for lung injury is higher.(12)
ARDS mortality rate has declined over the last 2 decades. In the 1980s, mortality rates were approximately 64–70%.(14–16) However, these rates must be interpreted with caution, as an ALI/ARDS consensus definition was not adopted until 1994. More recent studies now indicate a mortality risk of 29–42%.(1,17–19) The nature of the underlying clinical disorder is an important determinant of outcome. For example, sepsis has a higher mortality than major trauma (43 vs. 11%), whereas pneumonia and aspiration are intermediate risk factors (36 and 37%, respectively).(20) Other factors that influence mortality appear to be age and race. Rubenfeld and colleagues(1) found that mortality was significantly lower in patients 15–19 years of age (24%) compared to patients 85 years of age or older (60%). This finding was further supported by Flori et al's(21) prospective study of 328 pediatric patients with a reported mortality rate of 22%. Racial inequalities in disease burden also occur in African-Americans and Hispanics who have a higher 60-day mortality rate (33%) compared to Caucasians (27%.)(22) This increased risk of death is independent of age, gender, ventilation strategy, lung injury etiology, comorbidities, or degree of hypoxemia. For African-Americans, the severity of illness at presentation appeared to moderate this higher mortality risk. Despite these different mortality rates in specific age and racial groups, the overall trend has been a decline in mortality over the last 2 decades. The primary factors that seem to explain the reduction in mortality are the use of a lung protective ventilation strategy, a fluid conservative strategy, and other improvements in critical care including perhaps more effective treatment of sepsis. These factors will be discussed more in the section on treatment.
Pathogenesis
Acute lung injury is a disorder of acute inflammation that causes disruption of the lung endothelial and epithelial barriers. The alveolar–capillary membrane is comprised of the microvascular endothelium, interstitium, and alveolar epithelium. Cellular characteristics of ALI include loss of alveolar–capillary membrane integrity, excessive transepithelial neutrophil migration, and release of pro-inflammatory, cytotoxic mediators (Fig. 1).(12,23) Biomarkers found on the epithelium and endothelium and that are involved in the inflammatory and coagulation cascades predict morbidity and mortality in ALI (Table 1).
FIG. 1.
The normal alveolus (left-hand side) and the injured alveolus in the acute phase of ALI and the acute respiratory distress syndrome. In the acute phase of the syndrome (right-hand side), there is sloughing of both the bronchial and alveolar epithelial cell, with the formation of protein-rich hyaline membranes on the denuded basement membrane. Neutrophils are shown adhering to the injured capillary endothelium and marginating through the interstitium into the air space, which is filled with protein-rich edema fluid. In the air space, an alveolar macrophage is secreting cytokines, interleukin (IL)-1, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α, which act locally to stimulate chemotaxis and activate neutrophils. Interleukin-1 can also stimulate the production of extracellular matrix by fibroblasts. Neutrophils can release oxidants, proteases, leukotrienes, and other proinflammatory molecules, such as platelet-activating factor (PAF). A number of anti-inflammatory mediators are also present in the alveolar milieu, including IL-1-receptor antagonists, soluble TNF receptors, autoantibodies against IL-8, and cytokines such as IL-10 and IL-11 (not shown). The influx of protein-rich edema fluid into the alveolus has led to the inactivation of a surfactant. ALI, acute lung injury; MIF, macrophage inhibitory factor. Reprinted with the permission of the publisher.(12) Copyright 2000 Massachusetts Medical Society. All rights reserved.
Table 1.
Biomarkers in Clinical Trials of Acute Lung Injury and the Acute Respiratory Distress Syndrome
| Pathobiology | Biomarker | Abnormality in ALI/ARDS | Organ failure-free days | Ventilator-free days | Mortality |
|---|---|---|---|---|---|
| Endothelium | VWF | Increased | Reduced(31,32) | Reduced(31–33) | Predictive(31–33) |
| Endothelium and epithelium | ICAM-1 | Increased | Reduced(36) | Reduced(34,36) | Predictive(34–36) |
| SP-D | Increased | Reduced(51) | Reduced(51) | Predictive(51) | |
| RAGE | Increased | Reduceda(53) | Reduceda(53) | Predictivea(53) | |
| Inflammation | IL-6 | Increased | Reduced(25) | Reduced(25) | Predictive(24,25) |
| IL-8 | Increased | Reduced(25) | Reduced(25) | Predictive(24,25) | |
| Coagulation | Protein C | Decreased | Reduced(29) | Reduced(29) | Predictive(29) |
| PAI-1 | Increased | Reduced(29) | Reduced(29) | Predictive(29) |
Notes: VWF, von Willebrand factor antigen; ICAM-1, intercellular adhesion molecule; SP-D, surfactant protein D; RAGE, receptor for advanced glycation end-products; IL-6, IL-8, interleukins-6, -8; PAI-1, plasminogen activator inhibitor-1
Outcomes associated with high-tidal volume cohort only.
