Abstract
This study was undertaken to determine if the oral consumption of red beetroot food color would result in an inhibition of N-nitrosomethylbenzylamine (NMBA)-induced tumors in the rat esophagus. Rats were treated with NMBA and given either regular water ad libitum or water containing 78 μg/mL commercial red beetroot dye, E162. The number of NMBA-induced esophageal papillomas was reduced by 45% (P < .001) in animals that received the food color compared to controls. The treatment also resulted in reduced rates of cell proliferation in both precancerous esophageal lesions and in papillomas of NMBA-treated rats, as measured by immunohistochemical staining of Ki-67 in esophageal tissue specimens. The effects of beetroot food color on angiogenesis (microvessel density by CD34 immunostaining), inflammation (by CD45 immunostaining), and apoptosis (by terminal deoxynucleotidyl transferase dUTP nick end-labeling staining) in esophageal tissue specimens were also determined. Compared to rats treated with NMBA only, the levels of angiogenesis and inflammation in the beetroot color-consuming animals were reduced, and the apoptotic rate was increased. Thus, the mechanism(s) of chemoprevention by the active constituents of red beetroot color include reducing cell proliferation, angiogenesis, and inflammation and stimulating apoptosis. Importantly, consumption of the dye in the drinking water for a period of 35 weeks did not appear to induce any overt toxicity. Based on the fact that red beetroot color contains betanins, which have strong antioxidant activity, it is postulated that these effects are mediated through inhibition of oxygen radical-induced signal transduction. However, the sum of constituents of E162 has not been determined, and other components with other mechanisms may also be involved in antagonizing cancer development.
Key Words: red beetroot dye, esophageal carcinogenesis, rat
Introduction
The nitrosamine carcinogen N-nitrosomethylbenzylamine (NMBA) is a potent inducer of squamous cell papillomas in the rat esophagus.1,2 We have shown that freeze-dried black raspberries (Rubus occidentalis), which contain several anticancer agents such as ellagic acid, β-carotene, α-carotene, lutein, gallic acid, ferulic acid, p-coumaric acid, quercetin, β-sitosterol, stigmasterol, kaempferol, and anthocyanins, will reduce the number of NMBA-induced esophageal papillomas in rats when added to the diet at concentrations of 5% and 10%.3–6 When added at 20% of the diet, however, freeze-dried black raspberries do not further reduce papilloma multiplicity in this model.5,6 We have questioned, therefore, whether the esophageal tumor response to NMBA could be further reduced if the rats consumed other types of food-based anticancer agents. Purported anticancer chemicals not found in freeze-dried black raspberries are the betalains, which are composed of reddish to yellowish betacyanins7 and minor yellow to orange betacyanins. These pigments are prepared by mechanically crushing red beetroots and concentrating the water-soluble colored juice by spray-drying. The product is stabilized by the addition of ascorbic acid, citric acid and a diluent such as dextrin. The final product is sold by the food industry as red dye E162. Because they have exceptionally high radical scavenging activity, the betacyanins and other betalains are thought to be the most important anticancer constituents of E162.7–12 The only table foods containing these pigments are red beetroot, cactus pear, amaranth, pitaya, and Swiss chard.13 E162 prepared from red beetroot contains 0.35–0.5% dry weight of betalains. It also contains among many other constituents betaine at ∼1–1.2% dry weight. Betaine is known to methylate DNA by the transfer of a methyl group to S-adenosylmethionine and, ultimately, to the 5 position of cytosine in DNA.14–17 A common feature of tumors is hypomethylation, and Feinberg et al. and Kisseljova and Kisseljov recently reviewed the importance of hypomethylation in carcinogenesis and suggested that food constituents that antagonize hypomethylation should be cancer preventive.18,19 Thus, the betaine in E162 may also have chemopreventive activity. Finally, undefined components may also have anticancer activity.
Kapadia et al. have shown that carcinogen-treated mice given beetroot extract showed significant tumor inhibitory effect.8,9 They also reported that the mice that drank water containing 0.0025% E162 ad libitum had significantly reduced incidences of tumors in 7,12-dimethylbenz[a]anthracene-induced ultraviolet light B-promoted and (±)-(E)-4-methyl-2-[(E)-hydroxyamino]-5-nitro-6-methoxy-3-hexanamide-induced 12-O-tetradecanoylphorbol-13-acetate-promoted skin carcinogenesis and in N-nitrosodiethylamine-induced phenobarbital-promoted liver tumors. Because E162 has divergent anticancer constituents, we hypothesized that E162 might also inhibit NMBA-induced esophageal papilloma development in rats. Thus, we conducted the present study to determine the potential chemopreventive effects of E162 in the rat esophagus and to investigate the mechanism(s) of its action.
