SUMMARY
There is a discrepancy between the in vitro anergic state of CD4+CD25hiFoxP3+ regulatory T (Treg) cells and their in vivo proliferative capability. The underlying mechanism of this paradox is unknown. Here we show that the anergic state of Treg cells depends on the elevated activity of the mammalian target of rapamycin-(mTOR)-pathway induced by leptin: a transient inhibition of mTOR with rapamycin, before T-cell-receptor-(TCR)-stimulation, made Treg cells highly proliferative in the absence of exogenous interleukin-2 (IL-2). This was a dynamic and oscillatory phenomenon characterized by an early downregulation of the leptin-mTOR-pathway followed by an increase in mTOR activation necessary for Treg cell expansion to occur. These data suggest that energy metabolism, through the leptin-mTOR-axis, sets responsiveness of Treg cells that use this information to control immune tolerance and autoimmunity.
Keywords: Treg cell, metabolism, mTOR, leptin, immune tolerance
INTRODUCTION
A growing body of evidence suggests that the flexibility in T cell commitment is probably not an exception but rather the norm (Bluestone et al., 2009). The commitment to a specific T cell phenotype, including that of naturally occurring CD4+CD25hiFoxP3+ regulatory T (Treg) cells, depends on the integration of numerous environmental signals received by the T cell (Sakaguchi et al., 2008). It is well known that when some Treg cells lose the expression of the forkhead family transcription factor FoxP3, they also lose their suppressive capacity and can even acquire an effector T cell phenotype (Zhou et.al., 2009).
The degree of flexibility and plasticity of T cells may become clearer with a better understanding of the molecular actions of factors such as cytokines, antigen-presenting cells, transcription factors, that determine the fate towards defined T cell phenotypes. Here we test the possibility that cellular energy metabolism can be a key element in the plasticity of Treg cells (Zheng et al., 2009).
In humans and mice, natural Treg cells display classical surface markers of activated T cells and proliferate in vivo (Vukmanovic-Stejic et al., 2006). Yet, Treg cells are anergic in vitro and fail to proliferate in response to T-cell-receptor (TCR) ligation. This in vitro condition of the Treg cells appears reversible upon TCR stimulation, in the presence of high doses of interleukin-2 (IL-2) (Thornton and Shevach, 1998; Ng et al., 2001).
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved 289-kDa serine-threonine protein kinase that is inhibited by rapamycin. mTOR integrates environmental cues from nutrients, energy and growth factors to direct cell growth, proliferation and differentiation (Woods et al., 2008; Blouet et al., 2008). In vitro, an increase in adenosine triphosphate (ATP) increases mTOR signaling, and mTOR itself is thought to serve as an ATP sensor working as a checkpoint for cells to sense and decode changes in energy status, to accordingly determine the rate of cell growth and proliferation (Faivre et al., 2006; Thomson et.al., 2009). Although the list of downstream targets of mTOR is continuously expanding, the most studied readouts of mTOR function are the phosphorylation of p70S6 kinase (p70S6K) and the S6 ribosomal protein (S6), which are direct downstream translational regulators.
Rapamycin is an immunosuppressive drug that can promote the expansion of Treg cells in long-term cultures in the presence of supraphysiologic concentrations of IL-2 (Battaglia et al., 2005; Strauss et al., 2009). Considering that IL-2 activates mTOR (Bensinger et al., 2004; Zeiser and Negrin, 2008), the conundrum is how a concomitant inactivation of mTOR by rapamycin and IL-2-mediated activation of mTOR can result in Treg cells proliferation.
Changes in the microenvironment directly influence the intracellular energy status. Within this context, it has been hypothesized that leptin, a cytokine-like hormone mainly produced by adipocytes, might act as an endogenous “sensing” factor that could act as a critical link among environment (avaibility of nutrients), metabolism, and immune responses (Matarese and La Cava, 2004). Leptin is an anorexigenic molecule with pro-inflammatory activities that potentiates T helper 1 (Th1) pro-inflammatory immune responses and constrains Treg cell proliferation (Lord et al., 1998; De Rosa et al., 2007). The neutralization of leptin or its receptor (LepR) during TCR-engagement rapidly and efficiently reverses the in vitro hyporesponsiveness of Treg cells (De Rosa et al., 2007).
Here, we examined the role of the leptin-mTOR axis (Cota et al., 2006) in the integration of cellular energy status and leptin-related signalling in both human and mouse Treg cells and in leptin- and LepR-deficient Lepob/ob and Leprdb/db mice, respectively. Our findings identify a dynamic role for energy metabolism in driving the fate and responsiveness of Treg cells, suggesting targeted manipulation of this cell subset during immune responses.
RESULTS
Freshly isolated Treg cells have an activated nutrient and energy-sensing mTOR signalling pathway
Previous studies have indicated an important role of the mTOR-pathway in Treg cell activity (Sauer et al., 2008; Haxhinasto et al., 2008), yet the role of this energy-sensing pathway in Treg cell anergy and proliferation has not been explored. Here we analyzed mTOR activity in freshly isolated, purified human CD4+CD25hiCD127−FoxP3+ Treg cells (>95% pure by FACS analysis), using as readout the phosphorylation of the downstream translational regulators of mTOR, p70S6K and S6 (Blouet et al., 2008; Yang and Guan, 2007). The phosphorylation of mTOR, p70S6K and S6 were substantially higher in Treg cells as compared to CD4+CD25− effector T cells (Figure 1A), as confirmed by confocal microscopy (Figure 1B) and flow cytometry (Figure 1C). In vivo, mTOR phosphorylation in sections from non-reactive human lymph nodes was markedly increased in FoxP3+ T cells as compared to FoxP3− cells (Figure S1A). In agreement with the increased activation of the mTOR pathway in Treg cells, there was a reduced phosphorylation of the AMP-activated protein kinase (AMPK) (Figure S1B), a sensor of intracellular energy status which negatively regulates mTOR in relation to changes in the ATP/ADP ratio (Laplante and Sabatini, 2010). Confirming high mTOR activity and an enhanced metabolism in Treg cells, the intracellular amounts of ATP were found substantially elevated in freshly isolated Treg cells (Figure S1C). EGFP-Foxp3 reporter mice confirmed an increased expression of P-S6 by flow cytometry (Figure S1D). Taken together, these results indicate that freshly-isolated human Treg cells display an active mTOR pathway.
Figure 1. Freshly-isolated human Treg cells have active mTOR.
(A) Immunoblot for FoxP3, P-mTOR, P-p70S6K and P-S6 in human freshly-isolated Treg cells and in CD4+CD25− effector T cells. Graphs show the relative densitometric quantitation of the gels shown. One representative of five independent experiments. (B) Confocal microscopy of freshly-isolated human Treg cells and effector T cells stained for P-mTOR (green) and FoxP3 (red). Representative of three independent experiments. (C) P-S6 expression in freshly-isolated effector T and Treg cells, respectively. Representative of three independent experiments. The gray histogram represents the isotype-matched negative control.
