Abstract
The effect of SN-38 was evaluated on multiple lung cancer cell lines. It inhibits anchorage-dependent and –independent growth as monitored by MTT and soft agar colony assay, respectively. SN-38 collapsed the mitochondrial membrane potential (MMP), arrested cells in S- and G2-phases of the cell cycle, and induced apoptosis via activation of caspase 3 and PARP. A single injection of 2mg/kg body weight of SN-38 caused a significant reduction of lung cancer xenografts. These findings indicate that SN-38 induces apoptosis in the lung cancer cells effectively. Thus, SN-38 can potentially be an effective therapeutic agent against lung cancer.
Keywords: SN-38, adenocarcinoma, apoptosis, mitochondrial membrane potential, cell cycle arrest
I. INTRODUCTION
Despite rapid advances in diagnostic and operative techniques, lung cancer remains one of the most difficult human malignancies to treat. The American Cancer Society estimates that 214,440 persons in the United States developed lung cancer in 2009, with 159,390 deaths1. Lung cancer-dependent deaths constituted 30% (men) and 26% (women) of the estimated total cancer-related deaths in 2009(1). Data indicate that while the overall incidence of lung cancer is declining, it continues to rise in women1. The relative 5-year survival ratio of the patients that had lung or bronchus cancer from 1995 to 2001 was still quite low (15%) and was not improved very much compared to the 1970’s (12%). Therefore, it is clear that novel treatment strategies for lung cancer are urgently needed. The possible cures for lung cancer are surgery, chemotherapy, and radiotherapy. A major difficulty in lung cancer treatment is that with common surgical and radiation therapies there is considerable damage to normal tissue. Recently, multiple new chemotherapeutic agents have been developed and some are in clinical trials2, 3. Although some of them have produced promising results, their therapeutic spectrum is narrow.
Camptothecin derivatives have been widely used for cancer chemotherapy over the last two decades. Their mechanism of action is by inhibiting topoisomerase I activity4–6. Among camptothecin derivatives, irinotecan and topotecan are widely used clinically. Irinotecan in particular has been demonstrated to be superior to most broad-spectrum conventional cancer therapies7–9. The usual chemotherapy for extensive small cell lung cancer is etoposide plus cisplatin or this combination in alternation with a regimen of doxorubicin, vincristine, and cyclophosphamide10–13. In earlier studies, irinotecan hydrochloride, a topoisomerase I inhibitor, was effective against small-cell lung cancer14. Noda et al (2002) have shown that irinotecan plus cisplatin is an effective treatment for metastatic small-cell lung cancer8. Severe and unpredictable diarrhea caused by irinotecan has hampered its clinical use. Irinotecan undergoes metabolic activation by carboxylesterase to yield the active metabolite, SN-38, which has 100 to 1000-fold greater cytotoxicity compared to the parent compound6. This indicates SN-38 itself can be a very useful and potent anti-cancer drug.
Apoptosis is an important phenomenon in the cytotoxicity induced by anticancer drugs, and cell death can be induced by several mechanisms15–20. Many reports have indicated that mitochondria play a critical role in the commitment of cells to apoptosis. Anticancer drugs may damage the mitochondria by increasing the permeability of the outer mitochondrial membrane, which is associated with collapse of mitochondrial membrane potential (MMP)17, 18. Some anticancer drugs arrest cells in either S- or G2-phase, leading to apoptosis16, 19, 20. A few anticancer drugs also cause death to neighboring cells by a bystander mechanism by producing extracellular molecules such as reactive oxygen species (ROS)15.
The molecular mechanism of SN-38 action has been studied primarily using colon cancer 21–23 but very few studies have been conducted for lung cancer24–26. Accordingly, the present study was undertaken to test the effect of SN-38 on non-small-cell lung cancer (NSCLC). The present study focused on the effect of SN-38 on lung cancer cell lines from varieties of angles, such as anchorage-dependent and independent growth, mitochondrial membrane potential (MMP), cell cycle analysis, and a syngeneic mouse graft study. Our study showed that SN-38 causes S- and G2 cell cycle arrest and involvement of caspase-3 and PARP for induction of apoptosis/cell death in lung cancer cell line. It also has inhibited LLC tumor growth in syngeneic mice model.
II. MATERIALS AND METHODS
A. Cell Cultures
Lewis lung carcinoma (LLC) and human lung cancer cell lines A549 and H358 were purchased from American Type Culture Collection (Manassas, VA). Cells were maintained in DMEM (Gibco BRL) for LLC, Ham’s F-12 (Mediatech, Inc., Herndon, VA) for A549, and RPMI-1640 (Gibco BRL) media for H358. All media were supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA). The cells were incubated in 5% CO2 humidified at 37°C for growth.
B. Evaluation of cell proliferation
The number of viable LLC, A549, or H358 cells after SN-38 treatment was evaluated by the MTT (3-[4, 5-methylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide) assay. In brief, LLC, A549, and H358 cells (5 × 103 cells/well) were seeded in a 96 well plate and kept overnight for attachment. The next day the medium was replaced with fresh medium with various concentrations of SN-38 and cells were allowed to grow for 48 hrs. Four hours before completion of 48 hrs incubation, 10µl of MTT (5 mg/ml) was added in each well- and plates were returned to the incubator. After completing the incubation, 100 µl of solubilization buffer (10% SDS with 0.01 N HCl) was added to each well and incubated overnight at room temperature. Color developed after the reaction was measured at 550 nm and background absorbance was measured at 630 nm using the Molecular Devices Spectramax 190 plate reader (Global Medical Instrumentation, Inc. Ramsey, Minnesota).
C. Soft agar colony assay
Anchorage-independent cell growth inhibition was monitored by soft agar assay in 12 well tissue culture plates. A549 or H358 cells (500µl containing 5000 cells) suspended in 0.4% agar in appropriate culture medium containing 10 % serum were overlaid onto the bottom layer of 0.8% agar (750µl) in the same culture medium. Plates were incubated at 37°C overnight. The next day 150µl medium with different concentration of SN-38 was added in each well to get final concentrations of 10, 100 and 1000nM SN-38 and cells were allowed to form colonies. For monitoring colony formation, images were captured on day 10. The number of colonies > 1000 µm2 size was counted using an automated computer-controlled colony counter and an inverted microscope equipped with an image analysis computer (Olympus, Center Valley, PA).
