Abstract
Oxidized cytoplasmic and nuclear proteins are normally degraded by proteasome, but accumulate with age and disease. We demonstrate the importance of various forms of the proteasome during transient (reversible) adaptation (hormesis), to oxidative stress in murine embryonic fibroblasts. Adaptation was achieved by ‘pre-treatment’ with very low concentrations of H2O2, and tested by measuring inducible resistance to a subsequent, much higher ‘challenge’ dose of H2O2. Following an initial direct physical activation of pre-existing proteasomes, 20S proteasome, immunoproteasome, and PA28αβ regulator, all exhibited substantially increased de novo synthesis during adaptation over 24 hours Cellular capacity to degrade oxidatively damaged proteins increased with 20S proteasome, immunoproteasome, and PA28αβ synthesis, and was mostly blocked by 20S proteasome, immunoproteasome, and PA28 siRNA knock-down treatments. Additionally, PA28αβ knockout mutants achieved only half the H2O2 induced adaptive increase in proteolytic capacity of wild-type controls. Direct comparison of purified 20S proteasome and immunoproteasome demonstrated that immunoproteasome can selectively degrade oxidized proteins. Cell proliferation and DNA replication both decreased, and oxidized proteins accumulated, during high H2O2 challenge, but prior H2O2 adaptation was protective. Importantly, siRNA knock-down of 20S proteasome, immunoproteasome, or PA28αβ regulator blocked 50–100% of these adaptive increases in cell division and DNA replication, and immunoproteasome knock-down largely abolished protection against protein oxidation.
Keywords: Free Radicals, Ubiquitin-Proteasome System, Aging, Protein Degradation, Protein Oxidation, Hormesis
INTRODUCTION
Oxidatively damaged proteins represent a threat to viability and are rapidly degraded in mammalian cells, plant cells, yeast, mitochondria, and bacteria [1–16]. The Proteasome is mostly responsible for this selective proteolysis in the cytosol and nucleus of eukaryotes [1–6, 11–16]; the Lon protease performs a similar function in mitochondria and bacteria [7–10].
We [1–6, 15], and others, [11–14, 16, 17] have repeatedly shown that oxidized proteins are degraded by proteasome in an ATP-independent and ubiquitin-independent manner in mammalian cells. We have also published a direct comparison of the ability of purified 20S proteasome and 26S proteasome to degrade several oxidized and control protein substrates [6]. Our results clearly show that the 26S proteasome is extremely poor at degrading oxidized proteins and, in fact, shows no preferential recognition of oxidized proteins [6]. These results led us to assume that oxidized cytoplasmic and nuclear proteins are mostly degraded by the 20S proteasome. New work with the Immunoproteasome, and with proteasome regulators such as PA28, however, has made us re-evaluate this view and has encouraged us to more carefully test the possible contributions of the Immunoproteasome and the PA28 regulator to the removal of oxidized cellular proteins.
All forms of the proteasome, [see [18, 19] for reviews,] include a core, tube-like complex, consisting of four rings, stacked together in the order: alpha, beta, beta, alpha; the three proteolytic activities of the complex reside in the beta rings. Each ring contains seven different subunits. The core 20S proteasome can bind two Pa700 (19S) regulators (one to each alpha ring) thus forming the 26S proteasome which is responsible for ATP/ubiquitin mediated proteolysis. Alternatively, the alpha rings of the 20S core proteasome can be free, or can bind to the cytoplasmic PA28αβ (11S) regulators, or the nuclear PA28γ (REGγ) or PA200 regulators. A special form of the core proteasome is synthesized by substituting the proteolytically catalytic β1 (or X), β2 (or Y), and β5 (or Z), subunits with β1i (Lmp2), β2i (Mecl-1), and β5i (Lmp7) subunits, thus forming the so called ‘Immunoproteasome [20–22]. The Immunoproteasome has often been linked to the cytoplasmic PA28αβ (11S) regulator in the literature, since both are induced by cell treatment with interferon-gamma [20–22]. Similarly, it is widely accepted that the Immunoproteasome (as the name implies) and, perhaps, PA28αβ (11S) are required for the generation of peptides of the correct length for MHC class 1 (self) antigen processing [20–22].
Although it is quite clear that mammalian cells can transiently adapt to increased levels of oxidative stress, through signaling pathways and altered gene expression [23–26], most of the literature on proteasome and oxidative stress deals with the proteasome as a static, pre-formed proteolytic ‘machine.’ Several studies, however, clearly reveal that both age and disease can alter the activity of the proteasome [6, 9, 12, 13, 27, 28], and studies by Ferrington et al. [29–31] indicate that altered subunit composition and altered regulator/activator binding, may underlie at least some of these changes. Furthermore, exciting studies from the group of Kalyanaraman et al. [32–34] show that both intracellular hydrogen peroxide (H2O2) and nitric oxide (NO• ) can induce increased proteasome activity. Despite these encouraging initial reports, the inducibility of the proteasome by oxidative stress, and the specific subunit and regulator/activator composition of such ‘stress-induced’ proteasomes, have not been carefully studied.
Previously, we demonstrated that mammalian cells (as well as bacteria and yeast) can transiently and reversibly adapt to mild oxidative stress by altering gene expression over several hours [23–26] in a process that is sometimes called hormesis. For such experiments, one first finds a challenge dose of oxidant (e.g. H2O2) that normally causes an easily measurable negative effect on cell growth and division. The challenge dose should not be so strong, however, that it causes massive cell death from apoptosis or necrosis. Separately, one finds a much lower pre-treatment dose (or adaptive dose) of the same oxidant and allows a suitable time-lag to permit adaptive gene expression to occur. When the normally toxic challenge dose is applied to pre-adapted cells, we find that they are transiently resistant to the stress. [23–26]. This model of transient and reversible oxidative stress adaptation seemed ideal to test the possibility that various forms of the proteasome, and its regulators, might be involved in stress-protection, and that the synthesis of some subunits might be inducible during stress adaptation.
Our present investigations were designed to answer four questions: 1) Which forms of the proteasome (20S, 26S, immunoproteasome) are actually important in degrading oxidized intracellular proteins following an oxidative stress? 2) Which form(s) of the proteasome are induced during transient adaptation to an oxidative stress? 3) Which proteasome regulators/activators are induced during transient adaptation to an oxidative stress, and how much do these regulators/activators contribute to proteolytic capacity? 4) Does induction of proteasome subunits and/or regulators actually contribute to the increased stress-resistance of oxidative stress-adapted cells?
MATERIALS AND METHODS
Materials
Materials were purchased from VWR unless otherwise stated. Murine Embryonic Fibroblasts (MEF), were purchased from ATCC (Manassas, VA) catalog #CRL-2214. In addition Wild-type MEF and PA28αβγ- MEF cells [35] were used. HT1080 Human fibroblast cells (catalog #CCL-121) were purchased from ATCC (Manassas, VA). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM), catalog #10-013-CV, purchased from Mediatech (Manassas, VA) and supplemented with 10% Fetal Bovine Serum (catalog #SH30070.03) from Hyclone (Logan, UT); henceforth referred to as ‘complete media.’ Cells were typically incubated at 37ºC under 5% CO2 and ambient oxygen.