Transient mTOR inhibition with rapamycin, before TCR engagement, reverses the hyporesponsiveness of functional Treg cells
Chronic treatment with rapamycin (a selective mTOR inhibitor) promotes expansion of Treg cells in the presence of supraphysiologic concentrations of IL-2 in long-term T cell cultures in vitro (Battaglia et al., 2005; Strauss et al., 2009). The molecular events leading to in vitro expansion of Treg cells are not clear, also because rapamycin and IL-2 display opposite effects on mTOR activity (Zeiser and Negrin, 2008).
We evaluated the responsiveness of Treg cells after transient inhibition of mTOR with rapamycin (without IL-2), prior to anti-CD3 plus anti-CD28 stimulation. Acute mTOR inhibition resulted in the reversal of Treg cell anergy and a robust proliferation with conspicuous cell clustering by 60-72h (Figures 2A and 2B). Wheras both acute and chronic treatment with rapamycin reduced the proliferation of effector T cells (Figures S2A and S2B), Treg cells proliferation did not occur during chronic rapamycin treatment when exogenous IL-2 was not present (Figure S2E). Thus, Treg cells appear more sensitive to a short or transient perturbation of the mTOR-pathway than effector T cells. To confirm specificity for mTOR, we transiently inhibited the mitogen-activated protein kinase (MAPK) pathway with UO126, and no substantial induction of Treg cell proliferation was observed (Figure S2F).
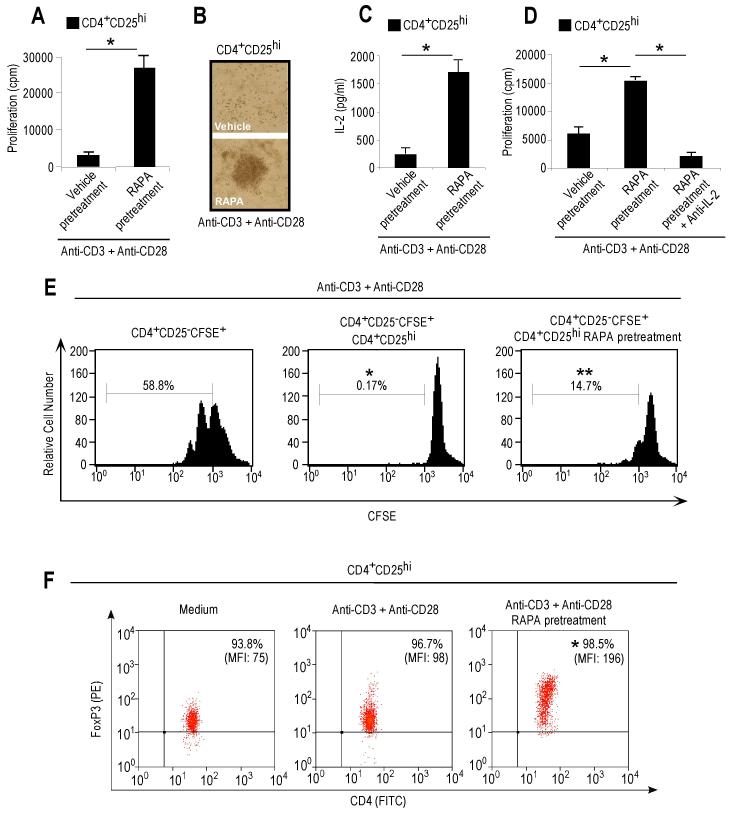
Figure 2. Transient mTOR inhibition with rapamycin, before anti-CD3 plus anti-CD28 stimulation, associates with proliferation of functional human Treg cells which upregulate FoxP3 expression.
(A) Proliferation of Treg cells pretreated or not with rapamycin, before anti-CD3 plus anti-CD28 mAb stimulation. Data are shown as mean ± SD (n = 20, *p < 0.0001). (B) Contrast-phase microscopy showing clustering and activation of Treg cells pretreated or not with rapamycin. Representative experiment of ten. (C) IL-2 secretion by Treg cells pretreated or not with rapamycin, upon anti-CD3 plus anti-CD28 stimulation. The data are shown as mean ± SD (n = 4, *p < 0.001). (D) Proliferation of Treg cells pretreated or not with rapamycin in the presence or absence of IL-2 neutralizing mAb. The data are shown as mean ± SD (n = 4, *p < 0.001). (E) Proliferative response at 36h of CFSE-labelled effector T cells alone (left panel) or in co-culture with untreated (middle panel) or rapamycin-pretreated (right panel) unlabeled Treg cells. Representative of three independent experiments. (*p < 0.0001, **p < 0.001 when compared to CD4+CD25−CFSE+). (F) FoxP3 expression in Treg cells pretreated or not with rapamycin, after 36h stimulation with anti-CD3 plus anti-CD28. Representative of six independent experiments (*p < 0.01, as compared with anti-CD3 plus anti-CD28).
The reversal of Treg cell anergy after acute reatment with rapamycin associated with an increased production of IL-2 by the Treg cells, and it was abolished by the addition of an IL-2 neutralizing antibody (Figures 2C and 2D), indicating IL-2 dependency for the rapamycin-induced Treg cell expansion. Opposite phenomena were observed in effector T cells (Figures S2C and S2D). The effects were specific for the Treg cells and could not be ascribed to IL-2 secretion by non-Treg cells, as indicated by flow cytometry for intracellular IL-2 production at early time points (Figure S2G and S2H). Also, the mTOR-treated proliferating Treg cells increased the expression of FoxP3 at 36-48h and maintained their suppressive phenotype (Figure 2E and 2F). The expression of the activation marker CD25 on CFSE-labelled effector T cells (Figure S2I) and that of CD39, CD71, CTLA-4, GITR and CCR7 were upregulated on the proliferating Treg cells after transient mTOR-inhibition in the 36h cultures (Figure S2J). All together, these data suggest that transient mTOR inhibition with rapamycin, before anti-CD3 plus anti-CD28 stimulation, associates with proliferation of functional human Treg cells which upregulate FoxP3 expression.