Following infection or trauma, upregulation of proinflammatory cytokines occurs as a direct response and/or as a marker of ongoing cellular injury. Meduri et al.(24) found that baseline and persistently elevated plasma levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α were strongly predicative of mortality. This finding was further supported by Parsons and colleagues'(25) large prospective study involving the ARDS Net trial of lower versus higher tidal volume. Even after adjustments for ventilator strategy, severity of illness and organ dysfunction, higher plasma levels of IL-6 and IL-8 were independently associated with fewer organ failure- and ventilator-free days, and elevated IL-6 and IL-8 independently predicted higher mortality. Several studies have demonstrated that lower tidal volume ventilation can attenuate the cytokine responses, potentially reflecting the ability to indirectly modulate the inflammatory response as well as decreasing ventilation-induced lung epithelial injury.(25–28) Alterations in coagulation and fibrinolysis also occur in lung injury, specifically protein C and plasminogen activator inhibitor-1. Ware et al.(29) measured plasma samples of these proteins taken as part of a large, prospective multicenter clinical trial. Compared to controls and patients with acute cardiogenic pulmonary edema, lower plasma levels of protein C and higher plasma levels of plasminogen activator inhibitor-1 were strong independent predictors of mortality, as well as ventilator-free and organ-failure-free days.
Microvascular endothelial injury leads to increased capillary permeability. This alteration in permeability permits the efflux of protein-rich fluid into the peribronchovascular interstitium, ultimately crossing the epithelial barrier into the distal airspaces of the lung.(30) Several studies have documented increased release of von Willebrand factor (vWf )(31–33) and upregulation of intracellular adhesion molecule-1 (ICAM-1)(34–36) following endothelial injury. Both of these biomarkers are independent predictors of mortality.
Transepithelial neutrophil migration is an important feature of acute lung injury because neutrophils are the primary perpetrators of inflammation. Excessive and/or prolonged activation of neutrophils contributes to basement membrane destruction and increased permeability of the alveolar–capillary barrier. Migrating groups of neutrophils result in the mechanical enlargement of paracellular neutrophil migratory paths.(37) Neutrophils also release damaging pro-inflammatory and pro-apoptotic mediators that act on adjacent cells to create ulcerating lesions.(37,38) One of the best studied neutrophil mediators, elastase, appears to degrade epithelial junctional proteins, possess pro-apoptotic properties, and perhaps have direct cytotoxic effects on the epithelium.(39–43) In some animal models, neutrophil depletion can be protective.(37,44–46) However, acute lung injury can also develop in the absence of circulating neutrophils indicating that neutrophil-independent pathways can also cause lung injury.(47)
Normally, type I and type II alveolar epithelial cells form tight junctions with each other, selectively regulating the epithelial barrier. Increased permeability of this membrane during the acute phase of lung injury leads to the influx of protein-rich edema fluid into alveolar space. Type I and II epithelial injury leads to disruption of normal fluid transport via downregulated epithelial Na channels and Na +/K +ATPase pumps, impairing the resolution of alveolar flooding.(12,30) In fact, Lee et al.(48) recently reported that alveolar edema fluid from ALI patients downregulated the expression of ion transport genes responsible for vectorial fluid transport in primary cultures of human alveolar epithelial type II cells. Conversely, gene expression for inflammatory cytokines IL-8, TNF-α, and IL-1β increased by 200, 700, and 900%, respectively. In functional studies, net vectorial fluid transport was also reduced (0.02 ± 0.05 vs. 1.31 ± 0.56 μL/cm2/h, p < 0.02). Alveolar epithelial type II cell injury also leads to a loss of surfactant production,(49) decreasing overall pulmonary compliance. Finally, type II epithelial cells normally drive the epithelial repair process; loss of this function can lead to disorganized, fibrosing repair.(50)
Alveolar epithelial biomarkers including surfactant D (SP-D) and the receptor for advanced glycation end-products (RAGE) are validated biomarkers for lung epithelial injury. SP-D, secreted by type II epithelial cells, has anti-inflammatory properties and promotes pathogen phagocytosis and neutrophil recruitment. A prospective study from the large ARDS Network low tidal volume ventilation cohort (563 patients) reported that higher baseline plasma SP-D levels were independently associated with mortality and fewer ventilator- and organ-failure free days after controlling for severity of illness, clinical covariates, and ventilator strategy.(51) RAGE, a transmembrane immunoglobulin primarily expressed on type I epithelial cells, is elevated in the plasma and edema fluid of patients with ALI compared to those with hydrostatic edema.(52) Calfee and colleagues(53) utilized the ARDS Network plasma samples from the low versus high tidal volume trial to further investigate the relationship of RAGE and ALI. This study reported that higher RAGE levels were associated with increased morbidity and mortality and fewer ventilator-free and organ-failure free days in the higher tidal volume cohort. These findings persisted after adjustment for age, gender, severity of illness, and the presence of sepsis or trauma. RAGE levels declined in both groups; however, there was a 15% greater reduction (p = 0.02) in day 3 RAGE levels in the lower tidal volume cohort.