Hydrogen Peroxide Adaptation
MEF cells were pre-treated with 250 nmol - 2 μmol H2O2 per 107 cells, for one hour at 37ºC under 5% CO2 to induce adaptation to oxidative stress. Cells were then washed twice with phosphate-buffered saline (PBS), which was finally replaced with fresh complete media
Western Blots
MEF cells were harvested from 75 cm2 flasks by trypsinization. Cells were washed twice with PBS to remove trypsin and then lysed in RIPA buffer (catalog #89901) from Thermo Fisher (Waltham, MA) supplemented with protease inhibitor cocktail (catalog #11836170001) from Roche (Nutley, NJ). Protein content was quantified with the BCA Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. For Western analysis, 5 μg – 20μg of protein was run on SDS–PAGE and transferred to PVDF membranes. Using standard Western blot techniques, membranes were treated with the following proteasome/PA28 subunit antibodies purchased from Biomol (Plymouth Meeting, PA): anti-β1i antibody, anti-β2i antibody, anti-β5i antibody, anti-β2 antibody, anti-PA28α antibody, anti-PA28β antibody, anti-α4 antibody (catalog #PW8205-0100, PW8150-0100, #PW8200-0100, PW9300-0025, PW8185-0100, PW8240-0100 and PW8120-0025). Other membranes were probed with anti-β1 antibody or anti-α3 antibody (catalog # sc-67345 and #sc-58414), from Santa Cruz Biotechnology (Santa Cruz, CA). Additionally some membranes were probed with anti-S4 antibody (catalog #539167) from calbiochem (San Diego, CA).
The blocking buffer employed for Western blotting was Startingblock buffer (catalog #37539) from Thermo Fisher (Waltham, MA) and the Wash buffer was 1x PBS containing 0.1% Tween 20. An enhanced chemiluminescence kit (Pierce, Rockford, IL), was used for chemiluminescent detection and membranes were developed onto either Kodak Biomax films (VWR, West Chester, PA) using the Kodak GBX developing system (VWR, West Chester, PA) or detected using the biospectrum imaging system (UVP, Upland, CA).
siRNA ‘Knock-down’ Treatments
siRNA was purchased from two different companies. Custom β5 and S4 siRNA, and the relevant control (non-silencing) scrambled siRNA’s were purchased from Qiagen (Huntsville, Al). MEF were grown to 50% confluence in 75 cm2 flasks, siRNA treatment was performed as described in the Qiagen product manual.
Other siRNA’s were from Santa Cruz biotechnology (Santa Cruz, CA). These included: β1 (catalog #sc-62865), Lmp2 (catalog #sc-35821), PA28α (catalog #sc-151977) and the relevant scrambled control siRNA (catalog #sc-29528). For experiments with these siRNA’s, MEF were seeded at a density of 100,000 cells per well in six well plates and grown to 20% confluence. siRNA treatment was then performed as described in the Santa Cruz Biotechnology product manual.
Proteasome and Immunoproteasome Purification
20S Proteasome was purified from MEF cells as previously described [36]. The 20S proteasome was then identified in samples using Native PAGE blots with suc-LLVY-AMC overlay. Samples were further analyzed using Native PAGE blots and Western blot analysis. Immunoproteasome was purified identically from MEF cells pre-treated with interferon-γ, as previously described [37]. Additionally, in other experiments, purified erythrocyte 20S proteasome (catalog # PW8720) and purified spleen Immunoproteasome (catalog # PW9645) were purchased from Biomol (Plymouth Meeting, PA), and their purity was assessed by Western blots.
Fluorpeptide Proteolytic Assays
MEF were harvested by cell scraping in phosphate buffer, except for Figure. 2 in which cells were harvested from six-well plates by trypsinization. Cells were then re-suspended in 50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl2, (pH 7.5) and lysed by 1–5 freeze-thaw cycles. Protein was quantified by Bradford assay. From 0.02 to 5.0 μg of cell lysate was then transferred to 96 well plates. Next, 2 μM of either N-succinyl-Leu-Leu-Val-Tyr-AMC (catalog # 80053-860) purchased from VWR (Chester, PA,); or Z-Leu-Leu-Glu-AMC (Product code: ZW9345-0005) or Bz-Val-Gly-Arg-AMC (catalog # BW9375-0005) both from Biomol (Plymouth Meeting, PA), were added to the plates. Plates were incubated at 37ºC and mixed at 300 rpm for 4 hours. Fluorescence readings were taken at 10 minute intervals using an excitation wavelength of 355nm and an emission of 444 nm. Fluoresence units were converted to moles of free AMC, with reference to an AMC standard curve of known amounts of AMC (Merck, Whitehouse Station, NJ, catalog #164545), following subtraction of background fluorescence. In some experiments, cells were treated with 1 μM of the proteasome inhibitors MG132 (catalog #474790) or lactacystin (catalog #426100), both from Merck (Whitehouse Station, NJ), 30 minutes prior to incubation and addition of substrates.
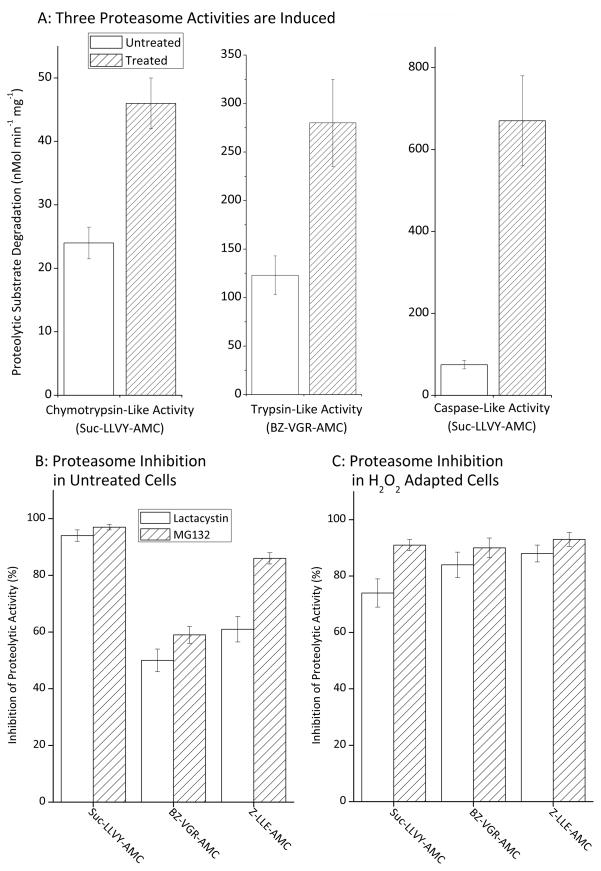
Figure 2. Proteasome Capacity is Increased During Transient Adaptation to H2O2.
Panel A. MEF cells were grown to 20% confluence then transiently adapted to oxidative stress by pre-treatment with 250 nmol of H202 per 107 cells in complete media and incubated at 37ºC under 5% CO2. After one hour, the cells were washed twice with PBS and fresh complete media added. Twenty four hours after exposure, the cells were harvested. Cells were then lysed and suspended in 50mM Tris, 25mM KCl, 10mM NaCl, 1mM MgCl2 (pH 7.5). Proteolytic activity assays for degradation of suc-LLVY-AMC, Bz-VGR-AMC, and Z-LLE-AMC were then performed, as described in Materials & Methods. Values are means ± SE, n = 6. Panel B. MEF cells were prepared, harvested and lysed as described in figure 2A but not pre-treated with H2O2. Samples were then incubated with 1 μM of either MG312 or lactacystin for 30 minutes after which proteolytic activity assays for degradation of suc-LLVY-AMC, Bz-VGR-AMC, and Z-LLE-AMC were performed. Values (means ± SE, n = 3) represent the percent reduction in proteolytic activity following treatment with inhibitors. Panel C. MEF cells were prepared, transiently adapted to oxidative stress by pre-treatment with 250 nmol of H2O2 per 107 cells (as per Panel A), harvested and lysed. Samples were then incubated with 1 μM MG312 or lactacystin for 30 minutes, and proteolytic activity assays for suc-LLVY-AMC, Bz-VGR-AMC, and Z-LLE-AMC were performed. Values (means ± SE, n = 3) are the percent reduction in proteolytic activities caused by treatment with proteolytic inhibitors.