In vivo transient inhibition of mTOR with rapamycin enhances Treg cell proliferation in EGFP-FoxP3 reporter mice, and ameliorates autoimmune encephalomyelitis
Treg cells, despite an in vitro hyporesponsiveness, can proliferate in vivo (Vukmanovic-Stejic et al., 2006). To evaluate whether acute and transient mTOR inhibition could also enhance in vivo Treg cell proliferation, we treated EGFP-FoxP3 reporter mice with BrdU, to follow in vivo Treg cell proliferation both in basal conditions and after antigen immunization with complete Freund’s adjuvant, CFA. We injected a single dose of rapamycin into naïve EGFP-FoxP3 mice and, 12h before CFA priming, into another group of immunized EGFP-FoxP3 mice (Figure 3A). Interestingly, the percentage and absolute numbers of EGFP-FoxP3 cells were increased by rapamycin pre-treatment, at a higher degree after immunization with CFA (Figure 3B). The increased numbers of EGFP-FoxP3 cells were due to proliferation, as suggested by BrdU incorporation (Figure 3C), both in the peripheral blood (Figure S3A) and in the draining lymph nodes (Figure 3C). Thus, also in vivo a transient mTOR inhibition enhances Treg cell expansion. Conversely, chronic treatment with rapamycin – in the absence of elevated IL-2 - did not induce proliferation of Treg cells in naïve and immunized mice (Figure S3B and S3C).
Figure 3. In vivo transient mTOR inhibition with rapamycin increases Treg cell proliferation in EGFP-FoxP3 mice and ameliorates RR-EAE.
(A) Schematic model of the experimental design. Briefly, EGFP-FoxP3 reporter mice were daily treated with BrdU in basal conditions and upon antigen immunization with CFA and were injected with a single dose of rapamycin or vehicle, 12h before CFA priming, following proliferation of EGFP-FoxP3 cells overtime. Blood samples were obtained at 5 days and draining lymph nodes were harvested at day 8 and 12, respectively. (B) Absolute number and percentage of EGFP-FoxP3 cells gated on CD4+ cells in the lymph nodes from mice pretreated or not with rapamycin, immunized or not with CFA. The data are shown as mean ± SD (n = 6, *p < 0.01). (C) Flow cytometry for BrdU incorporation in EGFP-FoxP3 cells from the lymph nodes of EGFP-FoxP3 mice in vivo pretreated (red line) or not (black line) with rapamycin, in unimmunized or after 8 and 12 days immunization with CFA. Representative of three independent experiments (*p < 0.01 when compared with vehicle, **p < 0.001 when comparet with vehicle). The gray histogram represents the isotype-matched negative control. (D) Mean clinical score of RR-EAE in SJL/J female mice immunized with PLP139-155 preceded either by vehicle or 12h rapamycin pretreatment. Data are representative of two independent experiments with similar results (n = 5 mice per group, *p < 0.01). (E) Percentage of FoxP3+ cells gated on CD4+ cells isolated from the lymph nodes of SJL/J mice immunized with PLP139-155 peptide, pretreated or not with rapamycin before PLP139-151 priming. The data are shown as mean ± SD (n = 5 mice per group, *p < 0.01).
To expand the findings to an autoimmune model, we evaluated whether acute rapamicin pre-treatment, 12h before priming with self-antigen, could affect clinical onset and progression of autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. Relapsing-remitting EAE (RR-EAE) susceptible SJL/J female mice were immunized with proteolipid protein peptide (PLP139-155) preceded either by PBS or rapamycin treatment. Transient mTOR inhibition, before autoantigen immunization, led to a statistically significant reduction in RR-EAE clinical score during the following 30 days of observation (Figure 3D). These beneficial effects on EAE onset and progression associated with an increased frequency of Treg cells that preceded the peak of the disease in rapamycin pre-treated mice (Figure 3E). These data indicate that also in vivo transient mTOR inhibition with rapamycin increases Treg cell proliferation in EGFP-FoxP3 mice and ameliorates the progression of RR-EAE.
Hyperactivation of mTOR in vitro results in hyporesponsiveness of Treg cells and is also necessary for their proliferation over time
The early molecular events induced by transient inhibition of the mTOR-pathway in human Treg cells were studied in 1h cultures (Figure 4). The extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) and the cell-cycle inhibitor p27kip1 reflected the anergic state of the Treg cells after treatment with anti-CD3 plus anti-CD28 (De Rosa et al., 2007), wheras after TCR stimulation the inhibition of mTOR induced the activation of ERK1 and ERK2 and the degradation of the pre-existing high amounts of p27kip1 (which associated with cell-cycle arrest in the Treg cells) (Figure 4A and Figure S4A). High phosphorylation of mTOR in Treg cells did not change after TCR stimulation, and rapamycin decreased the phosphorylation of mTOR, p70S6K and S6 (Figure 4B and Figure S4B). These data suggest that the reduction of mTOR activity, before TCR-engagement, is necessary to allow Treg cells to enter cell-cycle, exiting their anergic state.
Figure 4. Early and late molecular events induced by mTOR inhibition in human and mouse Treg cells.
(A) Immunoblot for P-ERK and p27Kip1, (B) P-mTOR, P-p70S6K, P-S6, and (C) P-Lck, P-Zap-70, P-AKT, P-STAT5 and FoxP3 on human Treg cells pretreated or not with rapamycin and then stimulated with anti-CD3 plus anti-CD28 for 1h. One representative out of five independent experiments. (D) Serial sections from non-reactive human lymph nodes (upper panels) or reactive human lymph nodes (lower panels) stained for Ki67, P-mTOR, and FoxP3. Number of FoxP3+ T cells (E) and expression of P-mTOR (F) in both reactive and non-reactive human lymph nodes. One representative out of three independent experiments is shown (*p < 0.005, **p < 0.001). (G) Proliferation of Treg cells pretreated or not with rapamycin, upon anti-CD3 plus anti-CD28 stimulation. The data are shown as mean ± SD (n = 20, *p < 0.0001). (H) Confocal microscopy on Treg cells pretreated or not with rapamycin, after 36h of anti-CD3 plus anti-CD28 stimulation, stained for P-mTOR (green) and FoxP3 (red). Representative of three independent experiments. (I) Flow cytometry for P-S6 expression in Treg cells pretreated (red line) or not (black line) with rapamycin, after 36h stimulation with anti-CD3 plus anti-CD28. Representative of three independent experiments (*p < 0.01 when compared with vehicle). The gray histogram represents the isotype-matched negative control. (J) Percentage of BrdU positive cells gated on CD4+EGFP-FoxP+ cells from lymph nodes of immunized EGFP-FoxP3 mice in vivo pretreated or not with rapamycin, and then in vitro stimulated with PPD. The data are shown as mean ± SD (n = 3, *p < 0.01). (K) Flow cytometry for P-S6 expression in EGFP-FoxP cells gated on CD4+ cells from lymph nodes of immunized EGFP-FoxP3 mice in vivo pretreated (red line) or not (black line) with rapamycin, and then stimulated in vitro with PPD. Representative of three independent experiments (n = 3, *p < 0.01 when compared with vehicle).