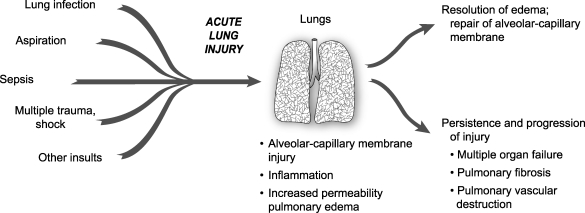
Resolution of ALI/ARDS is primarily dependent on a timely and orderly repair of the alveolar gas exchange apparatus. For gas exchange to improve, alveolar fluid transport must be upregulated, clearing the airspace of protein-rich edema fluid, and restoring the normal secretion of surface active material from alveolar type II cells (Fig. 2).(23,54)
FIG. 2.
The natural history of ALI/ARDS includes resolution and repair versus persistence and progression. Clinical and epidemiologic studies demonstrate that ALI/ARDS resolves with return of alveolar function to normal or near normal in some patients, whereas in others there is persistence and/or progression of injury. The outcomes of persistence and progression include multiple organ failure, fibrosing alveolitis, pulmonary vascular obliteration with pulmonary hypertension, and death. The genetic, cellular, molecular. and iatrogenic factors that contribute to each of these outcomes remain largely unknown. In addition, rational mechanism-based strategies that favorably influence repair of the alveolar–capillary membrane are undefined. Reprinted with the permission of the publisher.(23) Copyright 2005 American Thoracic Society. All rights reserved.
Treatment
Treatment of acute lung injury is based in both ventilatory and nonventilatory strategies. To date, the most significant advances in the supportive care of lung injury patients have been associated with improved ventilator management. Several clinical trials have shown that a large number of pharmacologic strategies have not been effective in reducing mortality.
The best evidence for the value of a lung protective strategy in patients with ALI is the National Heart, Lung, and Blood Institute (NHLBI) ARDS network's multicenter, randomized controlled trial of 861 patients with ALI/ARDS.(55) In this study, patients were randomized to 6 mL/kg tidal volume versus 12 mL/kg tidal volume with plateau pressure restrictions (<30 vs. <50 cm H2O). Mortality in the low tidal volume group was significantly lower than the high tidal volume group (31 vs. 40%, p = 0.007). Patients ventilated with low tidal volume also had more ventilator free and nonpulmonary organ failure-free days. Clinical risk factors including sepsis, aspiration, pneumonia, and trauma did not affect the efficacy of the low tidal volume strategy.(20) This strategy even attenuated the inflammatory response (IL-6 and IL-8) associated with acute lung injury.(25)
Optimal fluid management has been a controversial topic. In 2006, the NHLBI ARDS Network published the findings of their prospective, randomized controlled trial of fluid conservative versus fluid liberal management strategy.(56) Although there was not a significant difference in mortality, the fluid conservative strategy improved oxygenation and severity of lung injury as well as reduced the duration of mechanical ventilation. The incidence of nonpulmonary organ failure, specifically renal failure, and shock did not increase. These findings match well with previously published animal and human model studies.(56–60) This study also reported that the use of a pulmonary artery catheter (PAC) versus a central venous catheter (CVC) to guide fluid strategy was not associated with improved clinical outcomes.(61)
Recent advances in the understanding of the pathophysiology of acute lung injury have led to investigations of numerous potential pharmacologic treatments. Despite earlier encouraging preclinical evidence, phase III trials have not supported the use of exogenous surfactant, inhaled nitric oxide, intravenous prostaglandin E1, glucocorticoids, Ketoconazole, Lisofylline, N-acetylcysteine, and activated protein C as treatments for ALI (Table 2).