Proteolytic Assay of Radiolabeled Proteins
Tritium-labeled hemoglobin ([3H]Hb) and ezrin ([3H]ezrin) were generated in vitro as previously described [4–6, 15] using the [3H]formaldehyde and sodium cyanoborohydrate method of Jentoft and Deaborn [38], and then extensively dialyzed. Prior to dialysis, some purified, radiolabeled proteins were oxidatively modified by exposure to 2.0 mM H2O2 for 1 hour in order to generate oxidized substrates. All substrates were then incubated with cell lysates to measure proteolysis. Percent protein degraded (for both Hb and ezrin) was calculated by release of acid-soluble (supernatant) counts, by liquid scintillation after addition of 20% TCA and 3% BSA (as carrier) to precipitate remaining intact proteins [5, 12, 15], in which % Degradation = (acid-soluble counts – background counts) x 100.
BrdU Assay for DNA Replication and Cell Division
Bromodeoxyuridine (BrdU), a synthetic thymidine analogue, can be incorporated into newly synthesized DNA providing a test of DNA replication, as an indirect measure of cell division. The assay was performed as described in the product (catalog #2750) manual from Millipore (Billerica, MA). BrdU incorporation was detected by addition of peroxidase substrate. Spectrophotometric detection was performed at a wavelength of 450 nm.
Cell Counting Assay
Cells were seeded at 100,000 cells per ml in 24 well plates. Cells were harvested 24 hours after seeding, using trypsinization, and 100 μl of cell suspension was combined with 10 ml of diluent isoton (catalog #8546719) from Beckman Coulter (Fullerton, CA) in a cuvette. Cell counts were obtained using a Cell Counter purchased from Beckman Coulter (Fullerton, CA).
Oxyblot Assay for Protein Carbonyls (protein oxidation)
An Oxyblot kit for detection of protein carbonyls (catalog #S7150) was purchased from Millipore (Billerica, MA) and assays were performed as described in the product manual.
RESULTS
Increased proteolytic capacity following oxidative stress adaptation
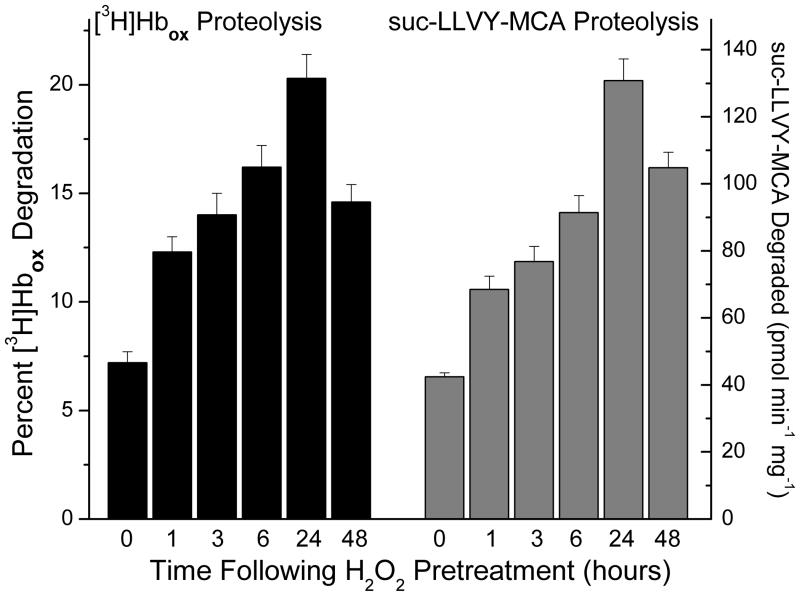
Since proteolysis (especially by the proteasome) plays a vital role in the removal of damaged proteins during oxidative stress [1–6, 11–16], we reasoned that the intracellular capacity to degrade oxidized proteins might increase significantly during stress adaptation. To begin to test this hypothesis, we pre-treated MEF cells with a mild (adaptive) dose of hydrogen peroxide, as previously [23–26], and then allowed a suitable adaptive period of 1–48 hours. Successful transient adaptation (peaking at about 24 hours and then declining) was again confirmed by increased capacity to cope with a subsequent challenge dose of H2O2 that was normally sufficient to significantly decrease cell proliferation in non-adapted cells (confirmatory data not shown), as previously described [23–26]. We then prepared cell lysates and added oxidized, tritium-labeled, hemoglobin ([3H]Hbox) or the fluoropeptide proteolysis substrate suc-LLVY-AMC (a measure of the chymotrypsin-like activity of the proteasome) to the extracts, and measured changes in proteolytic capacity. We observed a significant, progressive increase the capacity to degrade both [3H]Hbox and suc-LLVY-AMC after H2O2 pre-treatment (Fig. 1). Both activities reached a peak of 3–4 fold increases (p < 0.01), compared to untreated controls, 24 hours following pre-treatment and then began to decline.
Figure 1. Proteolytic Capacity Increases During Transient Adaptation to H2O2.
MEF cells were grown to 50% confluence and exposed, in PBS, to an adaptive pre-treatment of 2 μmol H202 per 107 cells. Successful transient adaptation (peaking at about 24 hours and then declining) was confirmed by increased capacity to survive a subsequent (much higher) challenge dose of H2O2 that, without adaptation, significantly decreased cell proliferation and DNA replication, and significantly increased accumulation of oxidized proteins (confirmatory data not shown at this point, but given as part of Figure. 7) as previously described [25, 26]. At various time points after exposure, cells were harvested and lysed then suspended in 50mM Tris, 25mM KCl, 10mM NaCl, 1mM MgCl2, (pH 7.5). Proteolytic activity assays for degradation of either [3H]Hbox [5, 15] or suc-LLVY-AMC [48, 49] were performed as described in Materials & Methods. Values are means ± SE, n = 3. The experiment was repeated in MEF cells grown to only 20% confluence and adapted by pre-treatment with 250 nmol of H202 per 107 cells, with very similar results (data not shown.)
Increased proteasome activity following oxidative-stress adaptation
We next sought to characterize the proteolytic enzyme(s) responsible for the increased proteolytic capacity observed in Figure. 1. Since previous work strongly suggested that proteasome was the most likely candidate [1–6, 11–16], we measured all three proteasome-dependent proteolytic activities, the chymotrypsin-like, trypsin-like and caspase-like activities of cell lysates, 24h after hydrogen peroxide pre-treatment. We observed significant (p < 0.01) twofold increases in trypsin-like and chymotrypsin-like activites, and a ten-fold increase in caspase-like activity (Fig. 2A). To test the proteasomal identity of these activities, we repeated the experiments using the proteasomal inhibitors lactacystin and MG132, which blocked the majority of all three activities in both control lysates (Figure. 2B), and were even more effective in H2O2 adapted lysates (Figure. 2C).
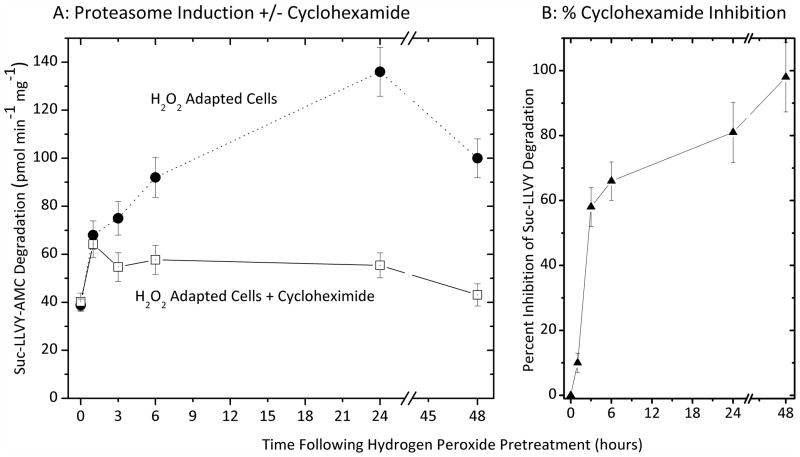
We have previously reported that nuclear proteasome can undergo direct activation by poly-ADP-ribose-polymerase [5], and other direct mechanisms of activating (existing) proteasome complexes, may also play a role during stress, without need for de novo synthesis of new proteasomes. To differentiate between direct activation of pre-existing proteasome complexes and de novo synthesis of proteasome (i.e. induction), we first pre-incubated cells with cycloheximide to block protein synthesis, and then exposed both cycloheximide-blocked and unblocked cells to an adaptive dose of H2O2. As shown in Figure. 3, cycloheximide had only a 10% inhibitory effect on increased proteasome activity during the first hour of H2O2 adaptation, indicating that a significant proportion of the hour-one increased proteolysis reported in Figure. 1 (using identical conditions) was actually due to direct, physical activation of existing proteasome complexes; this could be due to poly-ADP-ribose activation of proteasome in the nucleus, and/or to the effects of various proteasome regulators such as 19S, PA28αβ (or 11S), PA28γ (or REGγ), PA200, HSP90, etc. We plan to further pursue such direct activation mechanisms in a future report. Cycloheximide inhibited the increase in proteasome activity by 58% after three hours of H2O2 adaptation, by 82%, after 24 hours, and by 95% after 48 hours (Fig. 3). From these results we can conclude that there is a two-stage response to hydrogen peroxide pre-treatment, an initial translation-independent physical activation of existing proteasomes, followed by a progressive increase in proteasome transcription/translation.
