Abstract
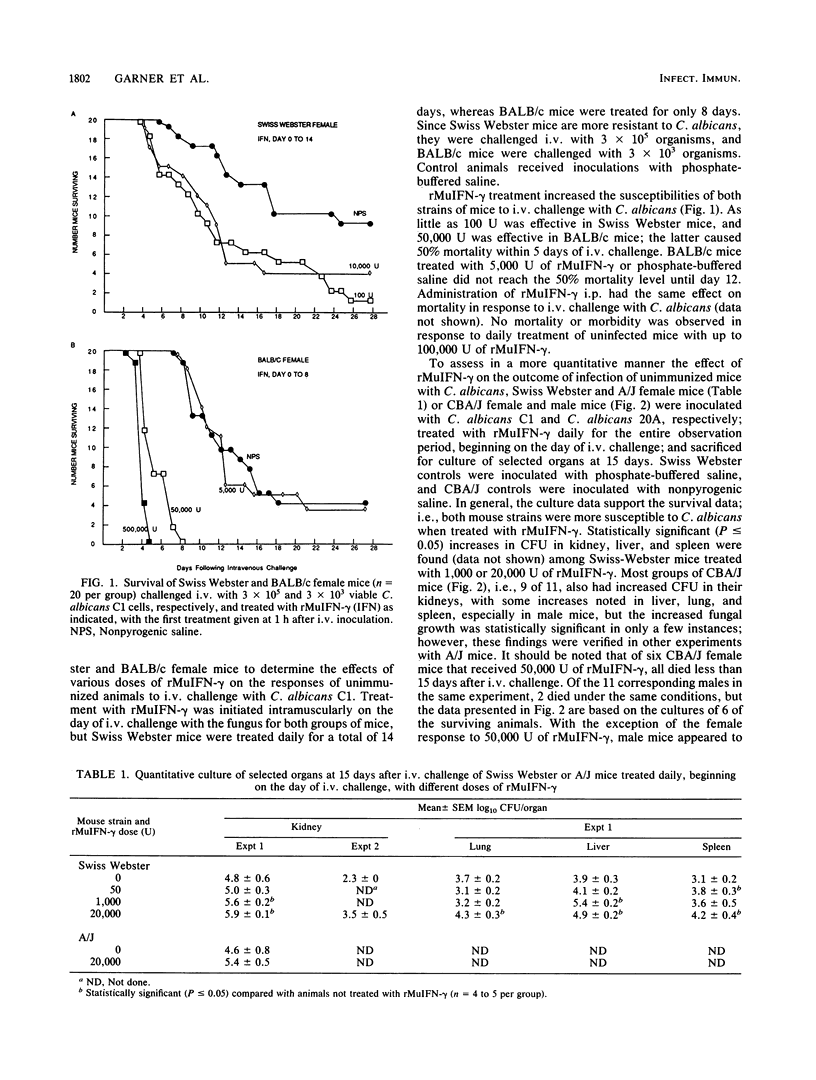
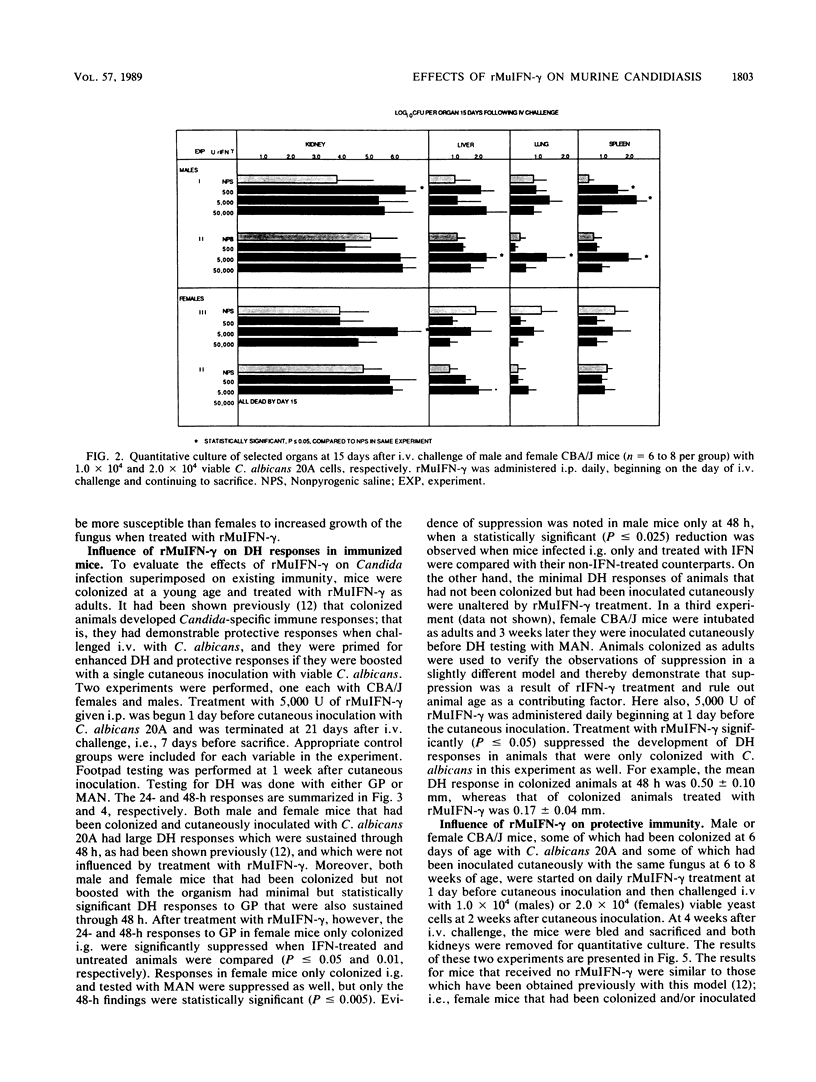
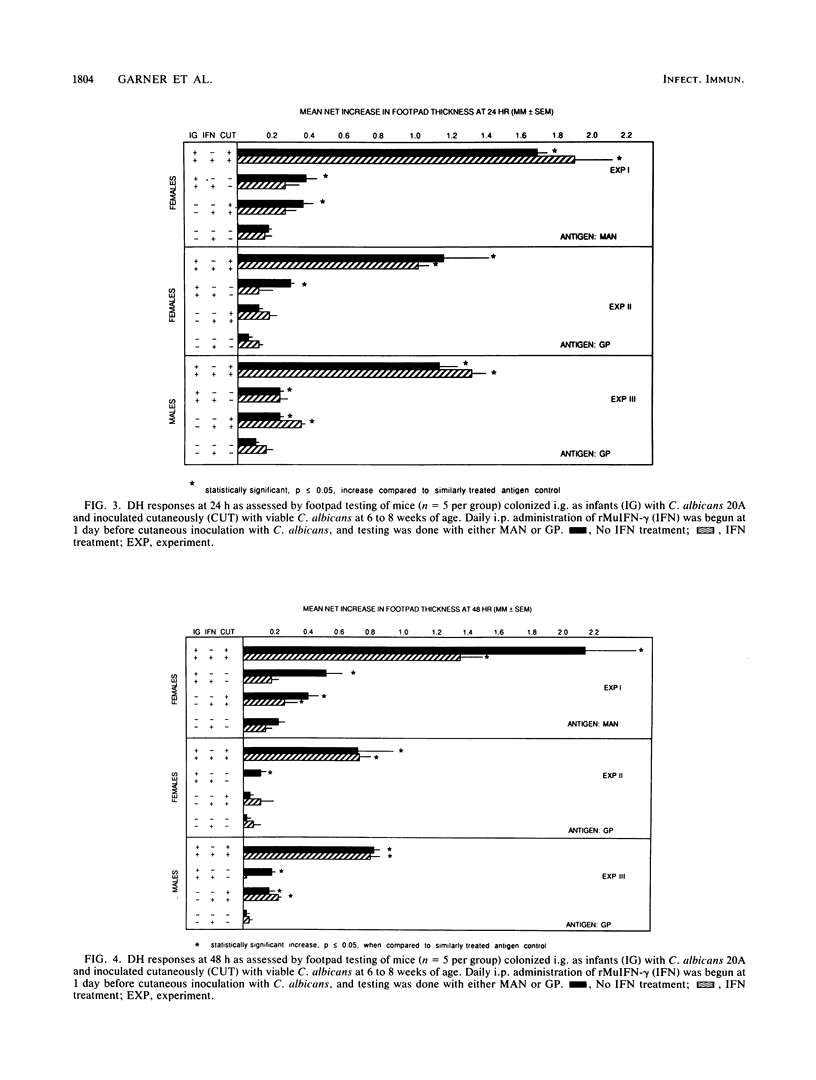
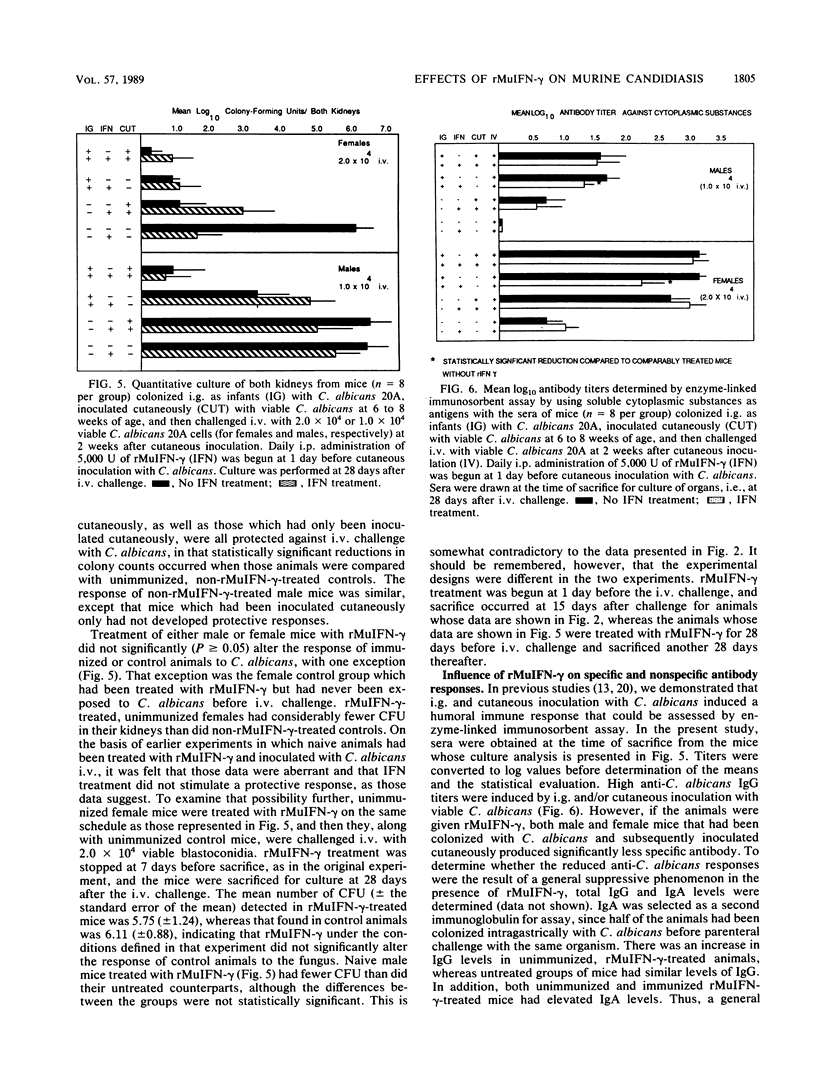
The immunologic effects of in vivo administration of recombinant murine gamma interferon (rMuIFN-gamma) were determined in a murine model of candidiasis. Naive mice were given graded doses of rMuIFN-gamma and then challenged intravenously with Candida albicans. Increased morbidity and mortality were noted in four different strains of mice, viz., BALB/c, A/J, Swiss Webster, and CBA/J, providing the mice had not been immunized with C. albicans before challenge. Quantitative culture of selected organs of Swiss Webster and CBA/J mice surviving treatment with rMuIFN-gamma revealed elevated numbers of C. albicans cells, particularly in the kidneys, but also in the liver, lungs, and spleen. The lungs, livers, and spleen of female CBA/J mice were more protected from increased multiplication of the fungus than were those of males of the same species or female Swiss Webster mice. On the basis of these initial findings, the effect of treatment with 5,000 U of rMuIFN-gamma on immune responses in a gastrointestinal model of candidiasis was determined. CBA/J mice that had been colonized with C. albicans as infants were boosted with a cutaneous inoculation of the fungus when 6 to 10 weeks old; development of delayed hypersensitivity (DH), antibodies, and protective responses was assayed at intervals thereafter. Daily treatment with rMuIFN-gamma (beginning 1 day before cutaneous inoculation) suppressed weak immune responses but had little effect on responses which were strong. For example, DH and anti-C. albicans