Table 2.
Selected Results of Clinical Trials of Pharmacologic Treatment for Acute Lung Injury and the Acute Respiratory Distress Syndrome
| Treatment | Year | Type of Study | No. of Patients | Findings | Study |
|---|---|---|---|---|---|
| Glucocorticoids (acute phase) | 1987 | Phase 3 | 99 | No benefit | Bernard et al.(90) |
| Glucocorticoids (acute phase) | 1988 | Phase 3 | 75 | No benefit | Luce et al.(91) |
| Surfactant | 1996 | Phase 3 | 725 | No benefit | Anzueto et al.(92) |
| N-acetylcysteine | 1997 | Phase 2 | 42 | No benefit | Domenighetti et al.(93) |
| Glucocorticoids (late phase) | 1998 | Phase 3 | 24 | Decreased mortality | Meduri et al.(94) |
| Inhaled nitric oxide | 1998 | Phase 2 | 177 | No benefit | Dellinger et al.(95) |
| Inhaled nitric oxide | 1999 | Phase 3 | 203 | No benefit | Payen et al.(96) |
| Liposomal PGE 1 (high dose) | 1999 | Phase 3 | 350 | No benefit | Abraham et al.(97) |
| Ketoconazole | 2000 | Phase 2 | 234 | No benefit | NIH ARDS Network(98) |
| Liposomal PGE 1 (low dose) | 2001 | Phase 3 | 102 | No benefit | Vincent et al.(99) |
| Lisofylline | 2002 | Phase 2-3 | 235 | Stopped for futility | NIH ARDS Network(100) |
| Glucocorticoids (late phase) | 2005 | Phase 3 | 180 | No benefit | Steinberg et al.(101) |
| Salbutamol IV | 2006 | Phase 2 | 40 | Reduced EVLW, improved survival trend | Perkins et al.(70) |
| Procysteine | 2008 | Phase 3 | 215 | Stopped for futility | Morris et al.(102) |
| Activated Protein C | 2008 | Phase 2 | 75 | Stopped for futility | Liu et al.(103) |
| Inhaled Albuterol | 2008 | Phase 3 | 279 | Stopped for futility | ARDS network: unpublished data |
There has been considerable preclinical data supporting the potential value of β-2 agonist therapy for the treatment of ALI.(62–68) These studies reported that β-2 agonists accelerate the resolution of pulmonary edema by decreasing inflammation and upregulating alveolar salt and water transport, hastening the resolution of alveolar edema. Recently, a large, multicenter, randomized clinical trial of an aerosolized β-2 agonist, albuterol, was stopped early for futility. In this NHLBI ARDS Network trial, the aerosolized β-2 agonist may not have been effective due to the severity of alveolar epithelial injury or suboptimal drug delivery to the injured alveoli.(69) There is a large, randomized clinical trial underway in the United Kingdom using intravenous salbutamol, a β-2 agonist. A small, single-center, randomized trial in the UK recently demonstrated that intravenous salbutamol significantly lowered extravascular lung water.(70)
The ARDS Network is currently investigating the potential benefits of initial trophic enteral feeding followed by advancement to full-calorie enteral feeding versus early advancement to full-calorie enteral feeding in patients with ALI/ARDS. Most clinicians agree that enteral nutrition is preferable over parenteral nutrition; however, the optimal timing, composition, and quantity of enteral feeding remains controversial. Some studies suggest enteral feeding within 48 hours of initiation of mechanical ventilation reduces mortality in patients with ARDS, although these findings are not conclusive.(71–73) Low to moderate volumes of enteral feed appear to reduce infections and mortality by maintaining intestinal microvilli height and structure and reducing inflammation by stimulating secretion of brush border enzyme, endogenous peptides, secretory IgA, and bile salts.(74–76)
A novel approach to ALI includes HMG-CoA reductase inhibitors (statins). Normally used for the prevention or treatment of cardiovascular disease, statins also possess significant anti-inflammatory, immunomodulatory, and antioxidant effects. However, it is uncertain how these properties will translate to the human ALI/ARDS population. Several observational studies in the human sepsis model, a known risk factor for ALI, have reported statin users have a decreased severity of sepsis and mortality despite having higher baseline comorbidities.(77–81) Preliminary data from two prospective randomized controlled trials involving statins and sepsis support these observational studies. Choi and colleagues(82) noted that hospital mortality was reduced in the statin group compared to placebo (27.3 vs. 55.9%; p = 0.026). Although Montoya and colleagues(83) did not report a difference in survival, hospital length of stay was shortened and C reactive protein (CRP) levels decreased in the simvastatin group. These findings need to be validated in prospective studies. Kor and colleagues(84) noted no difference in morbidity and mortality in a retrospective observational study of 178 patients with ALI/ARDS, 45% of whom had received statin therapy.