Figure 3. Inhibition of Proteasome induction by cyclohexamide.
Panel A. MEF cells were incubated with 100 μg/ml of cyclohexamide (or not treated) in an attempt to block H2O2 induced expression of proteolytic enzymes. Cells were then grown to 50% confluence and exposed (in PBS) to a transient adaptive pre-treatment 2 μmol of H202 per 107 cells, harvested, and lysed, as described in the legend to Figure. 1. Proteolytic activity assays for degradation of suc-LLVY-AMC were then performed, as per Figs. 1 and 2. Panel B. Inhibition of proteasome induction plotted as the percent inhibition (means ± SE, n = 3) exerted by cycloheximide against the H2O2 induced (adaptive) proteasome activity of panel A.
20S Vs. 26S proteasome in the degradation of oxidized proteins
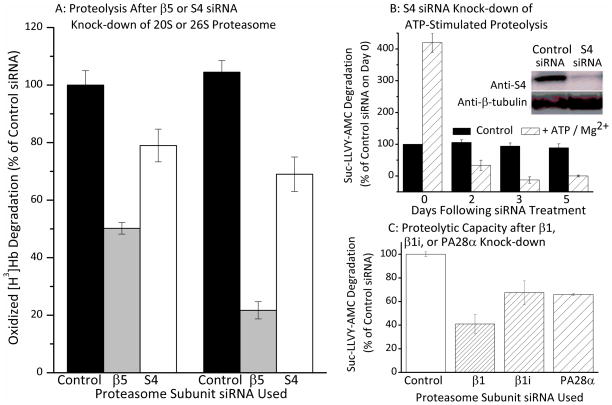
Since the proteasome exists in so many different forms (e.g. 20S, 26S, Immunoproteasome, hybrid proteasomes, etc.) we next wanted to determine which proteasome ‘species’ might be synthesized during H2O2 adaptation. As a first step, we tested the relative importance of the core 20S proteasome and the ATP/ubiquitin-dependent 26S proteasome using siRNA targeted to specific subunits of each complex (Fig. 4A). As shown in Figure. 4B, two days of treatment with siRNA directed against the β5 subunit of the 20S ‘core’ proteasome blocked some 50% of the increased capacity to degrade oxidized hemoglobin, that was induced by H2O2 adaptation. In contrast, two days of treatment with siRNA directed against the S4 subunit of the 19S regulator of the 26S proteasome blocked only 20% of the H2O2 adaptation-induced increase in capacity to degrade oxidized hemoglobin. After five days of β5 subunit ‘knock-down,’ induction of [3H]Hbox proteolysis was inhibited by 80%, whereas S4 subunit knock-down for five days still only caused a 30% inhibition.
Figure 4. ATP-Independent Degradation of Oxidized Proteins by the Proteasome.
Panel A. MEF cells were grown to 50% confluence and treated with β5, S4 or control (scrambled) siRNA. Cells were grown for a further 5 days then harvested, lysed, and suspended in 50mM Tris, 25mM KCl, 10mM NaCl, 1mM MgCl2 at (pH 7.5). Proteolytic activity assays for degradation of [3H]Hbox were performed as in Fig. 1 [15, 48, 49], on control samples and samples for which cells were grown for a period of 2 or 5 days following siRNA treatment, Values are means ± SE, n = 3. Panel B. The inset shows a Western blot for S4 knock-down. Quantification of triplicate blots, in comparison with standards, revealed an average 90% decrease in S4 subunit content relative to control siRNA treatments. The main portion of Panel B shows a comparison of ATP-stimulated and ATP-independent proteasomal chymotrypsin-like activity over a 5-day S4 siRNA knock-down time course. Cells were prepared as described in panel A and treated with either S4 or control (scrambled) siRNA. Cells were grown for a further 1 to 5 days and were then harvested, lysed, and suspended in 50mM Tris, 25mM KCl, 10mM NaCl, 1mM MgCl2 at (pH 7.5). Proteolytic activity assays for ATP-stimulated degradation of suc-LLVY-AMC, were then performed in the presence and absence of 10mM ATP. Addition of ATP produced a 4.2-fold increase in proteolysis in control samples (not treated with siRNA), or treated with control (scrambled) siRNA, on Day 0. By Day 2 of S4 siRNA treatment, however, ATP stimulation of proteolysis was only 10% of control values, and by Day 5, ATP completely failed to stimulate degradation (all data are means ± SE, n = 4). Panel C. MEF cells were grown to 20% confluence and then treated with control (scrambled) siRNA, or with siRNA’s directed against β1, β1i (Lmp2), PA28α. ATP-independent proteasomal chymotrypsin-like activity (capacity to degrade suc-LLVY-AMC) was then measured in all samples as described in Panel B. Values are means ± SE, n = 4.
Although Fig. 4A indicates a major role for β5 (core 20S proteasome) and only a minor role for S4 (ATP-stimulated 26S proteasome) in the degradation of oxidized proteins, our results might also be explained by ineffective or incomplete S4 knock-down. To test this possibility, we performed Western blots for the S4 subunit and quantified results in comparison with standards. The inset to Fig. 4B shows a typical S4 Western blot, revealing major loss of the S4 subunit following S4 siRNA treatment; quantification of gel triplicates revealed an 85–90% average decrease in S4 protein. Having ascertained that S4 had actually been successfully knocked-down, we next tested the ability of the S4 siRNA-treated cells to conduct ATP-stimulated proteolysis – an activity that depends upon the 19S S4 subunit. Addition of ATP produced a 4.2-fold increase in proteolysis in control samples not treated with siRNA, or treated with control (scrambled) siRNA (Fig. 4B). ATP-stimulated proteolysis was severely compromised by S4 siRNA, thus demonstrating the effectiveness of the S4 knock-down procedure, whereas ATP-independent activity was only mildly affected, (Fig. 4B). We suggest that the results of Figs. 4A and 4B make it reasonable to conclude that (some form of) the core 20S proteasome must have a highly important role in removal of oxidized proteins from the cell whereas the ATP/ubiquitin-dependent 26S proteasome appears to play a relatively minor role, consistent with previous findings [1–6, 11–16].
It should also be noted that interference with 26S proteasome function, e.g. by S4 subunit knock-down, is actually known to impede synthesis of the 20S proteasome: thus S4 knock-down would eventually be expected to limit 20S proteasome activity anyway [39], which may even explain why S4 knock-down does exert a small effect on the degradation of oxidized hemoglobin in Fig. 4A. The 19S regulator has been demonstrated to have important and diverse roles in transcription, [reviewed in [40, 41]]. While exact mechanism(s) are unclear, it has been observed that several subunits within the 19S regulator have important roles in the recruitment of RNA polymerase [42]. It has also been shown that the 19S proteasome regulator is required for recruitment of RNA polymerase II to promoter sites on many genes, and absence or insufficiency of the 19S results in decreased gene expression [43, 44].