antibody production were suppressed in animals colonized with C. albicans but not boosted by cutaneous inoculation, and DH was suppressed in uncolonized animals that had been inoculated once cutaneously with the fungus as well. There was no rMuIFN-gamma-induced suppressive effect of DH in mice which had been stimulated maximally with C. albicans, i.e., colonized animals that had been boosted cutaneously with the organisms. Collectively, these data indicate that naive mice or mice with minimal levels of anti-C. albicans sensitivity, females somewhat more so than males, were sensitive to suppressive effects of in vivo treatment with rMuIFN-gamma when challenged with C. albicans. In contrast, under conditions similar to those of humans, in whom underlying immunity to C. albicans is usually present, suppression of host responses to C. albicans was not observed in immunized mice in response to treatment with rMuIFN-gamma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect Immun. 1987 Dec;55(12):2951–2955. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Suzuki Y., Wheelock E. F. Interferon-gamma synergizes with tumor necrosis factor and with interleukin 1 and requires the presence of both monokines to induce antitumor cytotoxic activity in macrophages. J Immunol. 1987 Dec 15;139(12):4096–4101. [PubMed] [Google Scholar]
- Cho S. Y., Choi H. Y. Opportunistic fungal infection among cancer patients. A ten-year autopsy study. Am J Clin Pathol. 1979 Oct;72(4):617–621. doi: 10.1093/ajcp/72.4.617. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Host genotype influences immunomodulation by interferon. Nature. 1980 Mar 13;284(5752):173–175. doi: 10.1038/284173a0. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J., Vandeputte M. Inhibition by interferon of delayed-type hypersensitivity in the mouse. Proc Natl Acad Sci U S A. 1975 May;72(5):1753–1757. doi: 10.1073/pnas.72.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Domer J. E., Hector R. F. Enhanced immune responses in mice treated with penicillin-tetracycline or trimethoprim-sulfamethoxazole when colonized intragastrically with Candida albicans. Antimicrob Agents Chemother. 1987 May;31(5):691–697. doi: 10.1128/aac.31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Kamoun M., Gresser I., Bono R. Enhanced expression of HLA antigens and beta 2-microglobulin on interferon-treated human lymphoid cells. Eur J Immunol. 1979 Jun;9(6):446–449. doi: 10.1002/eji.1830090606. [DOI] [PubMed] [Google Scholar]
- Fulton A. M., Levy J. G. The induction of nonspecific T suppressor lymphocytes by prostaglandin E1. Cell Immunol. 1981 Mar 15;59(1):54–60. doi: 10.1016/0008-8749(81)90433-0. [DOI] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., De Maeyer-Guignard J., Tovey M. G., De Maeyer E. Electrophoretically pure mouse interferon exerts multiple biologic effects. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5308–5312. doi: 10.1073/pnas.76.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B., Lagrange P. H. Comparative effects of carrageenan on systemic candidiasis and listeriosis in mice. Clin Exp Immunol. 1981 May;44(2):355–358. [PMC free article] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Fiers W., Strom T. B. Cloned human interferon-gamma, but not interferon-beta or -alpha, induces expression of HLA-DR determinants by fetal monocytes and myeloid leukemic cell lines. J Immunol. 1984 Jan;132(1):240–245. [PubMed] [Google Scholar]