One promising new treatment for ALI is bone marrow-derived mesenchymal stem cells (MSCs). These cells possess the ability to differentiate into many types of cells, including vascular endothelium and alveolar epithelium. MSCs also secrete paracrine factors that reduce the severity of ALI,(85–88) including growth factors, factors that regulate barrier permeability, and anti-inflammatory cytokines. Gupta and colleagues(89) reported the MSCs' anti-inflammatory properties in both in vivo and in vitro. In a mouse model, Escherichia coli endotoxin was instilled into the distal airspaces of the lung, followed by direct intrapulmonary administration of MSCs 4 h later. MSCs decreased extravascular lung water, alveolar–capillary permeability, and mortality. These results were independent of the MSC's ability to engraft into the lung, a property suggestive of a paracrine mechanism of action. The pro-inflammatory response was downregulated, whereas the anti-inflammatory response upregulated. Several investigators are working on translating these experimental studies to phase I and II clinical trials of patients with severe ALI.
Finally, delivery of potential therapies via aerosol to the distal air spaces of the lung remains a viable delivery route for both small molecules and proteins. Depending on the treatment modality, aerosol delivery may avoid systemic effects and more specifically target the lung. Considerable expertise has been developed to optimize delivery of small and large molecules by the aerosol route in mechanically ventilated patients. This delivery method should be considered in future investigations of potential pharmacologic treatments.
Acknowledgments
This work was supported by the NHLBI HL51856 and HL51854 grants (MAM). The authors thank Diana Lim for her assistance.
Author Disclosure Statement
No conflicts of interest exist.
References
- 1.Rubenfeld GD. Caldwell E. Peabody E. Weaver J. Martin DP. Neff M. Stern EJ. Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Dowdy DW. Eid MP. Dennison CR. Mendez-Tellez PA. Herridge MS. Guallar E. Pronovost PJ. Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG. Bigelow DB. Petty TL. Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 4.Bernard GR. Artigas A. Brigham KL. Carlet J. Falke K. Hudson L. Lamy M. LeGall JR. Morris A. Spragg R. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 5.Esteban A. Fernandez-Segoviano P. Frutos-Vivar F. Aramburu JA. Najera L. Ferguson ND. Alia I. Gordo F. Rios F. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141:440–445. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson ND. Meade MO. Hallett DC. Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28:1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD. Caldwell E. Granton J. Hudson LD. Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 8.Meade MO. Cook RJ. Guyatt GH. Groll R. Kachura JR. Bedard M. Cook DJ. Slutsky AS. Stewart TE. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 9.Goss CH. Brower RG. Hudson LD. Rubenfeld GD. Network A. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 10.Hudson LD. Milberg JA. Anardi D. Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 11.Johnston CJ. Rubenfeld GD. Hudson LD. Effect of age on the development of ARDS in trauma patients. Chest. 2003;124:653–659. doi: 10.1378/chest.124.2.653. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB. Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 13.Hudson LD. Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest. 1999;116:74S–82S. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 14.Villar J. Slutsky AS. The incidence of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:814–816. doi: 10.1164/ajrccm/140.3.814. [DOI] [PubMed] [Google Scholar]
- 15.Fowler AA. Hamman RF. Good JT. Benson KN. Baird M. Eberle DJ. Petty TL. Hyers TM. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98:593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 16.Milberg JA. Davis DR. Steinberg KP. Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA. 1995;273:306–309. [PubMed] [Google Scholar]