Materials and Methods
Animals, diet, and E162-water treatment
Male F344 rats, 4–5 weeks old (Harlan Sprague-Dawley, Indianapolis, IN, USA), were housed under standard conditions (20 ± 2°C; 50 ± 10% relative humidity; 12-hour light/dark cycle). The animals were provided AIN-76A synthetic diet (Dyets, Inc., Bethlehem, PA, USA) and either deionized water or E162-water. The E162-water was prepared by dissolving 78 μg/mL E162 dye powder (single batch provided by Chr. Hansen, Inc., Milwaukee, WI, USA) in water. The typical formation (percentage dry weight) of E162 is as follows: ash, 12.3 ± 0.1; protein, 12.9 ± 0.6; crude fiber, 0.3 ± 0.1; total sugars, 45.2 ± 0.04; sucrose, 34.0 ± 0.6; pigments (betaalins) 0.5 ± 0.15; and betaine, 1.0 ± 0.3. All animals received their water via bottles that were exchanged with fresh water or freshly prepared E162-water three times weekly. The animals were caged in a room with yellow lights. The dry food dye was weighed out using a certified balance. As the half-life of betacyanins is known20 to exceed 100 days at 16°C and at pH >3, the level of these constituents in the drinking water remained essentially constant. Body weight and food and water consumption per cage were recorded weekly. All experimental protocols were in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of The Ohio State University.
The rats were randomized into four experimental groups of 18 animals each. Groups 1 (control) and 3 (E162) rats were injected subcutaneously with 0.2 mL of 20% dimethyl sulfoxide (DMSO) in water (NMBA vehicle) three times per week for 5 weeks. Group 2 and 4 rats were injected subcutaneously with NMBA (0.3 mg/kg of body weight) (Ash Stevens, Inc., Detroit, MI, USA) in 0.2 mL of NMBA vehicle three times per week for 5 weeks. Groups 1 and 2 animals were provided regular drinking water, whereas groups 2 and 4 animals were provided E162-water ad libitum. The rats were sacrificed at 35 weeks by CO2 asphyxiation. The esophagus of each rat was opened longitudinally, and the surface tumors were mapped, counted, and sized. Lesions greater than 0.5 mm in a single dimension were classified as tumors. One-half of each esophagus and the remaining tumors were fixed in 10% neutral buffered formalin for histopathologic (hematoxylin and eosin and immunohistochemistry) evaluations; the remaining tissues were snap-frozen in liquid nitrogen.
Immunohistochemistry
Sections (4 μm) of the entire esophagus and all tumors from five rats per group were stained with antibodies for antigens that serve as biomarkers for proliferation (Ki-67), angiogenesis (CD34), inflammation (CD45), and apoptosis (terminal deoxynucleotidyl transferase dUTP nick end-labeling [TUNEL]). Ki-67, CD34, and TUNEL were evaluated using paraffin sections; CD45 was assessed using frozen sections. Antigens were retrieved by placing the slides in a vegetable steamer in target retrieval solution (Dako, Carpinteria, CA, USA) for 25 minutes. All slides were then incubated for 5 minutes with 3% hydrogen peroxide to block endogenous peroxidase. The slides were then stained using a Dako Autostainer. Staining conditions for the primary antibodies were as follows: Ki-67 (catalog number M7248, Dako), diluted 1:10, 1 hour at room temperature; CD34 (catalog number AF4117, R&D Systems, Minneapolis, MN, USA), diluted 1:800, 30 minutes at room temperature; and CD45 (catalog number 550566, BD Biosciences, Franklin Lakes, NJ, USA), diluted 1:25, 1 hour at room temperature. The slides were then incubated with the respective secondary antibodies as suggested by the manufacturer. The ApopTag Plus kit (catalog number S7101, Chemicon, Billerica, MA, USA) was used for the TUNEL staining following the manufacturer's instructions. All slides were counterstained with hematoxylin and viewed and photographed at × 200 with a bright-field microscope mounted with a high-resolution spot camera. The camera was interfaced with a computer containing a matrix frame grabber and Simple PCI Imaging Systems image analysis software (Compix Media, Torrance, CA, USA). Twenty fields per entire esophagus and 30 fields of all of the tumors per group were analyzed for their staining intensities. The staining data were compared using analysis of variance and an unpaired t test (StatView®, SAS Institute, Cary, NC, USA). A value of P < .05 was considered to be statistically significant.