D. Analysis of apoptosis
Apoptosis was determined by acridine orange-ethidium bromide staining (AO-Et staining). In brief, 2.5×105 A549 cells were plated in 6-well tissue culture plates and kept overnight for attachment. The next day, the old medium was replaced with fresh medium with various concentrations of SN-38 and incubated for 48 hrs. Cells were harvested by trypsinization and washed with PBS. The extent of apoptosis was monitored by either fluorescence microscope or flow cytometer. For fluorescence microscope analysis, 1 × 106 cells were suspended in 1 ml PBS, 25µl of the cell suspension aliquot was mixed with 1µl of AO-Et Dye Mix (100 µg/ml acridine orange and100 µg/ml ethidium bromide), and the mixture was observed under the microscope. Examination of the sample was carried out using epi-illumination and a fluorescence filter combination suitable for observing fluorescein (ie, with a 495 nm primary filter and a 515 nm secondary filter). Apoptosis was determined according to the following criteria: normal viable cell: bright green chromatin with organized structure; necrotic cell: bright orange chromatin with organized structure; apoptotic cell with intact membrane: bright green chromatin which is highly condensed or fragmented; apoptotic cell with damaged membrane: bright orange chromatin which is highly condensed or fragmented. For flow cytometry, 0.5 ml cells (5 × 105) were incubated at room temperature with 0.10 µM acridine orange and 0.25 µM ethidium bromide containing PBS for 5 minutes, then analyzed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) using FL1 and FL3 set-up of filter.
E. Assessment of Mitochondrial Membrane Potential
For assessing the mitochondrial membrane potential, A549 cells (2.5 × 105/well) were cultured in 6-well plates and incubated with either 10 or 100 nM SN-38 for 24 hrs. The medium was removed and the cells were collected by trypsinization. The cells were pelleted by centrifugation, resuspended in 1 ml of rhodamine 123 solution (10 µg/ml PBS), incubated for 30 min at room temperature, washed with PBS and finally resuspended in PBS. The samples were analyzed for fluorescence (FL-1 detector, filter 530/30 nm band pass) on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) using the Cell Quest software.
F. Cell cycle analysis
To analyze the effect of SN-38 on the cell cycle, A549 cells (2.5 × 105/well) were incubated with 10 and 100 nM SN-38 for 48hrs and the DNA content was measured. In brief, cells were harvested, fixed in 70% pre-chilled ethanol, and incubated in PBS containing 40 µg/ml propidium iodide and 100 µg/ml RNase A for 1hr at room temperature. The fluorescence (excitation at 488 nm and emission at 585/42 nm) of 20,000 cells from each sample was measured with a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA).
G. Radioactivity release assay
To test whether SN-38 can induce cell death by a bystander mechanism as has been shown for other anticancer drugs15, a radioactivity release assay was carried out. In brief, 5×103 A549 cells were plated in a 12 well culture plate and kept overnight for attachment. Cells were radio-labeled with 2µCi [3H]-thymidine for 24 hrs in CO2 incubator. Radio-labeled cells were washed twice with PBS and then co-cultured for an additional 48 hrs with another set of A549 cells treated with either 100 or 500nM SN-38 for 4 hrs. After incubation, a portion of the culture medium (350µl) was collected and radioactivity released was counted using the Packard liquid scintillation counter Tri-Carb 2100TR (Perkin Elmer Life Science Boston, MA).
H. Western blot analysis
Half a million A549 cells were cultured in 6-well plates and allowed to grow 24 hrs for attachment. The cells were incubated with 10, 100 and 500 nM SN-38 for 48 hrs. Total cell lysate was prepared using 150 µl lysis buffer (1% TritonX-100, 0.1% SDS, 0.25M sucrose, 1mM EDTA, 30mM Tris-HCl (pH 8.0)) supplemented with protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). Proteins were separated by a 6 or 12% SDS-PAGE gel, electroblotted onto nitrocellulose membrane (Amersham Bioscience), and blocked overnight at 4°C with 5% nonfat dry milk in 0.1% Tween 20 in TBS (TBST). The membrane was washed and incubated with antibodies against caspase-3 and PARP (poly(ADP-ribose) polymerase) at a 1:1000 dilution with 5% nonfat dry milk in TBST for overnight at 4°C and then with a horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (Amersham Biosciences). The protein expression signal was detected with Pierce ECL Western Blotting substrate (Pierce, Rockford, IL). GAPDH was used as the sample loading control by re-probing with an anti-GAPDH antibody at a 1:8000 dilution (Santa Cruz Biotechnology, Inc.).
I. Effect of SN-38 on intraperitoneal LLC tumor model
To study the effect of SN-38, an intraperitoneal LLC tumor model was used. Wild-type 6–8 week old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, MA) were used for this study. All animal experiments were done under strict adherence with the Institutional Animal Care and Use Committee protocol as set by Kansas State University. In brief, each mouse was transplanted with 1×106 LLC cells per 200µl PBS in the peritoneal cavity. Five days after tumor transplantation mice were randomly assigned to groups: 1) Vehicle control, and 2) SN-38 group. There were 5 mice in each group. Each mouse was injected with either vehicle (0.4% DMSO in PBS) or 2mg/kg body weight SN-38. All the mice were kept under observation and were sacrificed at 15 days post tumor transplantation. All of the tumor masses were collected from the peritoneal cavity and combined weights were recorded. Changes in the tumor weight compared to vehicle-treated controls were used for determining the effect of SN-38.
J. Statistical analysis
Data are expressed as mean ± SE (standard error). For all in vitro experiments, statistical significance was assessed by the Tukey-Kramer Pairwise Comparisons test. If not otherwise stated, all experiments reported represent two independent replications performed in triplicate. For the in vivo experiment, the Mann-Whitney non-parametric statistical test was carried out. Statistical significance was set at * p < 0.01; ** p < 0.05; *** p < 0.001.