Although the main point of Fig. 4 was a comparison of the need for 20S versus 26S proteasome (to degrade oxidized proteins) it seemed useful to also add some measure of immunoproteasome, and PA28’s possible importance at this point in our studies. Therefore, we compared the relative importance of 20S, immunoproteasome, and the PA28 regulator under the same conditions used for Figs. 4A and 4B. For this, we measured the ATP-independent chymotrypsin-like activity of control cells, and siRNA-treated samples with depleted levels of either the core 20S proteasome β1 subunit, the Immunoproteasome β1i subunit, or the proteasome regulator PA28α subunit, all of which caused significant (p < 0.01) reductions in cellular proteolytic capacity (Fig. 4C), implying that they each have important roles. Please see Supplemental Figure 1 to see the effectiveness of siRNA’s.
Expression of the 20S proteasome, the Immunoproteasome, and the PA28αβ (11S) regulator following oxidative stress adaptation
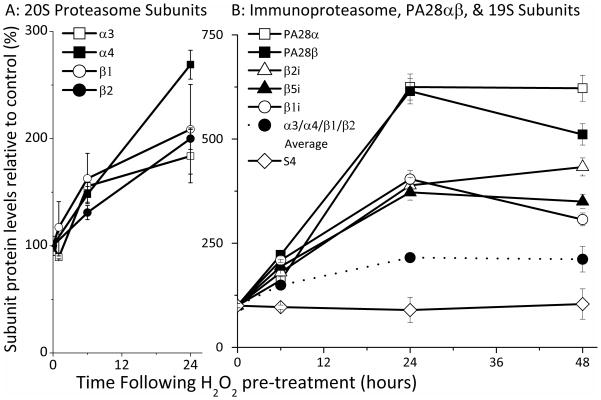
To test potential 20S proteasome induction during oxidative stress adaptation, we probed lysates from control and H2O2 adapted cells with antibodies against the α3, α4, β1, and β2 core 20S proteasome subunits. The four 20S subunits examined all exhibited a progressive rise of about two-fold (p < 0.01) during 24 hours of adaptation following mild H2O2 pre-treatment (Fig. 5A).
Figure 5. Expression of 20S Proteasome, 26S Proteasome, Immunoproteasome, and PA28αβ Regulator Subunits During Adaptation to H2O2.
Panel A. MEF cells were grown to 50% confluence and exposed (in PBS) to a transient adaptive pre-treatment of 2 μmol of H202 per 107 cells, then harvested as described in the legend to Figure. 1. Cells were then lysed and analyzed by Western blot, using antibodies against the 20S proteasome subunits β1, β2, α3 and α4. An enhanced chemiluminescence kit, (Pierce: Rockford, IL), was used for detection and membranes were developed onto Kodak Biomax films (VWR: West Chester, PA) using the Kodak GBX developing system. 20S proteasome subunit levels were quantified in comparison with standards. Panel B. MEF cells were prepared, H2O2 pre-treated (adapted), harvested, lysed and analyzed by Western blot, as described in Panel A. For Panel B, however, gels were probed with antibodies raised against the Immunoproteasome subunits β1i (Lmp2), β2i (Mecl-1), β5i (Lmp7) and S4 (26S proteasome subunit). An enhanced chemiluminescence kit (Pierce: Rockford, IL), was again used for detection, but signals were detected, and quantified in comparison with standards, using the biospectrum imaging system (UVP: Upland, CA). Also shown in Panel B, as a dotted line between solid circle symbols, is the arithmetic mean of 20S proteasome α3, α4, β1, and β2 subunit level values taken from Panel A. In both panels, values for percent change in subunit levels are means ± SE, n = 4, are reported as percent of control (non H2O2 adapted) levels.
Critically we observed no significant change in the level of 26S proteasome, as judged by the S4 subunit of the 19S regulator (Fig. 5B). The average change in the α3, α4, β1, and β2 core 20S proteasome subunits (mathematical mean) is shown as a dotted line between solid circle symbols in Fig. 5B, for comparison. These results, in conjunction with those of Figs. 3 and 4, suggest that the increased capacity to degrade oxidized proteins that is induced by oxidative stress adaptation is, at least, partly due to de novo synthesis of the 20S core proteasome and is independent of the ATP/ubiquitin stimulated 26S proteasome.
We next probed lysates from H2O2 adapted cells with antibodies directed against the three unique immunoproteasome subunits β1i (or Lmp2), β2 (or Mecl-1), and β5i (or Lmp7). Our interest in immunoproteasome under stress conditions stems both from our own PrOxI hypothesis [45], and reports by Kalyanaraman et al. [32–34] that immunoproteasome can be induced by both NO• and H2O2 and reports by Ferrington et al. [30] that injury can induce immunoproteasome expression. Importantly, we measured a three-to-four fold increase in immunoproteasome subunits and a six-fold increase in PA28α and PA28β subunits (Fig. 5B, p < 0.01). These large increases in immunoproteasome and PA28αβ subunits should be compared with the more modest (two-fold) increases in 20S core proteasome subunits, and the lack of any significant increase in the S4 subunit of the 26S proteasome.
Purified immunoproteasome degrades oxidized proteins, and the PA28αβ regulator is important for stress-adaptive increases in proteolytic capacity
Taken together, the results of Figures. 4 and 5 suggest a significant role for both immunoproteasome and pa28αβ in response to oxidative stress. It is important to note, however, that immunoproteasome has not been shown to be able to degrade oxidized proteins, and that the PA28αβ regulator has not been shown to enhance the degradation of oxidized proteins by either the 20S proteasome or the Immunoproteasome. We next proceeded to test both these possibilities.
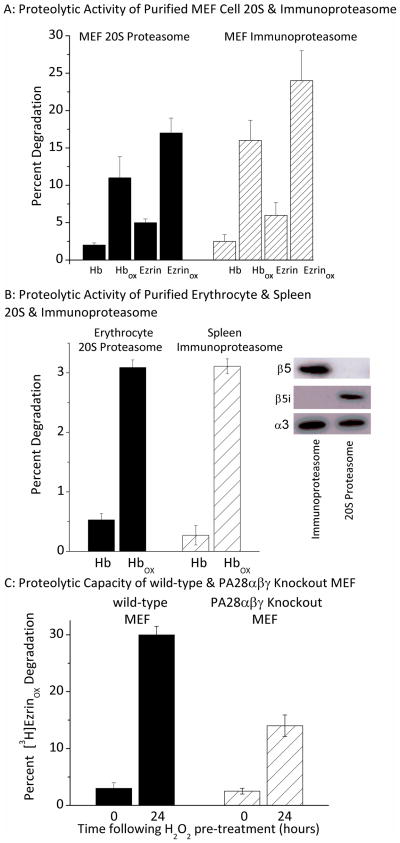
First, we purified both the core 20S proteasome and the Immunoproteasome from MEF cells and measured their ability to degrade both the control and oxidized forms of hemoglobin and ezrin (Fig. 6A). Additionally, to improve our confidence in the results, we repeated the assay using 20S proteasome and immunoproteasome purified from human erythrocytes and spleen, respectively (Fig. 6B). The purity of erythrocyte 20S proteasome (positive for β5 subunit but not for β5i), and of spleen immunoproteasome (positive for β5i subunit but not for β5) can be readily seen in the Western blot insert to figure 6B (in which both proteasome forms are appropriately positive for the α3 subunit). Oxidized hemoglobin is a good model substrate for oxidized proteins in general [2, 3] and ezrin undergoes substantial oxidation and proteasome-dependent degradation following exposure of cells to oxidants [46]. Our results show that the Immunoproteasome selectively degrades the oxidized forms of proteins, and that it is at least as efficient at degrading oxidized proteins as is the 20S core proteasome (Figs.. 6A and 6B, in which both proteasome and immunoproteasome degraded oxidized proteins significantly better than non-oxidized proteins: p < 0.01).