Results
The animals consumed approximately 15 mL of water daily. There were no significant differences in either water or food consumption or in body weights among all groups of animals during the bioassay (data not shown). The lesion data are presented in Table 1. There is a statistically significant (P < .01) difference in the numbers of dysplastic lesions per esophagus between the NMBA group and the NMBA + E162-water group. In addition, there is a statistically significant (P < .001) difference in the average number of tumors between the NMBA group (11.4) and the NMBA + E162-water group (6.2).
Table 1.
Effects of E162-Water on NMBA-Induced Precancerous Lesion Formation in the Esophagi of F344 Rats
| Group | Normal | Epithelial hyperplasia | Dysplasia | Papillomas |
|---|---|---|---|---|
| 1 (DMSO) | 97.6 ± 4.6 | 2.4 ± 4.6 | None | None |
| 2 (E162-water) | 92.9 ± 16.0 | 7.1 ± 16.0 | None | None |
| 3 (NMBA) | 22.8 ± 9.6 | 48.1 ± 12.5 | 29.1 ± 13.9 | 11.39 ± 0.97 |
| 4 (NMBA + E162-water) | 25.2 ± 11.4 | 52.7 ± 13.1 | 22.1 ± 15.4a | 6.21 ± 0.58b |
Data are mean ± SD values.
Statistically significant relative to the NMBA group as determined by analysis of variance: aP < .01, bP < .001.
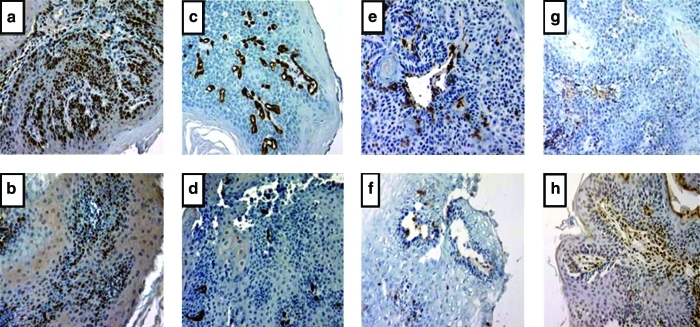
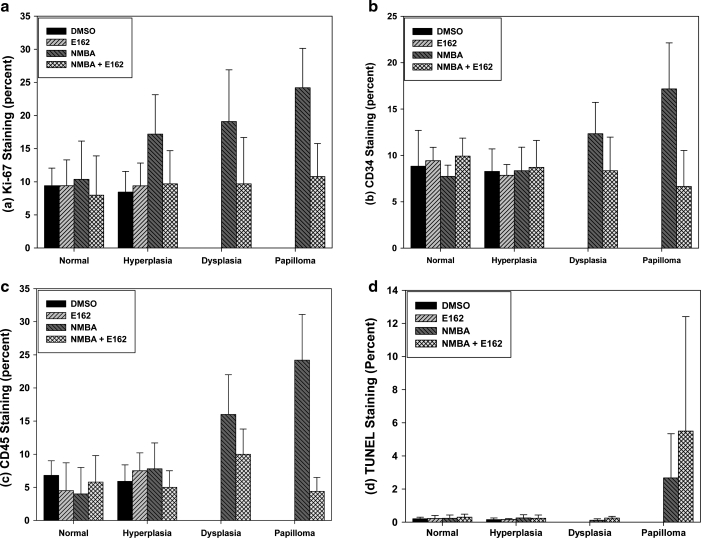
Examples of the biomarker staining for proliferation (Ki-67), angiogenesis (CD34), inflammation (CD45), and apoptosis (TUNEL) among the precancerous lesions and the tumors that developed in the NMBA group and the NMBA + E162-water group are shown in Figure 1, and the data developed from analyzing all of the images are summarized in Figure 2. Regarding Ki-67 (Fig. 2a), there was no difference in the rates of proliferation between the DMSO and E162 animals within the normal and hyperplastic regions of the esophagi. In contrast, the esophagi of NMBA-treated animals exhibited significantly (P < .005) elevated and progressively increasing Ki-67 staining within the hyperplastic, dysplastic, and papillomatous groups. In addition, the degree of staining in the papillomas was significantly greater (P < .005) than in the hyperplastic lesions. On the other hand, the level of Ki-67 staining in the corresponding lesions of the NMBA + E162 animals was not significantly different from the normal epithelium of the DMSO and E162 groups of rats. Thus, the rate of proliferation in the papillomas that arose in the NMBA animals was three times that of the control epithelium of the DMSO animals, whereas the papillomas that developed in the NMBA + E162 animals proliferated at a rate that was only slightly greater (30%; P < .04) than that of the normal epithelium of the DMSO animals.