III. RESULTS
A. SN-38 inhibits cell proliferation in two– and three- dimensional cell cultures
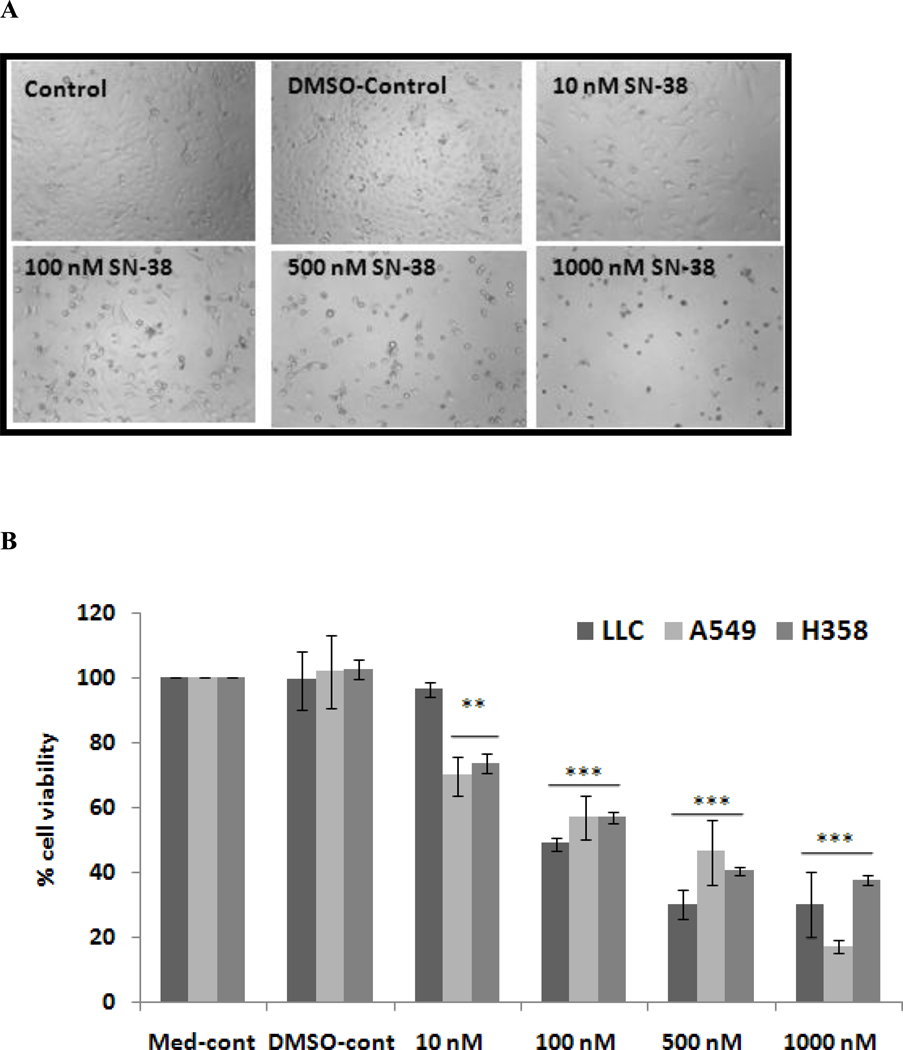
It is well known that the activity of mitochondrial dehydrogenase enzymes, detectable by catalyzing MTT reagent, correlates with viable cell number, which is used as a measure for cell proliferation in two–dimensional cell cultures. For studying the cell morphology, images were captured just before addition of MTT reagent. Analysis of the morphology and results of the MTT assay indicate that SN-38 began exhibiting an effect at the 10 nM concentration (Fig. 1) and induced cell death in approximately 50% of the cells at 100nM. This indicates that SN-38 is a very potent inhibitor of the growth of lung cancer cells.
Fig. 1.
Effect of SN-38 on LLC, A549 and H358 cells after incubation with different concentration of SN-38 for 48 hrs. A. Morphology of A549 cells in absence and presence of SN-38 (100 × magnification); B. SN-38 effect on proliferation of LLC, A549, and H358 cells was determined by MTT assay. **, p ≤ 0.01, ***, p ≤ 0.001 as compared to the level of DMSO control.
B. SN-38 inhibits anchorage-independent cell growth
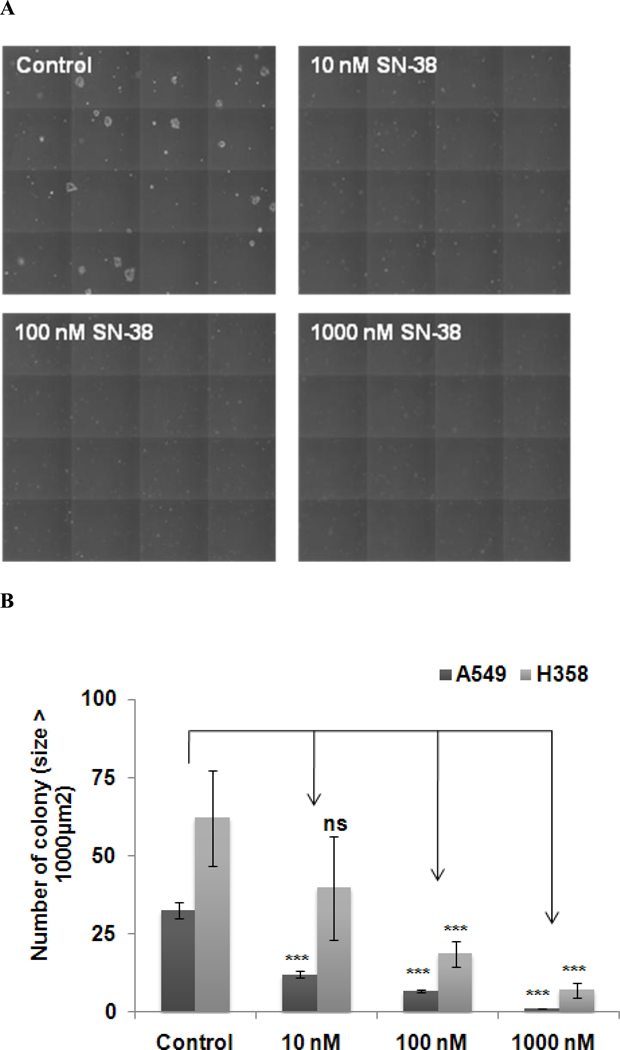
The soft agar colony assay is an in vitro model to mimic in vivo tumor growth. Since anchorage-independent growth is a hallmark of tumorigenesis, this assay was carried out to evaluate the effect of SN-38 on growth of these cells. Results showed that the presence of SN-38 significantly inhibited the growth of A549 and H358 colonies (Fig. 2). As shown in Fig 2, SN-38 dose dependently attenuated colony growth of both cell types. A549 cells were slightly more sensitive to the low concentration of SN-38 (10 nM). In addition, the effect of SN-38 was more pronounced in this colony formation assay, although this result was well corroborated with cell proliferation assay results (Fig. 1).