Figure 6. Importance of the Immunoproteasome and Pa28 for the Degradation of Oxidized Proteins.
Panel A. Proteolytic Activity of Purified MEF Cell 20S & Immunoproteasome. 20S proteasome was isolated from MEF cells and immunoproteasome was isolated after 2 days of cell treatment with IFNγ, as described by Tanakaa et al. [36, 37]. Purified 20S proteasome or purified immunoproteasome was then incubated for 60 minutes with [3H]Hb, [3H]ezrin, [3H]Hbox, or [3H]ezrinox. The percent protein substrate degraded was calculated, after addition of 20% trichloroacetic acid and 3% BSA to precipitate remaining intact proteins[5, 12, 15]. Percent protein degraded was determined by release of acid soluble counts in TCA supernatants using liquid scintilation in which % Degradation = (acid-soluble counts – background counts) x 100. Values are means ± SE, n = 3. Panel B: Proteolytic Activity of Purified Erythrocyte & Spleen 20S & Immunoproteasome. 20S proteasome purified from human erythrocytes, and Immunoproteasome purified from human spleen were studied exactly as per Panel A. Values are means ± SE, n = 3. The inset shows 20S proteasome and immunoproteasome samples of equal quantity, screened by Western blot with antibodies directed against β5, β5i (Lmp7) or α3 subunits, and demonstrates the purity of the 20S and immunoproteasome preparations. Panel C: Proteolytic Capacity of Wild-type & PA28αβγ Knockout MEF. Wild type MEF and PA28αβγ knockout cells developed by Yamano et al. [35] were transiently adapted to oxidative stress by pre-treatment with 4 μmol H2O2 per 107 cells. Control (0hr) and 24hr H2O2 adapted wild-type MEF and PA28αβγ knockout MEF cells were then harvested and lysed as described in the legend to Figure. 1. Lysates were then incubated for 60 minutes with [3H]ezrinox. Percent [3H]ezrinox protein degraded was determined as per Panel A. Values are means ± SE, n = 3.
To examine the importance of the PA28 regulator, we compared degradation of oxidized ezrin ([3H]ezrinox) in lysates from wild-type MEF cells, and from PA28αβγ knockout MEF cells. Lysates from both wild-type and PA28αβγ knockout cells were studied without H2O2 exposure, and after 24 hours of H2O2 adaptation. In wild-type MEF cell lysates we observed a 15-fold increase in ezrin degradation after 24 hours, whereas lysates from the PA28αβγ knockout cells exhibited only an 8-fold increase (Fig. 6C). These data (especially when considered with the results of Figs. 4C and 5B) indicate that, while the PA28 regulator may not be crucial for the increased proteolytic capacity associated with oxidative stress adaptation, it does seem to play an important role. Importantly, Yamano et al. [35] carefully characterized the cell lines used in our Fig. 6C and demonstrated that the 20S proteasome and Immunoproteasome contents are equal in control and PA28 knockout lines.
Importance of 20s core proteasome, immunoproteasome, and PA28 (11S) regulator induction to overall cell adaptation to oxidative stress
Finally, to determine if the observed inductions of 20S proteasome, immunoproteasome and PA28αβ were actually relevant, we assessed the importance of these proteins, in pre-treatment induced adaptation to oxidative stress. Cells were pre-treated with a mild (adaptive) dose of hydrogen peroxide and then challenged 24 hours later with a more severe dose. We have previously demonstrated that sub-lethal oxidative stress challenge causes a sharp decrease in DNA synthesis, transcription, translation, and rates of cell division, in (previously) divisionally competent cells [23–26]. In figures 7A, 7B, and 7C the doses of hydrogen peroxide challenge stress used were fairly mild (and more biologically relevant than extreme stress) and so the main effect of peroxide challenge was slow growth, rather than apoptosis. We confirmed these results by performing a caspase-3 assay on both challenged and unchallenged cells which showed only a 6% increase in caspase-3 activity (data not shown). To test the importance of various proteasome forms, our experiments were also conducted using siRNA against β1, β1i, and PA28α to block the induction of these subunits. As shown in Supplemental Figure 1, all three siRNA’s effectively completely blocked the adaptive induction of their respective proteasome subunits.
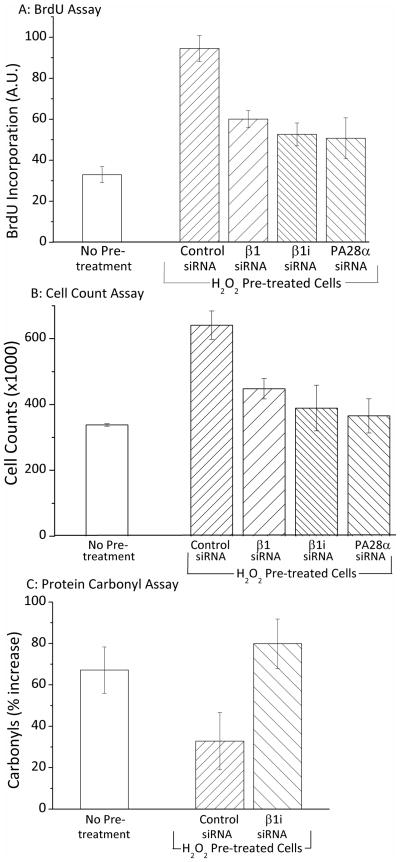
Figure 7. Blocking the Induction of 20S Proteasome, Immunoproteasome or PA28αβ Inhibits Adaptation in H2O2 Challenged Cells.
Panel A. MEF cells were grown to 20% confluence and then treated with β1, β1i (Lmp2), PA28α or control (scrambled) siRNA for 24 hours to block induction of the relevant proteasome subunits (see Supplemental Fig. 1 for proof of siRNA effectiveness). After siRNA exposure the media was replaced with fresh complete medium and after a further 24 hours (a total of 48 hours after initial siRNA exposure), some cells were transiently adapted to oxidative stress by pre-treatment with 2 μmol of H2O2 per 107 cells, while others were not adapted. Cells were incubated at 37ºC under 5% CO2 for 1 hour, after which the medium was replaced. Following a 24 hour adaptation period, both adapted and non-adapted cells were challenged by incubation with a high dose of 1 mM H2O2 (≈25 μmol H2O2 per 107 cells). Cells were then harvested and reseeded at 100,000 cells per ml on 96 well plates and the BrdU assay was then performed (as per Materials & Methods). BrdU results (means ± SE, n = 3) represent cellular BrdU incorporation into DNA in arbitrary units. On the X-axis, “No Pre-treatment” represents samples that were treated with control (scrambled) siRNA and challenged with high H2O2, but were not adapted by pre-treatment with low H2O2. All other samples were first treated with siRNA’s, adapted by pre-treatment with low H2O2, and then challenged by exposure to high (1.0mM) H2O2. Panel B. MEF cells were prepared, treated with siRNA’s, transiently adapted to oxidative stress 7 by pre-treatment with H2O2 (or not pre-treated), challenged with 1 mM H2O2 (≈25 μmol H2O2 per 107 cells), and harvested, exactly as described in Panel A. Samples were then seeded at a density of 100,000 cells per ml in 24 well plates. Cells were incubated for a further 24 hours, then cell counts were taken using a cell counter (see Materials & Methods). Values (means ± SE, n = 3) represent the cell proliferation in challenged cells which previously were either pre-treated with an adaptive dose of H2O2 or not pre-treated. Panel C. MEF cells were prepared, treated with siRNA, transiently adapted to oxidative stress by pre-treatment with H2O2 (or not pre-treated, challenged with 1 mM H2O2 (≈25 μmol H2O2 per 107 cells), and harvested, exactly as described in Panels A and B. Samples were then incubated for a further six hours, harvested, lysed, diluted based on protein content, and then assayed in an oxyblot for protein carbonyls (see Materials & Methods). Values (means ± SE, n = 3) represent the percent increase in protein oxidation (overall carbonyl intensity of anti-DNP antibody staining) of H2O2 challenged (1.0 mM) samples, both H2O2 pre-treated and non-pre-treated ± β1i siRNA’s.