FIG. 1.
Immunohistochemistry of regions with papilloma lesions: (a) NMBA Ki-67 staining; (b) NMBA + E162-water Ki-67 staining; (c) NMBA CD34 staining; (d) NMBA + E162-water CD34 staining; (e) NMBA CD45 staining; (f) NMBA + E162-water CD45 staining; (g) NMBA TUNEL staining; and (h) NMBA + E162-water TUNEL staining. Color images available online at www.liebertonline.com/jmf.
FIG. 2.
Summary of the immunochemistry analyses. (a) Effects of E162-water on Ki-67 labeling index as a biomarker of proliferation rate in preneoplastic and papillomatous rat esophagus. Data are mean ± SD values (n = 5). (b) Effects of E162-water on microvessel density in preneoplastic and papillomatous rat esophagus as determined by microvessel density reflected by CD34 staining. Data are mean ± SD values (n = 5). (c) Effects of E162-water on inflammation in preneoplastic and papillomatous rat esophagus determined by CD45 (leukocyte common antigen) staining. Data are mean ± SD values (n = 5). (d) Effects of E162-water on apoptotic index in preneoplastic and papillomatous lesions as measured by TUNEL staining. Data are mean ± SD values.
The effect of E162-water on angiogenesis in the lesions, as assessed by microvessel density, was evaluated by CD34 staining (Fig. 2b). In the group of rats treated with NMBA only, the microvessel density in the dysplastic lesions and in the papillomas was 160% and 220% higher (P < .001), respectively, compared with the normal epithelium of the DMSO and E162-water groups of animals. On the other hand, the respective lesions in the NMBA + E162-water group displayed no significant difference in CD34 expression compared with the normal epithelium of the DMSO and E162 groups of animals.
Inflammation was assessed by CD45 staining (Fig. 2c). The group of rats treated with NMBA only exhibited significantly (P < .001) increased levels of inflammatory cells in their dysplastic and papillomatous lesions compared to the normal epithelium and hyperplastic regions of their esophagi. In contrast, the NMBA + E162-water group displayed levels of inflammatory cells that were not significantly different from the normal epithelium of the DMSO and E162-water groups. Thus, the influx of inflammatory cells into the dysplastic and papillomatous lesions of the NMBA-treated animals was fourfold and sixfold greater, respectively, than the levels observed in the corresponding lesions that arose in the NMBA + E162 animals.
Lastly, we evaluated apoptosis (Fig. 2d). The only significant level of apoptosis detected by TUNEL staining was seen in the papillomas that arose in both the NMBA group and the NMBA + E162 group. The TUNEL staining indicated that the level of apoptosis was roughly twice (P < .05) as high in the NMBA + E162 group compared with the NMBA group, suggesting that the E162-water enhanced apoptosis.
