Abstract
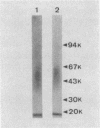
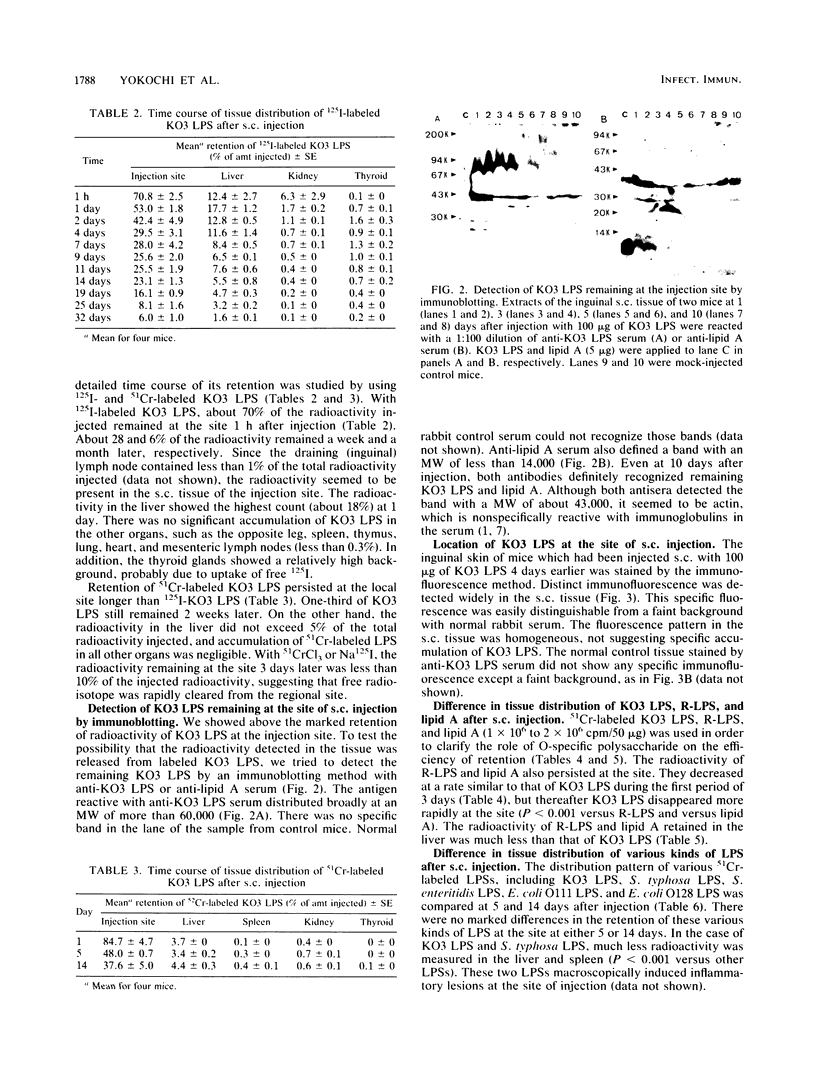
The tissue distribution of Klebsiella pneumoniae O3 lipopolysaccharide (KO3 LPS) was studied in mice injected subcutaneously (s.c.) or intraperitoneally (i.p.) with 125I-labeled KO3 LPS. Marked retention of KO3 LPS radioactivity could be found at the site of s.c. injection for several weeks. On the other hand, about 85% of the radioactivity rapidly disappeared from the peritoneal cavity within 6 h after i.p. injection. The long-term presence of KO3 LPS at the injection site was also supported by experiments with 51Cr-labeled KO3 LPS and immunoblotting and immunofluorescence staining methods. The R-form LPS lacking the O-specific polysaccharide chain of KO3 LPS and the lipid A fraction of KO3 LPS seemed to remain at the site in larger amounts and for longer times than KO3 LPS. There were no marked differences in the retention pattern at the injection site among KO3 LPS, Escherichia coli LPS, Salmonella typhosa LPS, and Salmonella enteritidis LPS. However, much less radioactivity accumulated in the livers and spleens of mice injected with either KO3 LPS or S. typhosa LPS compared with the other LPS preparations. It was suggested that retention of LPS at the site of s.c. injection may play an important role in the development of various biological actions of s.c. injected LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber B. H., Delovitch T. L. The identification of actin as a major lymphocyte component. J Immunol. 1979 Jan;122(1):320–325. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Alterations in rats in vivo of the chemical structure of lipopolysaccharide from Salmonella abortus equi. Eur J Biochem. 1985 Oct 15;152(2):353–359. doi: 10.1111/j.1432-1033.1985.tb09205.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ohta M., Mori M., Nakashima I., Kato N. The Klebsiella O3 lipopolysaccharide isolated from culture fluid: structure of the polysaccharide moiety. Microbiol Immunol. 1983;27(8):683–694. doi: 10.1111/j.1348-0421.1983.tb00631.x. [DOI] [PubMed] [Google Scholar]
- JONES R. S., RADCLIFFE I., LINKER A. Immunologic, chemical and isotopic identification of C-14-labeled bacterial polysaccharide in animal tissues. Proc Soc Exp Biol Med. 1962 Jun;110:323–326. doi: 10.3181/00379727-110-27505. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Kido N., Ohta M., Naito S. Comparative studies on adjuvanticity of Klebsiella O3 lipopolysaccharide and its lipid A and polysaccharide fractions. Immunology. 1985 Feb;54(2):317–324. [PMC free article] [PubMed] [Google Scholar]
- Kato N., Nakashima I., Ota M. Interferon production in mice by the capsular polysaccharide of Klebsiella pneumoniae. Infect Immun. 1975 Jul;12(1):1–6. doi: 10.1128/iai.12.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Ohta M., Hasegawa T., Mori M., Yamaki K., Mizuta K., Nakashima I., Naito S. Further studies of the polysaccharide of Klebsiella pneumoniae possessing strong adjuvanticity. II. Serological relationship between the adjuvant polysaccharide and O3 antigen of Klebsiella. Microbiol Immunol. 1981;25(12):1317–1325. doi: 10.1111/j.1348-0421.1981.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Kato N., Ohta M., Kido N., Naito S., Nakashima I., Nagase F., Yokochi T. Strong adjuvanticity of bacterial lipopolysaccharides possessing the homopolysaccharides consisting of mannose as the O-specific polysaccharide chains. Med Microbiol Immunol. 1985;174(1):1–14. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOYES H. E., McINTURF C. R., BLAHUTA G. J. Studies on distribution of Escherichia coli endotoxin in mice. Proc Soc Exp Biol Med. 1959 Jan;100(1):65–68. doi: 10.3181/00379727-100-24525. [DOI] [PubMed] [Google Scholar]