Next, we evaluated whether mTOR inhibition could alter TCR signalling. Rapamycin pre-treatment reduced the phosphorylation of Lck Tyr505 (an inhibitory tyrosine of the TCR-Lck kinase activity) (Figure 4C and Figure S4C). This event associated with a concomitant increase in Zap-70 phosphorylation, in agreement with induction of Treg cell proliferation (Figure 4C and Figure S4C). These findings suggest that transient mTOR inhibition modulates the TCR-signalling in Treg cells.
Further, we examined the phosphoinositide-3-kinase (PI3K)-protein kinase B (PKB or AKT) pathway downstream of the TCR activation. Ser473 phosphorylation of AKT was increased by rapamycin pre-treatment upon TCR engagement (Figure 4C and Figure S4C). Because we previously showed an early IL-2 secretion by rapamycin pre-treated Treg cells, we analyzed STAT5 phosphorylation (as a major element of transduction for IL2-R signaling) (Burchill et al., 2007; Yu et al., 2009). Unstimulated Treg cells had high amounts of phospho-STAT5 that were maintained high upon TCR stimulation; on the contrary, rapamycin pre-treatment led to loss in STAT5 phosphorylation and a subsequent induction upon TCR engagement (Figure 4C and Figure S4C). These data suggest that the high metabolic rate of Treg cells, as testified by active mTOR pathway, correlates with high STAT5 activity and Treg cell hyporesponsiveness in vitro.
Concomitantly with the suppression of mTOR activity, FoxP3 expression was reduced in Treg cells after mTOR inhibition (Figure 4C and FigureS4C). Because FoxP3 suppresses IL-2-gene transcription (Schubert et al., 2001), the rapamycin-induced transient downregulation of FoxP3 could account for the observed IL-2 production and reversal of Treg cell anergy (Figures 2C and 2D), a result in agreement with an early downmodulation of STAT5 phosphorylation and subsequent induction by anti-CD3 plus anti-CD28 stimulation. In vivo, confocal microscopy of human non-reactive lymph nodes indicated that FoxP3+ T cells had high amounts of phosphorylated mTOR, whereas reactive (proliferating) lymph nodes, positive for ki67, displayed a marked reduction of mTOR phosphorylation in the substantially elevated numbers of FoxP3+ T cells (Figures 4D, 4E and 4F).
Late molecular events (at 60-72h) of mTOR activity in proliferating Treg cells, after rapamycin treatment (Figure 4G), were analyzed by confocal microscopy and flow cytometry (Figures 4H and 4I). In contrast to the initial phases, actively proliferating Treg cells displayed high amounts of mTOR and S6 phosphorylation (Figures 4H and 4I), suggesting that an increased mTOR activity is required to sustain Treg cell proliferation over time. This would be in agreement with the possibility of an increased requirement of nutrients for Treg cells during proliferation. To corroborate the findings in vivo, EGFP-FoxP3 mice were immunized with CFA, pre-treated or not with rapamycin 12h before antigen priming. After 8 days, draining lymph nodes from mice were cultured in the presence of BrdU after PPD stimulation for 48h. EGFP-FoxP3 cells from mice pre-treated in vivo with rapamycin had a substantially higher BrdU incorporation than EGFP-FoxP3 cells of control mice (Figure 4J), associated with higher amounts of S6 phosphorylation (Figure 4K). To address whether an oscillation of the mTOR pathway (early downmodulation and subsequent upregulation) associates with induction and maintenance of Treg cell proliferation, we silenced mTOR expression in Treg cells through infection with lentivirus encoding shRNA sequences targeting the Frap1 gene (that encodes for mTOR). mTOR silencing did not allow Treg cells to unlock their hyporesponsive state upon anti-CD3 plus anti-CD28 stimulation (Figure S4D), due to inability to reactivate mTOR - essential for the maintenance of Treg cell proliferation (Figure S4D). This finding is in agreement with the evidence that chronic mTOR inhibition (and not transient) does not induce Treg cell proliferation in the absence of high amount of exogenous IL-2 (Figure S2E). The above findings suggest the existence of an oscillatory loop in the mTOR pathway which plays a role in Treg cell responsiveness.
Leptin contributes to the activation of the mTOR pathway in Treg cells
We have previously shown that leptin produced by Tregs cells constrains their proliferation, and that neutralization of leptin during the activation of Treg cells reverses their state of hyporesponsiveness (De Rosa et al., 2007). Because this phenomenon resembles the reversal of Treg cell anergy induced by the transient inhibition of mTOR with rapamycin (Figure 5A), we studied whether leptin could influence rapamycin-induced Treg cell proliferation. The addition of leptin to rapamycin-expanding Treg cells inhibited their proliferation (Figure 5B). To also address whether the high metabolic rate of freshly-isolated human Treg cells was associated with mTOR activation secondary to leptin secretion, we measured the amount of phosphorylation of S6 before and after leptin neutralization of unstimulated Treg cells. Interestingly, the high amounts of active S6 were substantially reduced by leptin blockade (Figure 5C). Specificity was confirmed by partial reversal of inhibition after the addition of recombinant leptin to leptin-neutralized Treg cells (Figure 5C). These results suggest a link between an autocrine secretion of leptin and mTOR activation in Treg cells (Figure 5C). To further evaluate the effects of leptin on mTOR (Figure 5D and Figure S5A), we treated unstimulated Treg cells with recombinant leptin - with or without pre-treatment with rapamycin. In both cases, leptin activated mTOR, increased S6 phosphorylation, and had little effect on p70S6K.
Figure 5. Leptin contributes to mTOR pathway activation in human and mouse Treg cells.