Discussion
Kapadia et al. noted more than 10 years ago that water containing red beetroot extract (E162 food color) significantly inhibited the induction of lung, skin, and liver tumors in carcinogen-treated mice.8,9 Now, by coupling our rat esophagus results with theirs, it can be concluded that E162-water has a broad spectrum of chemopreventive potency. Specifically, it is anticarcinogenic in rats and mice, both males and females, and for multiple anatomic sites. Furthermore, the anticancer activity of E162 is manifested at a low concentration in drinking water (0.0025% and 0.0078% for mice and rats, respectively) and without overt toxicity to the animals. In contrast, prevention by freeze-dried black raspberries of NMBA-induced tumors in rats requires that the animals consume a daily diet consisting of 5% freeze-dried berries, or 0.4–4 g/kg ellagic acid, or 0.1 μmol/kg phenylethyl isothiocyanate.21 Thus, although E162 is not a pure chemical, as is ellagic acid and phenylethyl isothiocyanate, it is evident that it is a mixture of constituents that have potent chemopreventive activity. Numerous investigators have credited betacyanins and other betalains as having the anticancer activity in E162 preparations.7–13 As E162 is reported by the manufacturer to contain 0.35–0.5% betacyanins and the rats consumed ∼1.1 mg of E162 daily, it can be calculated that the animals received a steady-state daily dose of ∼5 μg (∼20 μg/kg) of betacyanins. Thus, if betacyanins are the primary active constituent of E162, it can be inferred that they have very potent cancer chemoprevention activity.
Betacalins are antioxidants. Stintzing et al. reported that betacyanins are twofold more active as antioxidants than anthocyanins,22 and Kanner et al. and Gliszczyńska-Swigło et al. noted that they were >10-fold more effective than α-tocopherol in inhibiting peroxidation.10,11 Thus, they are expected to antagonize inflammation. Inflammation-causing neutrophils and macrophages are frequently recruited into tumors and produce exogenous reactive oxygen species.23–26 In keeping with the reports that betacyanins are antioxidants, we compared the level of inflammatory lymphocytes in the lesions that developed in NMBA-treated rats that either did or did not drink E162-water using antibodies to the leukocyte common antigen CD45.27 Our data show that the tumors that developed in the animals that drank E162-water had significantly (P < .001) less lymphocyte infiltration, compared against the NMBA-alone animals. Furthermore, these experiments showed that the level of lymphocyte infiltration was not significantly different from the normal esophageal tissue of the DMSO (control) group. These results suggest that the betacyanins in E162 antagonize lymphocyte infiltration and reduce the level of inflammation. They also suggest that one of the mechanisms of E162 chemoprevention is reducing inflammation.
The xenotransplant studies of Zou et al. suggested that there was reduced angiogenesis in the tumors that developed in nude mice that received the cactus pear preparation of E162.28 Accordingly, we quantified microvessel densities in precancerous esophageal lesions and tumors in animals that drank E162-water, using the CD34 antibody.29 The data showed that the precancerous lesions and tumors that arose in the animals that drank E162-water had a microvessel density that was 40% lower than that in the esophagi of NMBA-alone animals. Thus, our results suggest that another cancer preventing activity of E162 is to antagonize angiogenesis. As angiogenesis is promoted by reactive oxygen species,30 some of the chemopreventive activity may be attributed to the antioxidant action of the betacyanins and other betalains.
Other in vitro observations suggest that betacyanins and other betalains depress proliferation. Sreekanth et al. showed that E162 caused a dose- and time-dependent decrease in cellular proliferation.31 Therefore, we quantified the levels of proliferation in precancerous lesions and in esophageal tumors, using Ki-67 staining. Our data show that the level of proliferation in these lesions in the NMBA + E162 rats remains at control levels, regardless of grade of pathology. In contrast, Ki-67 staining was significantly (P < .001) elevated in the hyperplastic, dysplastic, and papillomatous lesions that arose in the NMBA-only rats. Again, it can be hypothesized that the betacyanins and other betalains antagonized proliferation because their antioxidative activity reduced the level of reactive oxygen species to levels that were too low to stimulate proliferation via inappropriate signal transduction.32 Betacyanins and other betalains seemingly have also chemotherapeutic activities. Zou et al. showed that their E162-like extract of cactus pear was effective in slowing the growth of tumors after injection of SKOV3 human ovarian cancer cells in nude athymic mice.28 Their observation matches our Ki-67 data showing that the papillomas that developed in the NMBA + E162 animals proliferated more slowly compared to those that were found in the NMBA-alone rats. Alternatively, the reduced proliferation could be due to mechanisms that augment apoptosis.33 Accordingly, we evaluated apoptosis levels using the standard TUNEL assay. We found that the lesions in the esophagi from the animals that drank E162-water had relatively (P < .05) higher levels of apoptosis. Again, the antioxidant activity of the betacyanins in E162 may be responsible for this observation, as excessive reactive oxygen species can antagonize TP53-caused apoptosis by oxidative inactivation of the protein. As a consequence the lesion/tumor exhibits less apoptosis.34
Future purification studies are needed to affirm that betacyanins and other betalains are the primary chemopreventive constituents in E162. Another candidate is betaine.14–16 This C1 donor may constitute as much as 1.5% of the dry weight of E162. It is known that animals fed a methyl donor-deficient diet can develop hypomethylation and cancers.35 Thus, the betanin in E162 may antagonize cancer development by augmenting S-adenosylmethionine levels,36,37 which, in turn, antagonize the development of hypomethylation.18,19 In addition, betaine is anti-inflammatory.38 Future studies will look into this possible anti-hypomethylation or anti-inflammation role of betaine in the mechanism of E162 cancer chemoprevention.