- Nakashima I. Adjuvant action of capsular polysaccharide of Klebsiella pneumoniae on antibody response. I. Intensity of its action. J Immunol. 1972 Apr;108(4):1009–1016. [PubMed] [Google Scholar]
- Nakashima I., Kato N. Microbial adjuvant and autoimmunity. I. Induction of antibody responses to syngeneic tissue extracts in mice treated with capsular polysaccharide of Klebsiella pneumoniae. Jpn J Microbiol. 1975 Feb;19(1):13–18. doi: 10.1111/j.1348-0421.1975.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Kojima T., Kato N. Cellular aspects of non-specific stimulation of antibody production by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1976 Feb;30(2):229–240. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Nagase F., Matsuura A., Yokochi T., Kato N. Adjuvant actions of polyclonal lymphocyte activators. III. Two distinct types of T-initiating adjuvant action demonstrated under different experimental conditions. Cell Immunol. 1980 Jul 1;52(2):429–437. doi: 10.1016/0008-8749(80)90363-9. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Yokochi T., Kato N., Asai J. Microbial adjuvant and autoimmunity. II. Production of lesions in mice immunized with syngeneic tissue extracts together with the capsular polysaccharide of Klebsiella pneumoniae. Microbiol Immunol. 1977;21(5):279–288. doi: 10.1111/j.1348-0421.1977.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Ohta M., Kido N., Hasegawa T., Ito H., Fujii Y., Arakawa Y., Komatsu T., Kato N. Contribution of the mannan O side-chains to the adjuvant action of lipopolysaccharides. Immunology. 1987 Apr;60(4):503–507. [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Mori M., Hasegawa T., Nagase F., Nakashima I., Naito S., Kato N. Further Studies of the polysaccharide of Klebsiella pneumoniae possessing strong adjuvanticity. I. Production of the adjuvant polysaccharide by noncapsulated mutant. Microbiol Immunol. 1981;25(9):939–948. doi: 10.1111/j.1348-0421.1981.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N., Asai J., Iijima S. Adjuvant action of capsular polysaccharide of Klebsiella pneumoniae on antibody response. IX. Its effect on the histology of the regional lymph node and other lymphoid organs. Microbiol Immunol. 1980;24(10):933–944. doi: 10.1111/j.1348-0421.1980.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N. Effect of capsular polysaccharide of Klebsiella pneumoniae on the differentiation and functional capacity of macrophages cultured in vitro. Microbiol Immunol. 1977 Oct 20;21(10):601–610. doi: 10.1111/j.1348-0421.1977.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Kato N., Miyadai T., Yoshida K., Kimura Y. In vivo effects of bacterial lipopolysaccharide on proliferation of macrophage colony-forming cells in bone marrow and peripheral lymphoid tissues. Infect Immun. 1985 Feb;47(2):496–501. doi: 10.1128/iai.47.2.496-501.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokochi T., Nakashima I., Nagase F., Kato N., Ohta M., Fujii Y., Mizoguchi K., Isobe K., Saito M. Further studies of the polysaccharide of Klebsiella pneumoniae possessing strong adjuvanticity. III. Augmentation of the antibody response to subcutaneously injected sheep red blood cells by the adjuvant polysaccharide. Microbiol Immunol. 1982;26(9):843–852. doi: 10.1111/j.1348-0421.1982.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Oka K., Iwata H., Miyadai T., Kimura Y., Kato N. Characterization of autoantigens relevant to autoimmune ophthalmitis and thyroiditis in mice immunized with the syngeneic tissue extracts and Klebsiella O3 lipopolysaccharide. Microbiol Immunol. 1987;31(10):1025–1032. doi: 10.1111/j.1348-0421.1987.tb01335.x. [DOI] [PubMed] [Google Scholar]