24h following H2O2 challenge, BrdU incorporation, as a measure of DNA replication and an indication of cell division, decreased 3.6 fold in comparison with unchallenged cells, but was three fold higher (p < 0.01) in H2O2 pre-adapted cells than in non-pretreated cells (Fig. 7A). Blocking expression of β1, β1i, and PA28α with siRNA (supplemental Fig. 1) significantly reduced the adaptive improvement in BrdU incorporation conferred by H2O2 pre-treatment (Fig. 7A). Similarly, 24h following H2O2 challenge, cell counts were twice as high (p < 0.01) in H2O2 pre-adapted cells than in non-pretreated cells (Fig. 7B). Blocking expression of β1, β1i, and PA28α with siRNA (supplemental Fig. 1) significantly reduced this adaptive improvement in cell number conferred by H2O2 pre-treatment (Fig. 7B). Finally, 24h following H2O2 challenge, protein carbonyls (a measure of protein oxidation) were only half as high (p < 0.01) in H2O2 pre-adapted cells than in non-pretreated cells (Fig. 7C). Blocking expression of β1i with siRNA (supplemental Fig. 1) significantly reduced this adaptive decrease in accumulation of oxidized proteins conferred by H2O2 pre-treatment (Fig. 7C).
Importantly, Supplemental Fig. 1 shows that the brief siRNA treatments used for the experiments of Fig. 7A, B, and C, blocked the increased expression of β1, β1i, and PA28α induced by adaptation to H2O2 pre-treatment, but did not decrease the basal levels of these proteasome subunits/regulators. The results of Fig. 7 and Supplemental Fig. 1 reveal important roles for the core 20S proteasome, the Immunoproteasome, and the Pa28 (11S) regulator in overall cellular adaptation to oxidative stress.
DISCUSSION
Our studies indicate that the 20S proteasome, the Immunoproteasome, and the Pa28 (11S) regulator all play major roles in the degradation of oxidized proteins, whereas the 26S proteasome seems not to be involved. We also find that the Immunoproteasome is at least as capable of degrading oxidized proteins as is the 20S proteasome. We go on to demonstrate that the proteasome is a highly plastic system under mild oxidative stress, and that the 20S proteasome, the Immunoproteasome and the PA28αβ regulator are all induced during transient adaptation to oxidative stress. Furthermore all of these proteins were demonstrated to provide significant contributions to adaptation (Figs 4 – 6) and increased tolerance to oxidative stress (Fig. 7). We also provide new evidence of a highly significant role for the Immunoproteasome in stress-adaptation (Figs. 4–7).
During adaptation to H2O2 the proteasome undergoes a two-stage response: an initial direct activation of pre-existing proteasome during the first hour (by poly-ADP-ribose polymerase in the nucleus [5] and other proteasome regulators in the cytoplasm), followed by a much slower de novo synthesis of 20S proteasome, immunoproteasome, and PA28αβ subunits. After 24 hours, the cellular capacity to degrade oxidized proteins is increased more than three-fold, and essentially all of this increase can be blocked by proteasome inhibitors (Figs. 2B and 2C). These results demonstrate that proteasome is highly responsive to oxidative stress, being both activated and induced under stress-adaptive conditions. We plan to follow-up these findings with detailed studies of proteasome direct activation, and of the signal transduction pathway(s) involved in proteasome induction, in subsequent reports.
Proteasome can only be inactivated by H2O2 concentrations much higher than those used in the current work. Studies of purified 20S and 26S proteasomes, and intact cell studies, show that the 20S proteasome is rather resistant to oxidation, whereas the 26S proteasome is extremely sensitive. In fact, the I50 for 26S inactivation by peroxynitrite, hypochlorite, or H2O2 is an order of magnitude lower than that of the 20S proteasome [47]. Despite the relative resistance of 20S proteasome to direct oxidative inactivation, it is interesting to note that Taylor’s group has reported that even relatively low levels of H2O2 will inactivate both the E1 and E2 enzymes of the ubiquitinylation pathway, due to highly redox-sensitive sulfhydryl groups that are required for activity [14]; thus, further diminishing the importance of ATP- and ubiquitin-stimulated proteolysis (26S proteasome) in the degradation of oxidized proteins. Recently, Midicherla and Goldberg suggested that yeast degrade newly-synthesized oxidized proteins in an ATP- and ubiquitin-stimulated pathway. It is possible that the degradation of newly-synthesized proteins may be a special case. It is also possible (although unlikely) that yeast may handle oxidized proteins differently that do the mammalian cells which we have studied. We, and several other groups, have repeatedly shown that oxidized proteins are degraded, by proteasome in the cytoplasm and nucleus of mammalian cells, by an ATP- and ubiquitin-independent mechanism [1–6, 11–16, 48–50], and the current work strongly supports this view.
The Immunoproteasome has long been considered as a proteasome variant that generates peptides for MHC Class I processing. Although we had previously suggested that oxidation might be a common protein modification that the Immunoproteasome might recognize, the ‘PrOxI hypothesis,’ and although recent data show that immunoproteasome can be induced by oxidative stress [29–34], there has been no direct demonstration that immunoproteasome can truly degrade oxidized proteins, until now. Our current data may even indicate (although more detailed studies are needed) that immunoproteasome may actually be slightly more efficient than 20S proteasome, in recognizing the oxidatively modified forms of protein substrates such as hemoglobin and ezrin (Fig. 6). We suggest that the PrOxI hypothesis [45], which proposes that some fraction of all intracellular proteins undergoes oxidation, with subsequent processing for MHC Class I by immunoproteasome, now deserves much greater scrutiny and serious testing.
Although induction of 20S proteasome, immunoproteasome, and PA28αβ regulator synthesis during oxidative stress adaptation is certainly interesting, the important question is whether such induced proteolytic capacities actually contribute to the increased oxidative stress tolerance of adapted cells. Our data reveal that the increased capacity (as measured in Fig. 7 by BrdU incorporation, cell proliferation, and diminished accumulation of oxidized proteins) of adapted cells to withstand a high H2O2 challenge is, at least, partly dependent upon 20S proteasome induction, immunoproteasome induction, and PA28αβ regulator induction. These findings demonstrate the importance of 20S proteasome, immunoproteasome, and PA28αβ in overall adaptation to oxidative stress.
Supplementary Material
Proteasome subunit expression was blocked with siRNA as described in Fig. 7. After 48hr of siRNA exposure, cells were pre-treated with an adaptive dose of hydrogen peroxide and 24hr later cells were harvested, lysed, diluted based on results from a BCA assay then analyzed by Western blot. An enhanced chemiluminescence kit, (Pierce: Rockford, IL), was used for detection and membranes were developed onto Kodak Biomax films (VWR: West Chester, PA) using the Kodak GBX developing system. Panel A shows representative Western blots, with and without siRNA treatment, of core 20S Proteasome (as represented by Anti-β1), Immunoproteasome (as represented by Anti-Lmp7) or the Pa28αβ regulator (represented by Anti-Pa28α). Panel B shows the same proteasome subunit/regulator levels detected and quantified, in comparison with standards, using the biospectrum imaging system (UVP: Upland, CA). All intensities shown in Panel B were adjusted for on β-tubulin loading levels. Results shown are means ± SE, where n = 3.
The abbreviations used are
- H2O2
hydrogen peroxide
- MEF
murine embryonic fibroblasts
- PA28αβ
a proteasome regulator (also called the 11S regulator)
- PrOxI hypothesis
Protein Oxidation and Immunoproteasome hypothesis of MHC Class I antigen processing (see reference # 45)
- [3H]Hb
tritium-labeled hemoglobin
- [3H]Hbox
tritium-labeled oxidized hemoglobin
- [3H]ezrin
tritium-labeled ezrin
- [3H]ezrinox
tritium-labeled oxidized ezrin
- TCA
trichloroacetic acid
Footnotes
This research was supported by grant #RO1-ES003598, and by ARRA Supplement 3RO1-ES 003598-22S2, both from the NIH/NIEHS to KJAD.