- 17.Erickson SE. Martin GS. Davis JL. Matthay MA. Eisner MD. Network NNA. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeley E. McAuley DF. Eisner M. Miletin M. Matthay MA. Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambon M. Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 20.Eisner MD. Thompson T. Hudson LD. Luce JM. Hayden D. Schoenfeld D. Matthay MA. Acute Respiratory Distress Syndrome Network: Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 21.Flori HR. Glidden DV. Rutherford GW. Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 22.Erickson SE. Shlipak MG. Martin GS. Wheeler AP. Ancukiewicz M. Matthay MA. Eisner MD. National Institutes of Health National Heart, Lung, and Blood Institute, and Acute Respiratory Distress Syndrome Network: Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37:1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthay MA. Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meduri GU. Kohler G. Headley S. Tolley E. Stentz F. Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 25.Parsons PE. Eisner MD. Thompson BT. Matthay MA. Ancukiewicz M. Bernard GR. Wheeler AP. Network NARDSCT: Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 26.Stuber F. Wrigge H. Schroeder S. Wetegrove S. Zinserling J. Hoeft A. Putensen C. Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med. 2002;28:834–841. doi: 10.1007/s00134-002-1321-7. [DOI] [PubMed] [Google Scholar]
- 27.Ranieri VM. Suter PM. Tortorella C. De Tullio R. Dayer JM. Brienza A. Bruno F. Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 28.Levitt JE. Gould MK. Ware LB. Matthay MA. Analytic review: the pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009;24:151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 29.Ware LB. Matthay MA. Parsons PE. Thompson BT. Januzzi JL. Eisner MD. National Heart, Lung, and Blood Institute, and Acute Respiratory Distress Syndrome Clinical Trials Network: Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugin J. Verghese G. Widmer MC. Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 31.Ware LB. Conner ER. Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29:2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Ware LB. Eisner MD. Thompson BT. Parsons PE. Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 33.Flori HR. Ware LB. Milet M. Matthay MA. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2007;8:96–101. doi: 10.1097/01.PCC.0000257097.42640.6F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flori HR. Ware LB. Glidden D. Matthay MA. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2003;4:315–321. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 35.McClintock D. Zhuo H. Wickersham N. Matthay MA. Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calfee CS. Eisner MD. Parsons PE. Thompson BT. Conner ER., Jr Matthay MA. Ware LB. Network NARDSCT: Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35:248–257. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zemans RL. Colgan SP. Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Downey GP. Dong Q. Kruger J. Dedhar S. Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest. 1999;116:46S–54S. [PubMed] [Google Scholar]
- 39.Ginzberg HH. Cherapanov V. Dong Q. Cantin A. McCulloch CA. Shannon PT. Downey GP. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol. 2001;281:G705–G717. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- 40.Ginzberg HH. Shannon PT. Suzuki T. Hong O. Vachon E. Moraes T. Abreu MT. Cherepanov V. Wang X. Chow CW. Downey GP. Leukocyte elastase induces epithelial apoptosis: role of mitochondial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol. 2004;287:G286–G298. doi: 10.1152/ajpgi.00350.2003. [DOI] [PubMed] [Google Scholar]
- 41.Martin TR. Hagimoto N. Nakamura M. Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc. 2005;2:214–220. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matute-Bello G. Frevert CW. Liles WC. Nakamura M. Ruzinski JT. Ballman K. Wong VA. Vathanaprida C. Martin TR. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect Immun. 2001;69:5768–5776. doi: 10.1128/IAI.69.9.5768-5776.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matute-Bello G. Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shasby DM. Fox RB. Harada RN. Repine JE. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol. 1982;52:1237–1244. doi: 10.1152/jappl.1982.52.5.1237. [DOI] [PubMed] [Google Scholar]
- 45.Shasby DM. Vanbenthuysen KM. Tate RM. Shasby SS. McMurtry I. Repine JE. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982;125:443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- 46.Abraham E. Carmody A. Shenkar R. Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 47.Martin TR. Pistorese BP. Chi EY. Goodman RB. Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JW. Fang X. Dolganov G. Fremont RD. Bastarache JA. Ware LB. Matthay MA. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem. 2007;282:24109–24119. doi: 10.1074/jbc.M700821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene KE. Wright JR. Steinberg KP. Ruzinski JT. Caldwell E. Wong WB. Hull W. Whitsett JA. Akino T. Kuroki Y. Nagae H. Hudson LD. Martin TR. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 50.Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am J Med. 1992;92:39S–343S. doi: 10.1016/0002-9343(92)90606-c. [DOI] [PubMed] [Google Scholar]