(A) Proliferation of human Treg cells pretreated or not with rapamycin or anti-leptin neutralizing mAb or both, before anti-CD3 plus anti-CD28 stimulation. The data are shown as mean ± SD (n = 20, *p < 0.0001). (B) Proliferation of Treg cells pretreated or not with rapamycin before treatment with leptin (100 ng/ml) before anti-CD3 plus anti-CD28 stimulation. The data are shown as mean ± SD (n = 10, *p < 0.0001, **p < 0.001 ). (C) P-S6 expression (black line) in unstimulated Treg cells, freshly isolated (left panel), anti-leptin pretreated in the absence (middle panel) or in the presence of exogenous leptin (right panel), at 1h timepoint. Representative of three independent experiments (*p < 0.01 when compared with vehicle). The gray histogram is the isotype-matched negative control. (D) Immunoblot for P-mTOR, P-p70S6K and P-S6 on unstimulated Treg cells pretreated or not with rapamycin and then treated or not with leptin for 1h. One representative out of five independent experiments. (E) Ex-vivo P-S6 expression in Treg cells from Lepob/ob mice treated or not with leptin after 2h from intreperitoneal injection (n = 3 mice/group, representative of three independent experiments). (F-G) Time course of fold changes in P-S6 expression in Treg cells from WT (F) or leptin-deficient Lepob/ob mice (G), intraperitoneally injected with rapamycin (red line) or leptin (black line), as compared with vehicle-treated mice. Representative of three independent experiments (*p < 0.01 when compared with vehicle). (H) Percentage of EGFP-FoxP3 cells gated on CD4+ cells from lymph nodes of ad libitum fed, 48h fasted and 48h fasted + leptin EGFP-FoxP3 mice. Representative of two independent experiments (n = 3 mice/group). (I) P-S6 expression (black line) in EGFP-FoxP3 cells gated on CD4+ cells from lymph nodes of ad libitum fed, 48h fasted and 48h fasted + leptin EGFP-FoxP3 mice. Representative of two independent experiments (n = 3 mice/group, *p < 0.01 when compared with ad libitum fed; ** p < 0.05 compared with 48h fasted). The gray histogram is the isotype-matched negative control. (J) Flow cytometry for BrdU incorporation in EGFP-FoxP3 cells from the lymph nodes of ad libitum fed, 48h fasted and 48h fasted + leptin EGFP-FoxP3 mice, and then stimulated in vitro with anti-CD3 plus anti-CD28. Representative of two independent experiments (n = 3, *p < 0.01 when compared with ad libitum fed; ** p < 0.05 compared with 48h fasted). The gray histogram represents the isotype-matched negative control.
The role of leptin on mTOR-pathway was then evaluated ex vivo by studying S6 phosphorylation in Treg cells isolated from leptin-deficient Lepob/ob mice treated or not with leptin (Figure 5E). A direct induction of P-S6 was detected at 2h in response to leptin. Time course experiments (0-12h) with vehicle, rapamycin, and leptin (Figures 5F and 5G) in wild-type (WT) and Lepob/ob mice showed that rapamycin reduced S6 phosphorylation in both groups of mice when compared to vehicle-treated mice. Conversely, leptin administration induced a significant activation of the mTOR pathway and increased phosphorylation of S6 only in Treg cells from Lepob/ob mice (Figure 5G).
As leptin is a metabolic parameter that reflects the nutritional state and the amount of food stored as fat, we evaluated in mice how 48h acute starvation - which dramatically reduces circulating leptin, body fat and immune function (Lord et al., 1998) - could influence Treg cell responsiveness in vivo and in vitro by modulating the mTOR pathway. To this purpose, EGFP-FoxP3 mice were starved for 48h in the presence or not of administered recombinant leptin, and compared with ad libitum fed mice. Starvation substantially decreased both body weight and serum leptin (Figure S5B and S5C). An increase in EGFP-FoxP3 cells (Figure 5H) associated with a dramatic reduction in S6 phosphorylation in starved mice, partly reversed by recombinant leptin replacement (Figure 5I), suggesting that also in vivo there is a direct link between leptin and the mTOR pathway in Treg cells. Strikingly, EGFP-FoxP3 cells from starved mice stimulated in vitro with anti-CD3 plus anti-CD28 had higher proliferation (as BrdU incorporation) when compared with ad libitum fed and starved mice treated with recombinant leptin (Figure 5J). Taken together, these data suggest that the modulation of leptin through acute starvation can be a strategy to unlock Treg cells hyporesponsiveness, by acting on the mTOR-pathway.
Transient inhibition of mTOR with rapamycin inhibits the expression of leptin and LepR in Treg cells
Unstimulated Treg cells expressed leptin and LepR, and pre-treatment with rapamycin reduced both the intracellular leptin and the cell surface expression of LepR (Figure 6A), as confirmed by mRNA expression by real time PCR (Figure 6B). This was even more evident after activation with anti-CD3 plus anti-CD28, where leptin increase (Figures 6A and 6B) associated with high mTOR activity (Figure 4B). Rapamycin pre-treatment reduced leptin and LepR expression in stimulated Treg cells (Figures 6A and 6B), as confirmed by ELISA (Figures 6C and 6D). These results suggest that rapamycin can unlock Treg cell hyporesponsivenes by reducing mTOR activation and inhibiting leptin secretion.
Figure 6. Rapamycin pre-treatment reduces the expression of leptin and LepR in Treg cells.
(A) Confocal microscopy of human Treg cells pretreated or not with rapamycin, in presence or absence of anti-CD3 plus anti-CD28 stimulation, stained for LepR (red) and leptin (green). Representative of three independent experiments (B) Realtime PCR for leptin in human Treg cell pretreated or not with rapamycin, in the presence or absence of anti-CD3 plus anti-CD28 stimulation. The data are shown as mean ± SD (n = 5, *p < 0.05; **p < 0.0001; ***p < 0.001). (C) Dot plots show beads-based ELISA for leptin in cell supernatant from Treg cells, pretreated or not with rapamycin, in the presence or not of anti-CD3 plus anti-CD28 stimulation. Representative of three independent experiments. (D) Graph shows quantitation of leptin production in cell supernatant from Treg cells in all the above conditions. The data are shown as mean ± SD (n = 3, *p < 0.0001).
LepR deficiency associates with a reduced mTOR activity and the proliferation of Treg cells
The early molecular events related to mTOR activity in Treg cells were evaluated in congenitally LepR-deficient Leprdb/db mice vs. Leprdb/+ heterozygous controls (Figure 7). Treg cells from Leprdb/db mice had higher expression of FoxP3 before and after TCR-mediated stimulation (Figure 7A and Figure S6A), confirming that LepR deficiency associated with elevated numbers of Treg cells (De Rosa et al., 2007). In Treg cells from heterozygous Leprdb/+ controls, mTOR activity was high before and after TCR stimulation, and this event associated with anergy of Treg cells, as confirmed by lack of proliferation, little ERK1 or ERK2 phosphorylation, and lack of p27kip1 degradation (Figures 7B, 7C and 7D and Figures S6A, S6B and S6C). Conversely, in Treg cells from Leprdb/db mice, mTOR, p70S6K and S6 phosphorylation were significantly reduced, and a lack of induction and maintenance of mTOR phosphorylation was found after anti-CD3 plus anti-CD28 stimulation, indicating a reduced mTOR activity in the absence of LepR responsiveness (Figure 7B and Figure S6B). These events in Leprdb/db mice associated with proliferation in Treg cells, together with higher ERK1 and ERK2 phosphorylation and increased degradation of p27kip1 (Figures 7C and 7D and Figure S6C).
Figure 7. Treg cells from LepR-deficient Leprdb/db mice have reduced mTOR activation concomitantly with a higher proliferative capacity.