Fig. 2.
Inhibition of anchorage-independent growth of A549 and H358 cells by SN-38. Soft agar colony assay was carried out as described in Materials and Methods section. A. Morphology of A549 cell colony on day 10; B. Colony number and size of A549 and H358 were evaluated after 10 days of treatment with various concentrations of SN-38 (10–1000 nM), *** p ≤ 0.001 as compared to the level of control.
C. Induction of apoptosis by SN-38 involves collapse of mitochondrial membrane potential (MMP) and S- and G2-phase cell cycle arrest
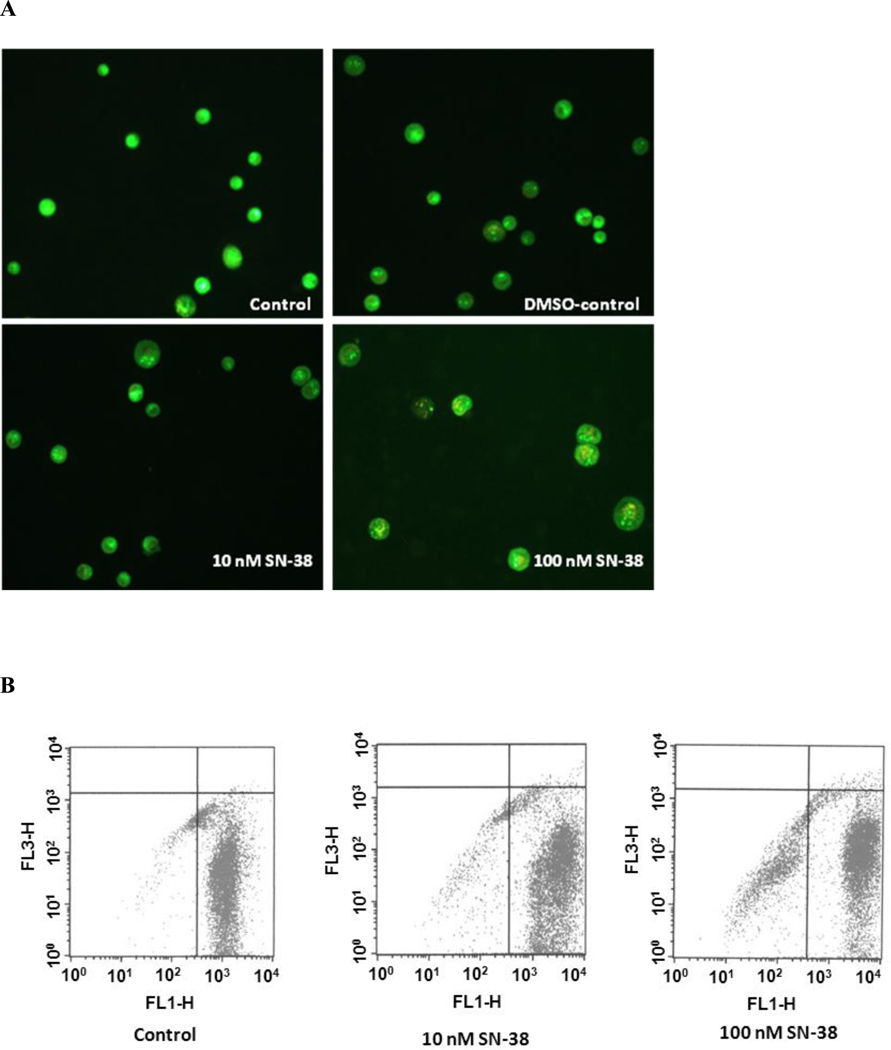
Having determined that SN-38 inhibits lung cancer cell growth and anchorage-independent growth, we sought to determine the mechanism by which SN-38 attenuates cell growth. Apoptosis is an important phenomenon in the cytotoxicity induced by anticancer drugs. Induction of apoptosis by SN-38 was evaluated using acridine orange-ethidium bromide staining. As shown in the Fig 3-A, SN-38 dose dependently induced apoptosis in A549 cells, which are characterized by highly condensed bright green or orange chromatin as observed under fluorescence microscope. A similar pattern of apoptosis was observed by flow cytometry analysis (Fig 3-B).
Fig. 3.
Effect of SN-38 on induction of apoptosis in A549 cell. Cells were incubated with either 10 or 100 nM SN-38 for 48 hrs. Cells were lifted after completion of incubation and stained with acridine orange-ethidium bromide dye mix as described in Materials and Methods. (A) A549 cells showing apoptotic cells (bright green or bright orange chromatin which is highly condensed) in SN-38 treated group; (B) Flow cytometer result of A549 cells showing apoptosis.