In conclusion, the goal of chemoprevention is to prevent or greatly slow the processes that lead to excessive replication of damaged cells that have arisen from many years of carcinogen insults. Another characteristic is that the compound must have little or no long-term toxicity. It must also be sufficiently potent to provide protection at low doses, easy to administer, and inexpensive. Our results with E162 using a rat esophageal squamous cell carcinoma model show that it can antagonize tumorigenesis. It is also known to be effective as a chemopreventive against mouse skin, lung, and liver cancer. The antagonism requires small amounts (<50 mg/day for a 70-kg human) incorporated into drinking water, it has no known toxicity,39 and it is inexpensive. Thus, E162 adequately satisfies the required characteristics to be a chemopreventive food product. Finally, as the active constituents in E162 (betalains and betaine) are different from those in freeze-dried black raspberries, future studies will determine if treatment of rats with both E162 and freeze-dried black raspberries will cause a further reduction in NMBA-induced esophageal tumors in rats.
Acknowledgments
This research was supported in part by American Institute for Cancer Research grant 07A104 (to J.F.L.) and National Institutes of Health grant CA103189 (to G.D.S.).
Author Disclosure Statement
All authors declare no compelling financial interests exist.
References
- 1.Stoner GD. Siglin JC. Morse MA. Desai DH. Amin SG. Kresty LA. Toburen AL. Heffner EM. Francis DJ. Enhancement of esophageal carcinogenesis in male F344 rats by dietary phenylhexyl isothiocyanate. Carcinogenesis. 1995;16:2473–2476. doi: 10.1093/carcin/16.10.2473. [DOI] [PubMed] [Google Scholar]
- 2.Siglin JC. Barch DH. Stoner GD. Effects of dietary phenethyl isothiocyanate, ellagic acid, sulindac and calcium on the induction and progression of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Carcinogenesis. 1995;16:1101–1106. doi: 10.1093/carcin/16.5.1101. [DOI] [PubMed] [Google Scholar]
- 3.Kresty LA. Morse MA. Morgan C. Carlton PS. Lu J. Gupta A. Blackwood M. Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 4.Stoner GD. Chen T. Kresty LA. Aziz RM. Reinemann T. Nines R. Protection against esophageal cancer in rodents with lyophilized berries: potential mechanisms. Nutr Cancer. 2006;54:33–46. doi: 10.1207/s15327914nc5401_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoner GD. Wang LS. Zikri N. Chen T. Hecht SS. Huang C. Sardo C. Lechner JF. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoner GD. Wang LS. Chen T. Chemoprevention of esophageal squamous cell carcinoma. Toxicol Appl Pharmacol. 2007;224:337–349. doi: 10.1016/j.taap.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlov A. Kovatcheva P. Tuneva D. Ilieva M. Bley T. Radical scavenging activity and stability of betalains from Beta vulgaris hairy root culture in simulated conditions of human gastrointestinal tract. Plant Foods Hum Nutr. 2005;60:43–47. doi: 10.1007/s11130-005-5098-z. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia GJ. Tokuda H. Konoshima T. Nishino H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996;100:211–214. doi: 10.1016/0304-3835(95)04087-0. [DOI] [PubMed] [Google Scholar]
- 9.Kapadia GJ. Azuine MA. Sridhar R. Okuda Y. Tsuruta A. Ichiishi E. Mukainake T. Takasaki M. Konoshima T. Nishino H. Tokuda H. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol Res. 2003;47:141–148. doi: 10.1016/s1043-6618(02)00285-2. [DOI] [PubMed] [Google Scholar]
- 10.Kanner J. Harel S. Granit R. Betalains—a new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 11.Gliszczyńska-Swigło A. Szymusiak H. Malinowska P. Betanin, the main pigment of red beet: molecular origin of its exceptionally high free radical-scavenging activity. Food Addit Contam. 2006;23:1079–1087. doi: 10.1080/02652030600986032. [DOI] [PubMed] [Google Scholar]