- 51.Eisner MD. Parsons P. Matthay MA. Ware L. Greene K. Acute Respiratory Distress Syndrome Network: Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchida T. Shirasawa M. Ware LB. Kojima K. Hata Y. Makita K. Mednick G. Matthay ZA. Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calfee CS. Ware LB. Eisner MD. Parsons PE. Thompson BT. Wickersham N. Matthay MA. NHLBI ARDS Network: Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthay MA. Folkesson HG. Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 55.De Campos T. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 56.National Heart, Lung, Blood Institute, and Acute Respiratory Distress Syndrome Clinical Trials Network. Wiedemann HP. Wheeler AP. Bernard GR. Thompson BT. Hayden D. deBoisblanc B. Connors AF., Jr Hite RD. Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 57.Reising CA. Wall PL. Paradise NF. Timberlake GA. Moorman DW. Continuous dose furosemide as a therapeutic approach to acute respiratory distress syndrome (ARDS) J Surg Res. 1999;82:56–60. doi: 10.1006/jsre.1998.5513. [DOI] [PubMed] [Google Scholar]
- 58.Schuller D MJ. Caladrino FS. Schuster DP. Fluid balance during pulmonary edema: is fluid gain a marker or cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 59.Humphrey H HJ. Sznajder I. Silverstein M. Wood L. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1900;97:1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 60.Simmons R. Berdine G. Seidenfeld J. Prihoda T. Harris G. Smith J. Gilbert T. Mota E. Johanson WJ. Fluid balance and the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:924–929. doi: 10.1164/arrd.1987.135.4.924. [DOI] [PubMed] [Google Scholar]
- 61.National Heart, Lung, Blood Institute, Acute Respiratory Distress Syndrome Clinical Trials Network. Wheeler AP. Bernard GR. Thompson BT. Schoenfeld D. Wiedemann HP. deBoisblanc B. Connors AF., Jr Hite RD. Harabin AL. Pulmonary artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 62.McAuley DF. Matthay MA. Is there a role for beta-adrenoceptor agonists in the management of acute lung injury and the acute respiratory distress syndrome? Treat Respir Med. 2005;4:297–307. doi: 10.2165/00151829-200504050-00001. [DOI] [PubMed] [Google Scholar]
- 63.Manocha S. Gordon AC. Salehifar E. Groshaus H. Walley KR. Russell JA. Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury. Crit Care. 2006;10:R12. doi: 10.1186/cc3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berthiaume Y. Staub NC. Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–343. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berthiaume Y. Broaddus VC. Gropper MA. Tanita T. Matthay MA. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol. 1988;65:585–593. doi: 10.1152/jappl.1988.65.2.585. [DOI] [PubMed] [Google Scholar]
- 66.Sakuma T. Folkesson HG. Suzuki S. Okaniwa G. Fujimura S. Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- 67.Saldias FJ LE. Comellas AP. Ridge KM. Rutschman DH. Sznajder JI. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 2000;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- 68.Vivona ML MM. Chabaud MB. Friedlander G. Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol. 2001;25:554–561. doi: 10.1165/ajrcmb.25.5.4420. [DOI] [PubMed] [Google Scholar]
- 69.Matthay M. Brower R. Thompson B. Schoenfeld D. Eisner M. Carson S. Moss M. Douglas I. Hite D. MacIntyre N. Liu K. Randomized, placebo-controlled trial of an aerosolized beta-2 adrenergic agonist (albuterol) for the treatment of acute lung injury. Am J Respir Crit Care Med. 2009;179:A2166. [Google Scholar]
- 70.Perkins GD. McAuley DF. Thickett DR. Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 71.Artinian V. Krayem H. DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006;129:960–967. doi: 10.1378/chest.129.4.960. [DOI] [PubMed] [Google Scholar]
- 72.Eyer S. Micon L. Konstantinides F. Edlund D. Rooney K. Luxenberg M. Cerra F. Early enteral feeding does not attenuate metabolic response after blunt trauma. J Trauma. 1993;34:639–643. doi: 10.1097/00005373-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim E. Mehringer L. Prentice D. Sherman G. Schaiff R. Fraser V. Kollef M. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. J Parenter Enteral Nutr. 2002;26:174–181. doi: 10.1177/0148607102026003174. [DOI] [PubMed] [Google Scholar]
- 74.Hernandez G. Velasco N. Wainstein C. Castillo L. Bugedo G. Maiz A. Lopez F. Guzman S. Vargas C. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999;14:73–77. doi: 10.1016/s0883-9441(99)90017-5. [DOI] [PubMed] [Google Scholar]
- 75.Groos S. Hunefeld G. Luciano L. Parenteral versus enteral nutrition: Morphological changes in human adult intestinal mucosa. J Submicrosc Cytol Pathol. 1996;28:61–74. [PubMed] [Google Scholar]