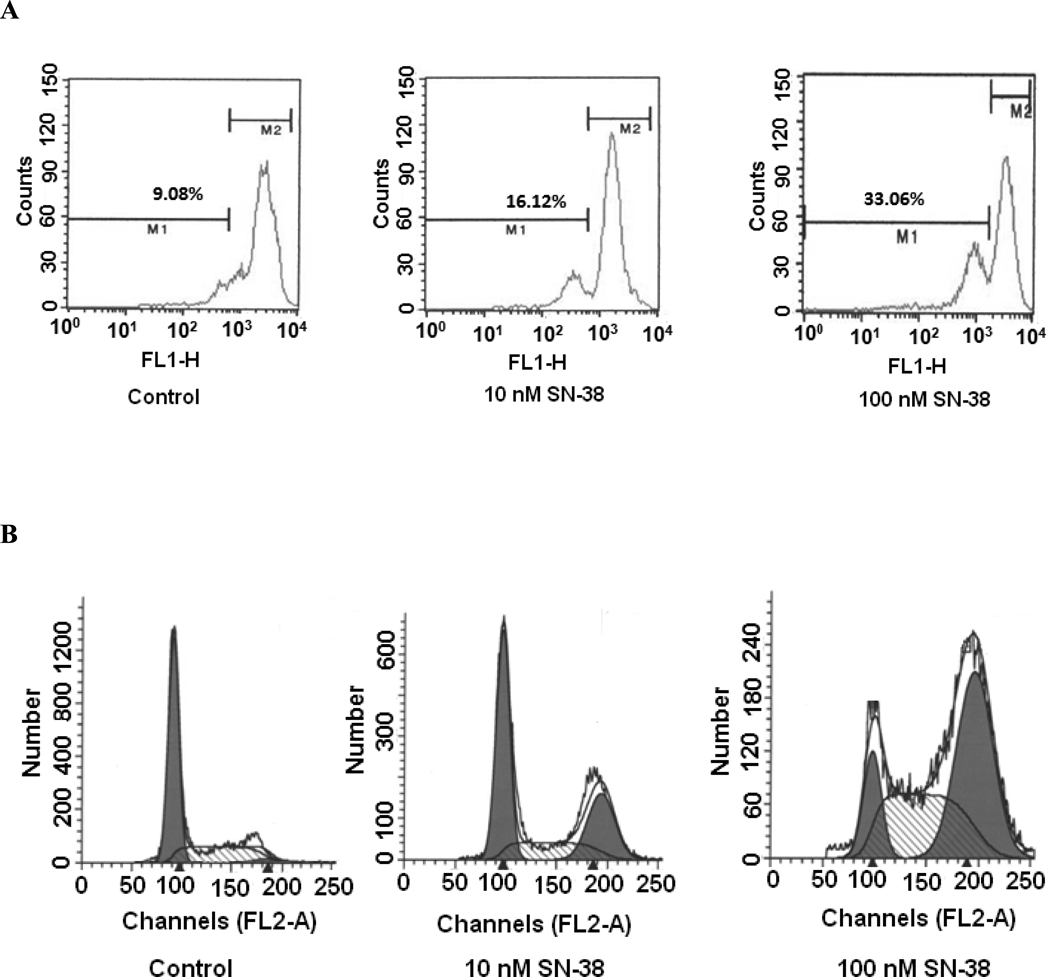
It is well known that a decline of mitochondrial potential is an early event in the process of cell death. Therefore, an investigation was carried out to determine whether change in the MMP is involved in SN-38 mediated cell death. A549 cells were treated with increasing concentrations of SN-38 for 24 hrs, and then the MMP was analyzed by flow cytometer after rhodamine 123 dye labeling(27). Cells treated with either 10 nM or 100 nM of SN-38 exhibited a marked reduction in cellular uptake of the fluorochrome (Fig. 4-A). The decrease of fluorescence intensity reflects the collapse of MMP, which generally defines an early and irreversible stage of apoptosis (28). These findings suggest that an alteration of MMP was caused by a low concentration of SN-38 and appears to be very early sign of apoptosis.
Fig. 4.
Effect of SN-38 on mitochondrial membrane potential and cell cycle in A549 cells. Cells were incubated with either 10 or 100 nM SN-38 for 48 hrs. Cells were lifted after completion of incubation and stained with rhodamine 123 for 30 min for determination of MMP (A), or fixed with 70 % ethanol and stained with 40µg/ml propidium iodide containing 100µg/ml RNase A for 1 hr for cell cycle analysis (B).
To further investigate the mechanism of apoptosis induction by SN-38, cell cycle analysis was carried out by propidium iodide staining. As shown in Fig. 4-B, after incubation of cells with SN-38 for 48 hrs, most cells were found in either S-phase or G2 phase of the cell cycle. This effect is more pronounced at a higher concentration of SN-38. These results clearly indicate that a major effect of SN-38 on the lung cancer cell lines is mediated via S- and G2 arrest and apoptosis.