- 12.Allegra M. Tesoriere L. Livrea MA. Betanin inhibits the myeloperoxidase/nitrite-induced oxidation of human low-density lipoproteins. Free Radic Res. 2007;41:335–341. doi: 10.1080/10715760601038783. [DOI] [PubMed] [Google Scholar]
- 13.Moreno DA. García-Viguera C. Gil JI. Gil-Izquierdo A. Betalains in the era of global agri-food science, technology and nutritional health. Phytochem Rev. 2008;7:261–280. [Google Scholar]
- 14.Castro R. Rivera I. Martins C. Struys EA. Jansen EE. Clode N. Graça LM. Blom HJ. Jakobs C. de Almeida IT. Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in HUVEC. J Mol Med. 2005;83:831–836. doi: 10.1007/s00109-005-0679-8. [DOI] [PubMed] [Google Scholar]
- 15.Zeisel SH. Mar MH. Howe JC. Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 16.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 17.Ueland PM. Holm PI. Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43:1069–1075. doi: 10.1515/CCLM.2005.187. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg AP. Ohlsson R. Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 19.Kisseljova NP. Kisseljov FL. DNA demethylation and carcinogenesis. Biochemistry (Mosc) 2005;70:743–752. doi: 10.1007/s10541-005-0179-z. [DOI] [PubMed] [Google Scholar]
- 20.Castellar MR. Obon JM. Fernandez-Lopez JA. The isolation and properties of a concentrated red-purple betacyanin food colourant from Oluntia stricta fruits. J Sci Food Agric. 2006;86:122–128. [Google Scholar]
- 21.Stoner GD. Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 22.Stintzing FC. Stintzing AS. Carle R. Frei B. Wrolstad RE. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agric Food Chem. 2002;50:6172–6181. doi: 10.1021/jf0204811. [DOI] [PubMed] [Google Scholar]
- 23.Abdalla SI. Sanderson IR. Fitzgerald RC. Effect of inflammation on cyclooxygenase (COX)-2 expression in benign and malignant oesophageal cells. Carcinogenesis. 2005;26:1627–1633. doi: 10.1093/carcin/bgi114. [DOI] [PubMed] [Google Scholar]
- 24.Knaapen AM. Güngör N. Schins RP. Borm PJ. Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Murakami A. Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 27.Holmes N. CD45: all is not yet crystal clear. Immunology. 2006;117:145–155. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou DM. Brewer M. Garcia F. Feugang JM. Wang J. Zang R. Liu H. Zou C. Cactus pear: a natural product in cancer chemoprevention. Nutr J. 2005;4:25–33. doi: 10.1186/1475-2891-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzzan B. Nicolas P. Cucherat M. Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2455. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 30.Blanchetot C. Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 31.Sreekanth D. Arunasree MK. Roy KR. Chandramohan Reddy T. Reddy GV. Reddanna P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia cell line-K562. Phytomedicine. 2007;14:739–746. doi: 10.1016/j.phymed.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg AS. Weinberg RA. Control of the cell cycle and apoptosis. Eur J Cancer. 1999;35:1886–1894. doi: 10.1016/s0959-8049(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 35.Pogribny IP. Ross SA. Wise C. Pogribna M. Jones EA. Tryndyak VP. James SJ. Dragan YP. Poirier LA. Irreversible global DNA hypomethylation as a key step in hepatocarcinogenesis induced by dietary methyl deficiency. Mutat Res. 2006;593:80–87. doi: 10.1016/j.mrfmmm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 37.Ueland PM. Holm PI. Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43:1069–1075. doi: 10.1515/CCLM.2005.187. [DOI] [PubMed] [Google Scholar]
- 38.Detopoulou P. Panagiotakos DB. Antonopoulou S. Pitsavos C. Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 39.WHO Food Additives Series 22. www.inchem.org/documents/jecfa/jecmono/v22je08.htm. [Jul;2007 ]. www.inchem.org/documents/jecfa/jecmono/v22je08.htm