- 76.Buchman A. Moukarzel A. Bhuta S. Belle M. Ament M. Eckhert C. Hollander D. Gornbein J. Kopple J. Vijayaroghavan S. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 77.Liappis AP. Kan VL. Rochester CG. Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 78.Almog Y. Shefer A. Novack V. Maimon N. Barski L. Eizinger M. Friger M. Zeller L. Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 79.Kruger P. Fitzsimmons K. Cook D. Jones M. Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 80.Hackam DG. Mamdani M. Li P. Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413–418. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- 81.Thomsen RW. Riis A. Kornum JB. Christensen S. Johnsen SP. Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med. 2008;168:2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 82.Choi HS PM. Kang HM. Kim YJ. Choi CW. You JH. Jo HY. Statin use in sepsis due to pneuomonia. Am J Respir Crit Care Med. 2008:A580. [Google Scholar]
- 83.Montoya CSC. Poblano M. Aguirre C. Olivera J. Marinez J. Franco J. Anti-inflammatory therapy with statins for septic patients. ECICS. 2008 abstract. [Google Scholar]
- 84.Kor DJ. Iscimen R. Yilmaz M. Brown MJ. Brown DR. Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009;35:1039–1046. doi: 10.1007/s00134-009-1421-8. [DOI] [PubMed] [Google Scholar]
- 85.Ortiz LA. Gambelli F. McBride C. Gaupp D. Baddoo M. Kaminski N. Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ortiz LA. Dutreil M. Fattman C. Pandey AC. Torres G. Go K. Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rojas M. Xu J. Woods CR. Mora AL. Spears W. Roman J. Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J. Woods CR. Mora AL. Joodi R. Brigham KL. Iyer S. Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 89.Gupta N. Su X. Popov B. Lee JW. Serikov V. Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 90.Bernard GR. Luce JM. Sprung CL. Rinaldo JE. Tate RM. Sibbald WJ. Kariman K. Higgins S. Bradley R. Metz CA. Harris TR. Brigham KL. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 91.Luce JM. Montgomery AB. Marks JD. Turner J. Metz CA. Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 92.Anzueto A. Baughman RP. Guntupalli KK. Weg JG. Wiedemann HP. Raventos AA. Lemaire F. Long W. Zaccardelli DS. Pattishall EN. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 93.Domenighetti G. Suter PM. Schaller MD. Ritz R. Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care. 1997;12:177–182. doi: 10.1016/s0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 94.Meduri GU. Headley AS. Golden E. Carson SJ. Umberger RA. Kelso T. Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 95.Dellinger RP. Zimmerman JL. Taylor RW. Straube RC. Hauser DL. Criner GJ. Davis K., Jr Hyers TM. Papadakos P. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Payen DVB. Genoa Group: Results of the French prospective multicentric randomized double-blind placebo-controlled trial on inhaled nitric oxide in ARDS. Intensive Care Med. 1999;25:S166. [Google Scholar]
- 97.Abraham E. Baughman R. Fletcher E. Heard S. Lamberti J. Levy H. Nelson L. Rumbak M. Steingrub J. Taylor J. Park YC. Hynds JM. Freitag J. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med. 1999;27:1478–1485. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 98.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 99.Vincent JL. Brase R. Santman F. Suter PM. McLuckie A. Dhainaut JF. Park Y. Karmel J. A multi-centre, double-blind, placebo-controlled study of liposomal prostaglandin E1 (TLC C-53) in patients with acute respiratory distress syndrome. Intensive Care Med. 2001;27:1578–1583. doi: 10.1007/s001340101077. [DOI] [PubMed] [Google Scholar]
- 100.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Steinberg KP. Hudson LD. Goodman RB. Hough CL. Lanken PN. Hyzy R. Thompson BT. Ancukiewicz M. National Heart, Lung, and Blood Institute, and Acute Respiratory Distress Syndrome Clinical Trials Network: Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 102.Morris PE. Papadakos P. Russell JA. Wunderink R. Schuster DP. Truwit JD. Vincent JL. Bernard GR. A double-blind placebo-controlled study to evaluate the safety and efficacy of L-2-oxothiazolidine-4-carboxylic acid in the treatment of patients with acute respiratory distress syndrome. Crit Care Med. 2008;36:782–788. doi: 10.1097/CCM.0B013E318164E7E4. [DOI] [PubMed] [Google Scholar]
- 103.Liu KD. Levitt J. Zhuo H. Kallet RH. Brady S. Steingrub J. Tidswell M. Siegel MD. Soto G. Peterson MW. Chesnutt MS. Phillips C. Weinacker A. Thompson BT. Eisner MD. Matthay MA. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]