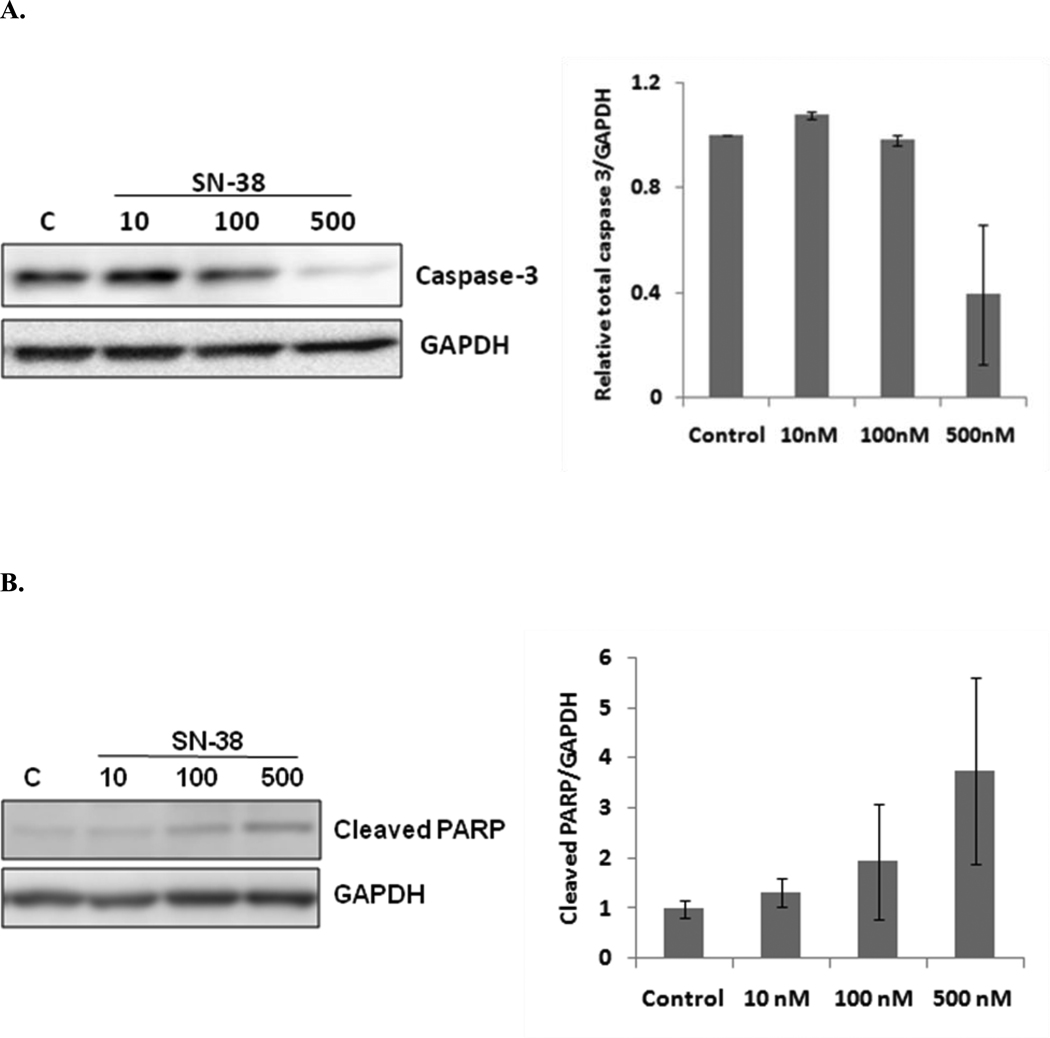
D. SN-38 induces apoptosis by activation of caspase-3 and poly (ADP-ribose) polymerase (PARP)
Caspase-3 plays a central role in the execution of the apoptotic cascade by cleaving poly (ADP-ribose) polymerase (PARP). From Fig. 5, it is clear that incubation of A549 cells with SN-38 causes a dose-dependent decrease of total caspase-3 (procaspase-3) and increase of cleaved PARP. These results indicate the involvement of the activation of caspase-3 and PARP in execution of SN-38 mediated apoptosis. On the other hand, it was found that SN-38 executes its antitumor effect without involvement of bystander molecules, which was confirmed by the radioactivity release assay (data not shown).
Fig. 5.
Effect of SN-38 treatment on A549 cells by Western blot analysis. A549 cells were cultured in 6-well plates and treated with SN-38. After 48 hrs of culture whole cell lysate was prepared and subjected to Western blot analysis. In each panel, the left side picture indicates Western blot analysis, whereas the right side bar graphs show semi-quantification of expressed proteins using scanning densitometry. SN-38 significantly decreased caspase-3 levels (A) and increased cleaved PARP levels (B) in a dose dependent manner. The average expression levels of caspase-3 and cleaved PARP were normalized by respective GAPDH control and are displayed in the histogram (n=3).
E. SN-38 inhibits growth of intraperitoneal LLC tumor
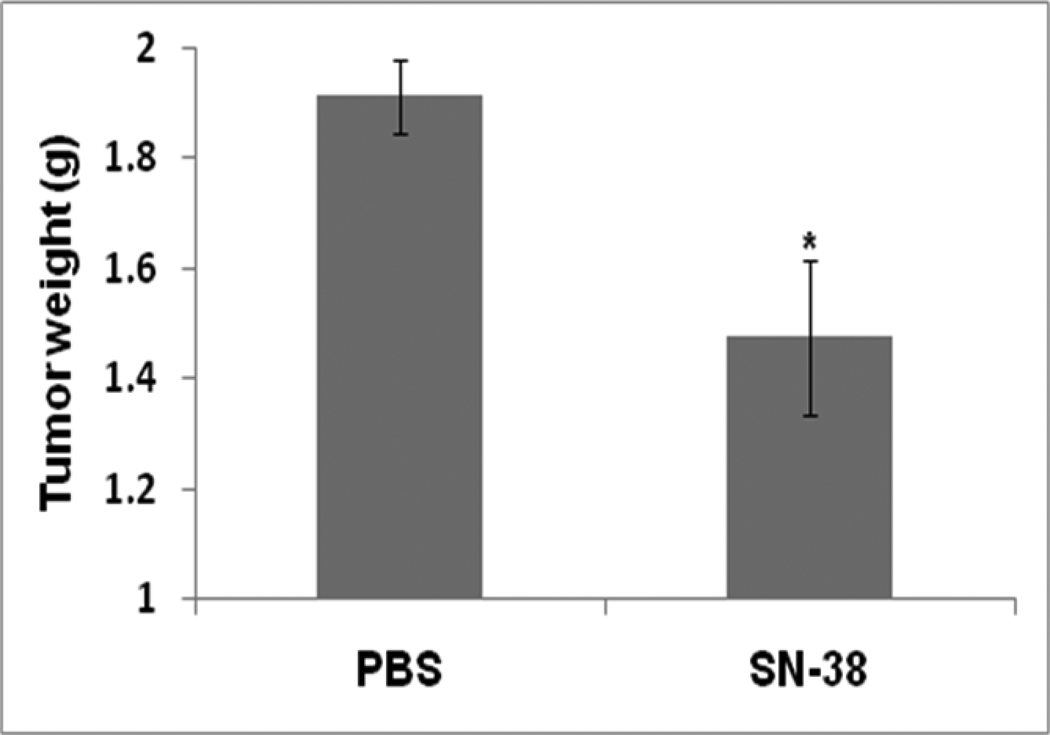
Since all in vitro cell culture studies clearly indicated that SN-38 potently induces apoptosis, thereby attenuating the growth of lung cancer cells, its effect was evaluated in immunocompetent mice using an intraperitoneal LLC xenograft model. In this model, a relatively small number of LLC cells (one million cells) effectively and primarily grew on the omentum. There was no invasion of organs in the abdominal cavity, such as liver, kidney, pancreas, or spleen. As shown in Fig. 6, a single intraperitoneal injection of SN-38 (2mg/kg body weight) significantly attenuated the growth of LLC tumors. It was observed that a single injection of SN-38 five days after LLC tumor transplantation reduced tumor growth 22.7 %.