Immunoblot for FoxP3 (A), P-mTOR, P-p70S6K, P-S6 (B), and P-ERK and p27Kip1 (C) in Treg cells from Leprdb/+ lean controls and leptin-receptor deficient Leprdb/db mice stimulated or not with anti-CD3 plus anti-CD28 for 1h. One representative out of four independent experiments. (D) Proliferation of Treg cells from Leprdb/+ and Leprdb/db mice, stimulated or not with anti-CD3 plus anti-CD28 mAb. The data are shown as mean ± SD (n = 10, *p < 0.0001). (E) P-S6 expression in Treg cells from Leprdb/+ (black line) or Leprdb/db (red line) mice in the presence or absence of anti-CD3 plus anti-CD28 in 36h culture. Representative of three independent experiments. The gray histogram represents the isotype-matched negative control. (F) P-S6 mean fluorescence intensity in both strains of mice with or without anti-CD3 plus anti-CD28 stimulation and (G) fold change in P-S6. (*p < 0.01 when compared with unstimulated cells). Leprdb/db mice had a much higher P-S6 induction than heterozygote controls during proliferation. Representative of three independent experiments.
At late time point of Treg cell proliferation (36-48h), higher induction of P-S6 was observed in Leprdb/db mice despite modest activation of this pathway at early stages (1h) (Figure 7E). Also, the 2-fold increase in S6 phosphorylation seen after TCR-mediated stimulation was not present in anergic Treg cells from Leprdb/+ ccontrol mice that maintained constantly high mTOR amounts (Figure 7F and 7G). These data suggest that alterations (reductions) of LepR-related signalling can enhance the proliferative potential of Treg cells, and render them responsive to TCR stimulation.
DISCUSSION
Here we show that a link between energy status and Treg cell responsiveness is the leptin-mTOR signalling pathway. Overexpression of leptin-mTOR in freshly isolated Treg cells is responsible for their state of hyporesponsiveness. While transient mTOR inhibition with rapamycin reduced proliferation of effector CD4+CD25− T cells, this condition led to proliferation of functional Treg cells.
One question is how rapamycin, which is an inhibitor of cell growth and proliferation used to block tumor cell growth and transplant rejection, can concomitantly reduce the proliferation of T cells and promote the proliferation of Treg cells. It is likely that dosage and timing may both be responsible in a scenario in which the intracellular metabolic state can control Treg cell responsiveness through the leptin-mTOR axis. As the metabolic state of a cell changes along with activation, rapamycin might differentially affect Treg cell reactivity depending on the metabolic state, and mTOR blockade on effector and Treg cells would associate with a differential outcome in the two cellular subsets. Treg cells have a high metabolic state, high ATP and mTOR activity, and are unresponsive to proliferative stimuli, whereas effector T cells have a low metabolic state associated with normal responsiveness. Of interest, the current findings suggested that the transient inhibition of the leptin-mTOR pathway, before TCR stimulation, promoted TCR-induced proliferation in Treg cells, yet at later time points an intact mTOR activity is necessary to sustain Treg cell proliferation. One possible explanation could be that Treg cells need a low metabolic rate to enter cell-cycle and start proliferation, whereas proliferation would be maintained by the presence of nutrients and growth factors that induce mTOR (which in turn controls their cellular transport and trafficking) (Faivre et al., 2006; Thomson et.al., 2009; Woods et al., 2008; Blouet et al., 2008; Yang and Guan, 2007).
The findings of this study support a mechanism of cross-regulation between metabolism and Treg cell responsiveness where an “oscillatory” switch in mTOR activity can control responsiveness of Treg cells . These results are in agreement with a recent report showing an evolutionarily-conserved mechanism of interaction between metabolism and genes of innate immunity in Drosophila, in which genes are activated under normal physiological conditions in response to the oscillating energy status of cell and tissues (Becker et al., 2010). The “oscillation” in the mTOR pathway activity may be necessary for Treg cell proliferation because when the pathway is constantly inhibited either by chronic rapamycin treatment or by mTOR gene silencing, cells are unable to re-activate it, so that proliferation does not occur.
Biochemical analyses showed that transient inhibition of mTOR “unlocked” Treg cell hyporesponsiveness via a rapid and early downmodulation of FoxP3 and the cell-cycle inhibitor p27kip1, together with phosphorylation of ERK1 and ERK2. Considering that FoxP3 inhibits Il2 gene transcription and p27kip1 maintains cell-cycle arrest in Treg cells (Schubert et al., 2001), the early downmodulation of these molecules would allow Treg cells to enter the G1/S phase of the cell-cycle and induce IL-2 gene transcription. At later time points (36h), the increased IL-2 secretion following proliferation would possibly induce FoxP3 expression in Treg cells. We also observed that in Treg cells there is a block in TCR signalling as suggested by the low Zap-70 phosphorylation and high amounts of the inhibitory Tyr505 Lck after TCR stimulation. Interestingly, transient mTOR inhibition unlocked TCR signalling by reducing the Lck inhibitory feedback and by restoring Zap-70 phosphorylation. These events were also able to induce a AKT signalling secondary to TCR engagement. Finally, we investigated the STAT5 molecule, as major element of IL-2R signal transduction (Burchill et al., 2007; Yu et al., 2009); Treg cells showed costitutively high amounts of phospho-STAT5 independently by TCR stimulation, in agreement with their high metabolic rate, high FoxP3 expression and high CD25 (IL-2Rα) expression. Rapamycin pre-treatment was able to affect STAT5 phosphorylation: it was firstly completely abolished and subsequently reinduced upon-TCR engagement, confirming also the increase in IL-2 secretion by proliferating Treg cells. Taken together, these events further confirm the need of perturbing the metabolic rate of Treg cells to allow their in vitro proliferation.
We have previously shown that Treg cells produce leptin that constrains their own proliferation (De Rosa et al., 2007). Here we further those findings by showing that leptin produced by Treg cells contributes to the activation of the mTOR-pathway in Treg cells. In the crosstalk between leptin and mTOR in Treg cells, rapamycin and subsequent transient mTOR inhibition substantially inhibit leptin secretion and LepR expression on Treg cells. Also, the evidence that EGFP-FoxP3 cells from starved mice proliferated in vitro, concomitantly with the transient reduction in serum leptin-mTOR-activation, suggests that leptin may link mTOR activity to the anergic state of Treg cells in vitro.