AUTHOR CONTRIBUTIONS
Andrew M. Pickering and Cheryl Y. Teoh performed most of the experiments, and were involved in project planning and data analysis as part of their Ph.D. studies. Alison L. Koop assisted with experiments, initially as an undergraduate researcher and subsequently as a research laboratory technician. Gennady Ermak and Tilman Grune assisted with project planning, data analysis, and writing the paper. Kelvin J. A. Davies (in whose laboratory all experiments were performed) oversaw the project, and led the planning and data analysis. Andrew M. Pickering and Kelvin J. A. Davies wrote the first draft of the paper, which was subsequently revised by them, Gennady Ermak and Tilman Grune, and eventually approved by all authors.
References
- 1.Davies KJ. Intracellular proteolytic systems may function as secondary antioxidant defenses: an hypothesis. J Free Radic Biol Med. 1986;2:155–173. doi: 10.1016/s0748-5514(86)80066-6. [DOI] [PubMed] [Google Scholar]
- 2.Davies KJ, Goldberg AL. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987;262:8227–8234. [PubMed] [Google Scholar]
- 3.Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J Biol Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 4.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJ. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci U S A. 1999;96:6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 7.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 8.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic Biol Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Bulteau AL, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci U S A. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 12.Fucci L, Oliver CN, Coon MJ, Stadtman ER. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 14.Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem J. 1995;307(Pt 1):297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shringarpure R, Grune T, Mehlhase J, Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 16.Whittier JE, Xiong Y, Rechsteiner MC, Squier TC. Hsp90 enhances degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2004;279:46135–46142. doi: 10.1074/jbc.M406048200. [DOI] [PubMed] [Google Scholar]
- 17.Ahn K, Erlander M, Leturcq D, Peterson PA, Fruh K, Yang Y. In vivo characterization of the proteasome regulator PA28. J Biol Chem. 1996;271:18237–18242. doi: 10.1074/jbc.271.30.18237. [DOI] [PubMed] [Google Scholar]
- 18.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Kasahara M. The MHC class I ligand-generating system: roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol Rev. 1998;163:161–176. doi: 10.1111/j.1600-065x.1998.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 21.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 22.Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 23.Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999;48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 24.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 25.Wiese AG, Pacifici RE, Davies KJ. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 26.Ermak G, Harris CD, Davies KJ. The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. Faseb J. 2002;16:814–824. doi: 10.1096/fj.01-0846com. [DOI] [PubMed] [Google Scholar]
- 27.Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. Faseb J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 28.Sitte N, Merker K, Von Zglinicki T, Davies KJ, Grune T. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II--aging of nondividing cells. Faseb J. 2000;14:2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- 29.Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. Faseb J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- 30.Ferrington DA, Hussong SA, Roehrich H, Kapphahn RJ, Kavanaugh SM, Heuss ND, Gregerson DS. Immunoproteasome responds to injury in the retina and brain. J Neurochem. 2008;106:158–169. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Kotamraju S, Tampo Y, Keszler A, Chitambar CR, Joseph J, Haas AL, Kalyanaraman B. Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: Role of ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 2003;100:10653–10658. doi: 10.1073/pnas.1933581100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas S, Kotamraju S, Zielonka J, Harder DR, Kalyanaraman B. Hydrogen peroxide induces nitric oxide and proteosome activity in endothelial cells: a bell-shaped signaling response. Free Radic Biol Med. 2007;42:1049–1061. doi: 10.1016/j.freeradbiomed.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotamraju S, Matalon S, Matsunaga T, Shang T, Hickman-Davis JM, Kalyanaraman B. Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radic Biol Med. 2006;40:1034–1044. doi: 10.1016/j.freeradbiomed.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Yamano T, Murata S, Shimbara N, Tanaka N, Chiba T, Tanaka K, Yui K, Udono H. Two distinct pathways mediated by PA28 and hsp90 in major histocompatibility complex class I antigen processing. J Exp Med. 2002;196:185–196. doi: 10.1084/jem.20011922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.TanakaaKeiji YH, Nobuyuki Tanahashia. Preparation of Proteasomes. In: ECJ, editor. Cell Biology. Elsevier Inc; 2006. pp. 91–97. [Google Scholar]
- 37.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, Tanaka K. Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem. 2000;275:14336–14345. doi: 10.1074/jbc.275.19.14336. [DOI] [PubMed] [Google Scholar]
- 38.Jentoft N, Dearborn DG. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979;254:4359–4365. [PubMed] [Google Scholar]
- 39.Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- 40.Collins GA, Tansey WP. The proteasome: a utility tool for transcription? Curr Opin Genet Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Lipford JR, Deshaies RJ. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol. 2003;5:845–850. doi: 10.1038/ncb1003-845. [DOI] [PubMed] [Google Scholar]
- 42.Swaffield JC, Bromberg JF, Johnston SA. Alterations in a yeast protein resembling HIV Tat-binding protein relieve requirement for an acidic activation domain in GAL4. Nature. 1992;357:698–700. doi: 10.1038/357698a0. [DOI] [PubMed] [Google Scholar]
- 43.Dennis AP, Lonard DM, Nawaz Z, O’Malley BW. Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. J Steroid Biochem Mol Biol. 2005;94:337–346. doi: 10.1016/j.jsbmb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Lipford JR, Smith GT, Chi Y, Deshaies RJ. A putative stimulatory role for activator turnover in gene expression. Nature. 2005;438:113–116. doi: 10.1038/nature04098. [DOI] [PubMed] [Google Scholar]
- 45.Teoh CY, Davies KJ. Potential roles of protein oxidation and the immunoproteasome in MHC class I antigen presentation: the ‘PrOxI’ hypothesis. Arch Biochem Biophys. 2004;423:88–96. doi: 10.1016/j.abb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Grune T, Reinheckel T, North JA, Li R, Bescos PB, Shringarpure R, Davies KJ. Ezrin turnover and cell shape changes catalyzed by proteasome in oxidatively stressed cells. Faseb J. 2002;16:1602–1610. doi: 10.1096/fj.02-0015com. [DOI] [PubMed] [Google Scholar]
- 47.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335(Pt 3):637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinheckel T, Grune T, Davies KJ. The measurement of protein degradation in response to oxidative stress. Methods Mol Biol. 2000;99:49–60. doi: 10.1385/1-59259-054-3:49. [DOI] [PubMed] [Google Scholar]
- 49.Pacifici RE, Davies KJ. Protein degradation as an index of oxidative stress. Methods Enzymol. 1990;186:485–502. doi: 10.1016/0076-6879(90)86143-j. [DOI] [PubMed] [Google Scholar]
- 50.Fagan JM, Waxman L, Goldberg AL. Red blood cells contain a pathway for the degradation of oxidant-damaged hemoglobin that does not require ATP or ubiquitin. J Biol Chem. 1986;261:5705–5713. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proteasome subunit expression was blocked with siRNA as described in Fig. 7. After 48hr of siRNA exposure, cells were pre-treated with an adaptive dose of hydrogen peroxide and 24hr later cells were harvested, lysed, diluted based on results from a BCA assay then analyzed by Western blot. An enhanced chemiluminescence kit, (Pierce: Rockford, IL), was used for detection and membranes were developed onto Kodak Biomax films (VWR: West Chester, PA) using the Kodak GBX developing system. Panel A shows representative Western blots, with and without siRNA treatment, of core 20S Proteasome (as represented by Anti-β1), Immunoproteasome (as represented by Anti-Lmp7) or the Pa28αβ regulator (represented by Anti-Pa28α). Panel B shows the same proteasome subunit/regulator levels detected and quantified, in comparison with standards, using the biospectrum imaging system (UVP: Upland, CA). All intensities shown in Panel B were adjusted for on β-tubulin loading levels. Results shown are means ± SE, where n = 3.