Fig. 6.
In vivo effect of SN-38 in a peritoneal LLC tumor model. Each mouse was transplanted with 1×106 LLC cells in its peritoneal cavity. Five days after tumor transplantation, mice were injected with either vehicle control or 2mg/kg body weight SN-38. All the mice were sacrificed 15 days post tumor transplantation. All tumor tissue in the peritoneal cavity was dissected from intestine and other organs and determined as tumor burden. *, p ≤ 0.05 as compared to the level of control.
IV. DISCUSSION
Lung cancer survival rates have not improved in the past 2 decades, so more effective treatments are urgently needed. Adenocarcinoma is a subset of non-small cell lung cancer (NSCLC); patients with adenocarcinoma comprise approximately 40% of the overall NSCLC population29. In search of a more effective anticancer drug against NSCLC, we have tested the effect of SN-38, an active metabolite of irinotecan, on LLC, A549, and H358 lung cancer cell lines. We found that SN-38 has a potent anticancer effect on all these cancer cell lines at nanomolar concentrations in in vitro cell proliferation and soft agar colony formation experiments. Since the majority of currently available anticancer treatments are effective at sub-micromolar to micromolar concentrations30, 31, SN-38 appears to be a more potent and potentially an effective anticancer drug..
It is well known that induction of apoptosis in cancer cells is a key mechanism for most anticancer therapies including radiotherapy, chemotherapy, immunotherapy, or cytokine gene therapy32, 33. Apoptosis pathways may be initiated through an intrinsic pathway (mitochondrial pathway) or extrinsic pathway (receptor pathway) resulting in activation of effector caspases34. The mitochondrial pathway plays a crucial role in drug-induced apoptosis35. During induction of apoptosis, mitochondrial pathway factors such as cytochrome c, apoptosis-inducing factor (AIF), or second mitochondria-derived activator of caspase (Smac)/DIABLO are released from mitochondria into the cytosol36. Cytochrome c triggers caspase-3 activation through formation of apoptosome complexes (cytochrome c/Apaf-1/caspase-9), whereas Smac/DIABLO promotes caspase activation by neutralizing the inhibitory effects of the inhibitor of apoptosis protein (IAP)36. In either case, activation of caspase-3 and cleavage of PARP are critical events for induction of apoptosis. Our study shows that SN-38 causes the disruption of MMP, activation of caspase-3 and cleavage of PARP, thereby inducing apoptosis in lung cancer cells. This indicates that disruption of MMP is an early event in apoptosis and appears to be associated with the release of mitochondrial factors, such as cytochrome c, which are responsible for formation of apoptosomes as described above. Caspase-3 has been shown to play a central role in the execution of the apoptotic program primarily by the cleavage of PARP37. An early transient burst of poly ADP-ribosylation of nuclear proteins were shown to be required for apoptosis to proceed in various cell lines, followed by cleavage of PARP, catalyzed by active caspase-3 (cleaved caspase-3)38. This inactivation of PARP is believed to prevent depletion of NAD (a PARP substrate) and ATP, which are thought to be required for later events in apoptosis39. Our finding also showed that treatment of cells with SN-38 leads to activation of caspase-3 which caused cleavage of PARP; this cleavage ultimately leads to apoptosis.
In addition to apoptosis, cell cycle modulation by various chemotherapeutic agents is gaining widespread attention because cell cycle regulation is the basic mechanism underlying cell fate, i.e., proliferation, differentiation, or acquired death40. Our study indicates that SN-38 arrests the cell cycle in S- and G2-phase. Thus, cell cycle arrest may be another antitumor mechanism for affected by SN-38 in the lung cancer cell lines.
Some anticancer agents also cause cell death by a bystander mechanism to neighboring cells by producing extracellular molecules such as reactive oxygen species (ROS)15. Our study with SN-38 indicated that SN-38-dependent cell death does not involve bystander molecules.
Translation of in vitro results to a peritoneal LLC syngeneic mouse model indicates that a single injection of SN-38 (2mg/kg body weight) decreases tumor burden by 22.7%. However, SN-38 is a water insoluble compound and has poor absorption, which may be the possible reason for the less dramatic effect we observed in vivo. The in vivo efficacy of SN-38 may be improved by using a drug delivery system incorporating micelles; liposomes, or nanoparticles. In support of this hypothesis, SN-38 micelles (an SN-38 covalently attached with the copolymer PEG-PGlu) have been shown to be very effective in attenuation of various pancreatic cancer cell xenografts41. However, it is important to design a cancer tissue-targeted SN-38 delivery since this highly potent cell death inducer also damages normal tissues.
In summary, the findings shown here confirm that SN-38 executes an antitumor effect by inducing apoptosis. The mechanism by which SN-38 causes cancer cell death is primarily due to the disruption of MMP, cell cycle arrest, and induction of apoptosis via activation of caspase-3 and cleavage of PARP. Thus, SN-38 is confirmed to be a very potent anticancer drug for lung cancer.