The so called “frugal phenotype”, in which the survival of chronically food-restricted mice is higher than in ad libitum fed mice, may fit with the possibility of a reciprocal influence between nutritional-metabolic state and immune responses (Matarese and La Cava, 2004; Matarese et al., 2008). In Lepob/ob and Leprdb/db mice, which have higher numbers of Treg cells secondary to the absence of leptin signalling (De Rosa et al., 2007), early kinetics (0-12h) of mTOR activation in Treg cells showed that leptin determined a 2-3 fold induction of P-S6 in Lepob/ob mice, rapidly subsiding after 12h when compared with WT mice. A different induction versus inhibition of the P-S6 pathway in WT versus Lepob/ob mice could be ascribed to the leptin already present in WT mice, whereas in Lepob/ob mice the chronic leptin deficiency would make the Treg cells more sensitive to mTOR activation after leptin administration. Interestingly, Treg cells in Leprdb/db mice had higher FoxP3 expression, lower mTOR activation and increased in vitro responsiveness, suggesting that the absence of LepR impairs mTOR activation and renders Treg cells responsive to in vitro TCR stimulation. Once Treg cell proliferation occurred, mTOR induction was higher in Treg cells from Leprdb/db mice than in Treg cells from WT animals.
Recent reports have shown an involvement of the mTOR pathway in the control of TCR-dependent FoxP3 expression (Sauer et al., 2008), in the de novo differentiation of Treg cells (Haxhinasto et al., 2008), and in the decision between effector T and Treg cell lineage commitment (Delgoffe et al., 2009). We suggest that the leptin-mTOR-axis might integrate cellular energy status with metabolic-related signalling in Treg cells, envisioning the possibility that a tuned control of energy could be used to manipulate Treg cells homeostasis. Indeed, in neural circuits the leptin-mTOR pathway drives the choices between food intake and fasting by controlling energy status (Gao and Horvath, 2008; Abizaid et al., 2006). In the hypothalamus, leptin inhibits food intake through mTOR activation, and mTOR inhibition with rapamycin prevents leptin-induced anorexia (Cota et al., 2006). Finally, neural cells are refractory to restimulation after a primary neural stimulation, which is somewhat similar to the finding of mTOR-mediated unresponsiveness after in vitro TCR stimulation of Treg cells that are metabolically active (McIntyre et al., 2004).
Current strategies to expand Treg cells with rapamycin employ ex vivo addition of supraphysiologic concentrations of IL-2 (500-1000 UI/ml) to cultured cells during TCR stimulation (Gregori et al., 2007). The addition of rapamycin to Treg cells during TCR stimulation (and not pre-treatment and removal, as we performed in this report) is not sufficient to induce proliferation of Treg cells but rather inhibits their expansion. Instead, Treg cells do expand in the presence of high doses of IL-2 and rapamycin for long time cultures (generally 7-10 days). Thus, on the one hand rapamycin lowers the metabolic state of Treg cells rendering them prone to proliferate. On the other hand, high doses of IL-2 induce mTOR activation that permits transport of nutrients, once the G1/S phase transition has been induced by TCR stimulation. In other terms, mTOR activation (high metabolic rate) determines Treg cell hyporesponsiveness but also maintens their expansion at later time points. This model is consistent with that of the mTOR pathway involvement in the control of proliferation of hematopoietic stem cells (HSCs) (Chen et al., 2008), where mTOR deletion drives HSCs from quiescence into cell-cycling and proliferation. All this suggests that a high metabolic rate contrasts cell-cycle transition from G1 to S phase, whereas a low metabolic rate (i.e. through rapamycin) promotes proliferation. The notion that the metabolic state in Treg cells can affect their proliferation and suppressive function is also supported by the high amounts of intracellular cyclic adenosine-3′-5′-monophosphate (cAMP) in Treg cells (cAMP participates in the metabolic activation of mTOR, resulting in an enhanced ATP production) (Kwon et al., 2004), and our data are in line with a report showing that Treg cells have high amounts of mTOR in a graft-verus-host murine model (Zeiser et al., 2008). The metabolic control of immune response in CD8+ T cells (Araki et al., 2009), the immune-mediated pathogenesis of obesity and obesity-related insulin resistance (Feuerer et al., 2009; Winer et al., 2009) has recently reinforced the concept that metabolism and proliferation of lymphocytes can impact, at different levels, the control of inflammation, autoimmunity and immune-mediated disorders (Matarese and La Cava, 2004; Matarese et al., 2008).
In conclusion, our findings may help to explain why Treg cells proliferate in vivo but are anergic in vitro, and why for the expansion of Treg cells in vitro there is the need of high doses of IL-2 (which activates mTOR kinase) together with the inhibitor of mTOR kinase rapamycin for long term cultures (Bour-Jordan and Bluestone, 2009). As the nutrient and energy sensing leptin-mTOR pathway sets the threshold for responsiveness of Treg cells, we hypothesize that the proliferating Treg cells in vivo (Vukmanovic-Stejic et al., 2006) can sense changes in the microenvironment when cultured in vitro, through the leptin-mTOR pathway which makes Treg cells unresponsive to TCR-mediated stimulation. More specifically, we hypothesize that the high proliferative rate in vivo of Treg cells would associate with continuous dynamic, “oscillatory” changes in mTOR activity depending on the fluctuations in the composition of the extracellular milieu - including amounts of leptin and nutrients such as amino acids, glucose and lipids. Alternatively, in vitro-cultured Treg cells, after isolation, would be exposed to a “static” milieu of the culture media characterized by constantly high concentration of leptin and nutrients (Sato et al., 2009), which could sustain mTOR activation and thus inhibit its dynamic, “oscillatory” changes required for Treg cell proliferation. The acute, transient inhibition of mTOR (either with rapamycin or nutrient starvation) in culture would reset in vitro the “oscillatory” fluctuations in mTOR activity rendering Treg cells able to respond to TCR stimulation. This would explain why constant mTOR inhibition, either with chronic rapamycin treatment or mTOR gene silencing, does not allow these dynamic changes and inhibits Treg cell expansion. When IL-2 at very high concentration is provided in vitro, the lowering of mTOR activity would be reversed and allow the dynamic changes required for Treg cells to expand.
Altogether, these findings indicate that the intracellular metabolic and energy status controlled by leptin-mTOR may control the choice between anergy and growth in Treg cells. This aspect can have influences in modulating immune responses in autoimmunity and transplantation.
EXPERIMENTAL PROCEDURES
Treg cell purification, cultures and proliferation assays
Human CD4+CD25hi and CD4+CD25− T cells were purified from PBMCs from buffy coats of human healthy donors by high-performance cell sorting (MoFlo, Dako) after staining with FITC anti-human CD4 (BD Pharmingen, clone RPA-T4), PE anti-human CD25 (BD Pharmingen, clone M-A251), APC anti-human CD127 (R&D Systems, clone 40131) or magnetic cell separation with Dynabeads Regulatory T Cell Kit (Invitrogen). Soon after isolation, CD4+CD25highCD127− cells were rapidly cleaned with Detach reagent (Invitrogen) to remove surface-bound CD25 mAb and beads. Antibody/bead-free cells were 95%–98% pure by FACS analysis, and >95% expressed FoxP3. For proliferation assays, purified cells were cultured (5×104 cells/well) as previously described (De Rosa et al., 2007).