- Khor M., Lowrie D. B., Coates A. R., Mitchison D. A. Recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin in experimental murine tuberculosis. Br J Exp Pathol. 1986 Aug;67(4):587–596. [PMC free article] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Granger D. L., Durack D. T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987 Aug;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Recombinant gamma interferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987 Jan;67(1):5–10. [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthe R. C., Andersen B. R., Cunningham B. L., Epstein R. B. Efficacy of granulocyte transfusions in the control of systemic candidiasis in the leukopenic host. Blood. 1978 Sep;52(3):493–498. [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz M., Manz G., Franke M. Einsatz von rekombinantem Human-Interferon gamma bei Patienten mit rheumatoider Arthritis. Z Rheumatol. 1986 May-Jun;45(3):93–99. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Worthington M., Hasenclever H. F. Effect of an interferon stimulator, polyinosinic: polycytidylic acid, on experimental fungus infections. Infect Immun. 1972 Feb;5(2):199–202. doi: 10.1128/iai.5.2.199-202.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Geelhoed G. W., Levine A. S. Fungemia with compromised host resistance. A study of 70 cases. Ann Intern Med. 1974 May;80(5):605–612. doi: 10.7326/0003-4819-80-5-605. [DOI] [PubMed] [Google Scholar]
- Youngner J. S. Properties of interferon induced by specific antigens. Tex Rep Biol Med. 1977;35:17–22. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]