ACKNOWLEDGEMENTS
The authors thank Ms. Marla Pyle (Department of Anatomy & Physiology, Kansas State University) for critical reading and constructive comments during the preparation of the manuscript. This work was supported in part by Kansas State University (KSU) Terry C. Johnson Center for Basic Cancer Research, KSU College of Veterinary Medicine Dean’s fund, NIH P20 RR017686 and KSU Targeted Excellence Research grant.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DH, Schiller JH. Novel therapies for the treatment of non-small cell lung cancer. Cancer Chemother Biol Response Modif. 2002;20:763–786. [PubMed] [Google Scholar]
- 3.Lynch TJ, Adjei AA, Bunn PA, Jr, DuBois RN, Gandara DR, Giaccone G, et al. Novel agents in the treatment of lung cancer: conference summary statement. Clin. Cancer Res. 2004;10:4199s–4204s. doi: 10.1158/1078-0432.CCR-040021. [DOI] [PubMed] [Google Scholar]
- 4.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 5.Hsiang Y-H, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]
- 6.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 7.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 8.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–89. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka MFKS N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst. 1991;83:855–861. doi: 10.1093/jnci/83.12.855. [DOI] [PubMed] [Google Scholar]
- 11.Roth BJJ, D.H. Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282–291. doi: 10.1200/JCO.1992.10.2.282. [DOI] [PubMed] [Google Scholar]
- 12.Ihde DC. Chemotherapy of lung cancer. N Engl J Med. 1992;327:1434–1441. doi: 10.1056/NEJM199211123272006. [DOI] [PubMed] [Google Scholar]
- 13.Aisner J. Extensive-disease small-cell lung cancer: the thrill of victory; the agony of defeat. J Clin Oncol. 1996;14:658–665. doi: 10.1200/JCO.1996.14.2.658. [DOI] [PubMed] [Google Scholar]
- 14.Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992;10:1225–1229. doi: 10.1200/JCO.1992.10.8.1225. [DOI] [PubMed] [Google Scholar]
- 15.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 16.Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004;23:6702–6711. doi: 10.1038/sj.onc.1207630. [DOI] [PubMed] [Google Scholar]
- 17.Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by betulinic Acid. Neoplasia. 2005;7:162–170. doi: 10.1593/neo.04442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Custodio JB, Cardoso CM, Madeira VM, Almeida LM. Mitochondrial permeability transition induced by the anticancer drug etoposide. Toxicol In Vitro. 2001;15:265–270. doi: 10.1016/s0887-2333(01)00019-4. [DOI] [PubMed] [Google Scholar]
- 19.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 20.Lu DY, Huang M, Xu CH, Yang WY, Hu CX, Lin LP, et al. Anti-proliferative effects, cell cycle G2/M phase arrest and blocking of chromosome segregation by probimane and MST-16 in human tumor cell lines. BMC Pharmacol. 2005;5:11. doi: 10.1186/1471-2210-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallin A, Svanvik J, Holmlund B, Ferreud L, Sun XF. Anticancer effect of SN-38 on colon cancer cell lines with different metastatic potential. Oncol Rep. 2008;19:1493–1498. [PubMed] [Google Scholar]
- 22.Souza V, Dong YB, Zhou HS, Zacharias W, McMasters KM. SW-620 cells treated with topoisomerase I inhibitor SN-38: gene expression profiling. J Transl Med. 2005;3:44. doi: 10.1186/1479-5876-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.te Poele RH, Joel SP. Schedule-dependent cytotoxicity of SN-38 in p53 wild-type and mutant colon adenocarcinoma cell lines. Br J Cancer. 1999;81:1285–1293. doi: 10.1038/sj.bjc.6694370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei XH, Nakanishi Y, Takayama K, Bai F, Kawasaki M, Tsuruta N, et al. Effect of CPT-11 in combination with other anticancer agents in lung cancer cells. Anticancer Drugs. 1997;8:231–237. doi: 10.1097/00001813-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 25.van Ark-Otte J, Kedde MA, van der Vijgh WJ, Dingemans AM, Jansen WJ, Pinedo HM, et al. Determinants of CPT-11 and SN-38 activities in human lung cancer cells. Br J Cancer. 1998;77:2171–2176. doi: 10.1038/bjc.1998.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtsuka K, Inoue S, Kameyama M, Kanetoshi A, Fujimoto T, Takaoka K, et al. Intracellular conversion of irinotecan to its active form, SN-38, by native carboxylesterase in human non-small cell lung cancer. Lung Cancer. 2003;41:187–198. doi: 10.1016/s0169-5002(03)00223-x. [DOI] [PubMed] [Google Scholar]
- 27.Scaduto JRC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 29.Auerbach O, Garfinkel L. The changing pattern of lung carcinoma. Cancer. 1991;68:1973–1977. doi: 10.1002/1097-0142(19911101)68:9<1973::aid-cncr2820680921>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CM, Chang KT, Chen JY, Chen YM, Chen MH, Perng RP. Cytotoxic effects of gemcitabine-containing regimens against human non-small cell lung cancer cell lines which express different levels of p185neu. Cancer Res. 1996;56:794–801. [PubMed] [Google Scholar]
- 31.Radhakrishna Pillai G, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004;208:163–170. doi: 10.1016/j.canlet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 33.Lowe SWL, A W. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 34.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 35.Debatin KM, Poncet D, Kroemer G. Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 36.van Loo GSX, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 38.Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem. 1998;273:13703–13712. doi: 10.1074/jbc.273.22.13703. [DOI] [PubMed] [Google Scholar]
- 39.Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. [PubMed] [Google Scholar]
- 40.Dobashi Y, Takehana T, Ooi A. Perspectives on cancer therapy: cell cycle blockers and perturbators. Curr Med Chem. 2003;10:2549–2558. doi: 10.2174/0929867033456495. [DOI] [PubMed] [Google Scholar]
- 41.Saito Y, Yasunaga M, Kuroda J, Koga Y, Matsumura Y. Enhanced distribution of NK012, a polymeric micelle-encapsulated SN-38, and sustained release of SN-38 within tumors can beat a hypovascular tumor. Cancer Sci. 2008;99:1258–1264. doi: 10.1111/j.1349-7006.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]