Mouse Treg cells were isolated with the Regulatory T Cell Isolation Kit (Miltenyi Biotec, Gladbach, Germany) and by Automacs ProSeparator (Miltenyi Biotec) or by high-performance cell sorting (MoFlo, Dako) for the expression of EGFP-FoxP3. Cells were then stimulated with Dynabeads mouse anti-CD3 plus anti-CD28 (0.5 bead/cell; 5 × 104 cells/well). Treg cells (> 95% pure by FACS analysis) were cultured as previously described (De Rosa et al., 2007).
In vitro rapamycin, leptin, anti-leptin and UO126 treatments
For transient mTOR inhibition, Treg and effector T cells were pretreated in vitro for 1h with rapamycin (Sigma-Aldrich) or with UO126 (Promega Corporation) at a final concentration of 100nM or 10μM, respectively. Cells were washed extensively with serum-free culture medium and cultured as described before. For chronic rapamycin treatment, the drug was added (at final concentration of 100nM) and left throughout the time of culture (72h). For in vitro leptin neutralization experiments, human leptin-neutralizing mAb (R&D Systems, Minneapolis, MN) was used at a final concentration of 20 μg/ml; human recombinant leptin was purchased from R&D Systems and it was used at the final concentration of 100 ng/ml.
Immunoblots and biochemical analyses
Total cell lysates and western blot analysis were performed as previously described (De Rosa et al., 2007). The antibodies used were the following: anti-p27Kip-1, anti-phospho-mTOR, anti-m-TOR, anti-phospho p70S6K,anti-p70S6K, anti-phospho-S6, anti-S6, anti-AMPK, anti-phospho-Lck (Tyr505), anti-phospho-Zap-70, anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-STAT5 (Tyr694) and anti-STAT5 (all from Cell Signaling Technology, Beverly, MA); anti-ERK1/2, and anti-phospho-ERK1/2 (all from Santa Cruz Biotechnology Inc., Santa Cruz, CA); anti-FoxP3 (eBioscience). The filters were also probed with a tubulin antibody (Sigma) to normalize for the amount of loaded protein. All filters were quantified as previously described (De Rosa et al., 2007).
Mice and in vivo experiments
Eight-to-ten weeks old female leptin-deficient C57BL6/J-ob/ob (Lepob/ob), C57BL6/J lean controls (WT), leptin-receptor deficient C57BL/Ks-db/db (Leprdb/db), and C57BL/Ks-db/+ lean controls (Leprdb/+) mice, SJL/J mice (H-2s), were purchased from Harlan Italy s.r.l. (Corezzana, Italy). B6.Cg-Foxp3tm2tch/J (EGFP-FoxP3) were purchased from Jackson Laboratory; these mice co-express EGFP and the regulatory T cell-specific transcription factor FoxP3 under the control of the endogenous promoter (Haribhai et al., 2007). Experiments were conducted in accordance with the animal welfare guidelines under an approved protocol of the Istituto Superiore di Sanità, Roma, Italy. Mice were age-matched for individual experiments and housed with a 12-hour light-dark cycle in the animal facility at the Università di Napoli “Federico II”.
Lepob/ob and WT mice were injected once intraperitoneally either with mouse recombinant leptin (R&D Systems) dissolved in 200μl of PBS at a dose of 100μg/mouse or with rapamycin at a dose of 100μg/mouse.
EGFP-FoxP3 reporter mice were daily treated with BrdU (1mg/mouse) in basal conditions and upon antigen immunization with CFA (complete Freund’s adjuvant Difco, BD Diagnostics — Diagnostic Systems) and were treated with a single dose of rapamycin (100μg/mouse) or vehicle, 12h before CFA priming, to follow proliferation of EGFP-FoxP3 cells overtime. Samples were obtained at 5 days from tail veins or from draining lymph nodes at days 8 and 12, respectively. For fasting experiments, daily treated with BrdU (1mg/mouse) EGFP-FoxP3 mice, were fasted for 48h in the presence or not of exogenous mouse recombinant leptin (R&D Systems) dissolved in 200μl of PBS at a dose of 1μg/g/mouse initial body weight twice daily at 9.00 AM and 6.00 PM, and compared with ad libitum fed mice (Lord et al., 1998). Draining lymph nodes from each group of mice were harvested after 48h starvation and stained for BrdU.
Statistical analyses
The Mann-Whitney U-test was used for unrelated two-group analyses and the Kruskal-Wallis ANOVA test for three or more groups, using StatView software (Abacus Concepts Inc.). Results are expressed as mean ± SD. P values < 0.05 were considered statistically significant.
Supplementary Material
Highlights.
1. The state of hyporesponsiveness of Treg cells depends on their high metabolic rate; 2. The high metabolic rate of Treg cells is due to an overexpression of the mTOR-pathway; 3. Leptin activates mTOR-pathway in Tregs cells; 4. Metabolism through leptin-mTOR sets responsiveness of Treg cells.
ACKNOWLEDGEMENTS
G.M. is supported by grants from the EU Ideas Programme, ERC-Starting Independent Grant “LeptinMS” n. 202579 and Telethon-JDRF Grant n. GJT08004. A.L.C. is supported in part by the NIH grant n. AR53239. T.L.H. is supported by the NIH Director’s Pioneer Award n. DP1OD006850. The authors thank Salvatore De Simone and Francesco D’Agnello for technical help in the cell-sorting MoFlo Core Facility. This work is dedicated to the memory of Eugenia Papa and Serafino Zappacosta.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abizaid A, Gao Q, Horvath TL. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron. 2006;51:691–702. doi: 10.1016/j.neuron.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Shultze JL, Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour-Jordan H, Bluestone JA. How suppressor cells led to anergy, costimulation, and beyond. J. Immunol. 2009;183:4147–4179. doi: 10.4049/jimmunol.0990078. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582:132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Bacchetta R, Passerini L, Levings MK, Roncarolo MG. Isolation, expansion, and characterization of human natural and adaptive regulatory T cells. Methods Mol. Biol. 2007;380:83–105. doi: 10.1007/978-1-59745-395-0_6. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53:S225–232. doi: 10.2337/diabetes.53.suppl_3.s225. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J. Cell Sci. 2010;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Matarese G, De Rosa V, La Cava A. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12–17. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin. Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sato K, Kondo M, Sakuta K, Hosoi A, Noji S, Sugiura M, Yoshida Y, Kakimi K. Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy. 2009;11:936–946. doi: 10.3109/14653240903219114. [DOI] [PubMed] [Google Scholar]
- Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Invest. 2006;116:2829–2830. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu. Rev. Nutr. 2008;28:295–311. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]
- Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7:458–462. doi: 10.4161/cc.7.4.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J. Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.