Abstract
Frequent mutations in the GNAQ, MMP8, Akt3, EGFR, and PIK3R1 genes have been reported in human cancers but mostly have not been well examined in thyroid cancer. Selected exons of GNAQ, MMP8, AKT3, EGFR, and PIK3R1 genes were sequenced in various thyroid cancers. We found a G2203A EGFR mutation, resulting in a G735S amino acid change, in one of 21 (5%) papillary thyroid cancer samples. We did not find any mutation in the MMP8 gene, but observed a frequent SNP A259G (K87E) genotype switch in various types of thyroid cancer samples. We did not find any mutation in the GNAQ, AKT3, and PIK3R1genes in various types of thyroid cancer. No mutation in these genes was found in 12 cell lines derived from various types of thyroid cancer. Therefore, unlike in other cancers, mutations in these genes are uncommon in thyroid cancer.
Keywords: Thyroid cancer, GNAQ, MMP8, AKT3, EGFR, PIK3R1
Introduction
Follicular epithelial cell-derived thyroid cancer is the most common endocrine malignancy with a high incidence worldwide [1, 2]. This cancer is histologically classified into papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC) [3]. Thyroid cancers frequently harbor activating mutations in the MAP kinase (MAPK) and phosphatidylinositol 3-kinases (PI3K)/Akt signaling pathways [4], as represented by RAS, BRAF, and RET/PTC mutations in the former and PIK3CA and PTEN mutations in the latter. As an important mechanism for the tumorigenesis of thyroid cancer and many other human cancers, aberrant activation of the two signaling pathways by such mutations can cause uncontrolled cell division, proliferation, and survival.
Somatic mutations of GNAQ, MMP8, Akt3, EGFR, and PIK3R1 genes have been recently reported in some human cancers with various prevalences and they can activate the MAPK and PI3K/Akt signaling pathways [5–10]. A particularly frequent somatic mutation of the GNAQ gene at codon 209, resulting in mutant GNAQQ209L, has been reported in uveal melanoma and blue nevi [5]. The GNAQ gene encodes a G-protein α subunit that mediates signals from G-protein-coupled receptors (GPCRs) to the MAPK pathway. The normal amino acid, glutamine, encoded by codon 209 of the GNAQ gene lies within the RAS-like domain of GNAQ (corresponding to residue 61 of Ras) and is essential for GTP hydrolysis. Recent studies found no mutation in GNAQ in PTC, MTC, and FTC, but it has not been analyzed in the more aggressive type of thyroid cancer, ATC [11–13]. Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade components of extra cellular matrix and basement membranes. Abnormalities of MMPs have been associated with cancer metastasis. Frequent mutations of the MMP8 gene have been observed in melanoma [6]. Most of the mutations in this gene have been observed in exon 2. All the mutants detected in this exon, including S50F, P78S, K87N, and G104R, were shown to be tumorigenic and the wild type has been shown to inhibit cell growth on soft agar and tumor formation in vivo [6]. A point mutation in the pleckstrin homology domain (E17K) and a point mutation in the regulatory C-terminal domain (E438D) of AKT3 were recently found in melanomas [7, 8]. Expression of the AKT3 E17K in A375 cells has been demonstrated to increase AKT phosphorylation as compared with the wild-type AKT3 [7]. A recent study reported an AKT3 mutation in PTC, but FTC and ATC were not examined in this study [14]. Varying frequencies of EGFR mutation in PTC had been reported in two studies [9, 15]. The status of somatic EGFR mutation is not known in this cancer in the American patients, while other types of cancers such as FTC and ATC have been reported [4]. The class IA PI3K lipid kinase has a catalytic subunit (p110α) and a regulatory subunit (p85α), which is encoded by PIK3CA and PIK3R1 genes, respectively. Somatic mutations of PIK3CA gene are common in human cancers. Recently, mutations have also been found in the PIK3R1 gene in human cancers [10]. These mutations in PIK3R1 are all shown to promote cell survival, anchorage-independent cell growth, and tumorigenesis through AKT activation in a p110-dependent manner [10]. The mutation status in the GNAQ, MMP8, AKT3, EGFR, and PIK3R1 genes has therefore mostly been incompletely examined or not been examined in thyroid cancers. We conducted the present study to investigate mutations in these genes in thyroid cancers.
Materials and Methods
Cell Lines, Tumor Samples, and DNA Extraction
The thyroid cancer cell lines (K1, K5, OCUT-1, OCUT-2, FB-1, SW1736, BCPAP, HTh7, HTh74, KAT 18, FTC133, and C643) and thyroid tumor, melanoma, and colon cancer samples used were as described previously with local Institutional Review Board approval [16]. Cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, streptomycin (100 μg/mL), penicillin (100 units/mL), and 2 mM glutamine. Genomic DNA from cell lines and tumors was isolated by standard phenol-chloroform extraction and ethanol precipitation procedures [16].
PCR Amplification and Sequencing of GNAQ, MMP8, AKT3, EGFR, and PIK3R1 Genes
The primer sequences and PCR conditions for the amplification of exon 5 of the GNAQ gene, exon 2 of the MMP8 gene, and exons 18, 19, and 21 of the EGFR gene are as described previously [5, 6, 17]. The primer sequences for the amplification of exon 2 and exon 12 of the AKT3 gene are as follows: (exon 2) AKT3-2F 5′-TGGAGGCCAGTGTTGTAGGAC-3′; AKT3-2R 5′-ATAGCCTAAGATATCTGACAC-3′, (exon 12) AKT3-12F 5′-AGCGACTCAGCATTGTAGACT-3′; AKT3-12R 5′-TCACTGTGGAATTTGATCTTG-3′. PCR reaction conditions were as follows, after initial denaturation, at 94°C for 2 min, amplification was performed at 94°C for 1 min, 60°C for 1 min for 35 cycles with final extension at 72°C for 7 min and the same PCR conditions were followed for the amplification of exon 12 of AKT3 except for the annealing temperature at 58°C. The primers sequences PIK3R1-14F 5′-AAACTGCTGGGAAACCATAGT-3′, PIK3R1-14R 5′-TAACTCATCCTGAATTGTAGC-3′, PIK3R1-16F 5′-AAGACAGCAAGGCAGGCTGAT-3′, PIK3R1-16R 5′-CTATGTCAAATCTTTGCCCCC-3′, PIK3R1-17F 5′-TGA-GACTGCACAATAATGCTT-3′ and PIK3R1-17R 5′-CTCAATTCACAGATCAGACTG-3′ were used for the PCR amplification of exon 14, 16, and 17, respectively. Annealing temperature was 57°C for exon 14 and 17 and 60°C for exon 16. The PCR products were directly sequenced using a Big Dye terminator v3.1 cycle sequencing ready reaction kit (Applied Biosystems). These exons were examined because they harbored most of the reported mutations in these genes. Gene Bank accession numbers are NM_002072.2 (GNAQ), NM_002424.2 (MMP8), NM_005465.3 (AKT3), NM_005228.3 (EGFR), and NM_181523.1 (PIK3R1).
Results
We examined exon 5 of the GNAQ gene for mutations in the present study since all of the known GNAQ mutations have been reported in codon 209 in this exon. Exon 2 of the MMP8 gene and exons 18, 19, and 21 of the EGFR gene were selected for sequencing as they have recently been shown to carry somatic mutations in other human cancers. Exons 2 and 12 of AKT3 were similarly chosen for analysis for their carrying mutations in other cancers. Exons 14, 16, and 17 of the PIK3R1gene were selected for analysis also because they were the most mutated exons in PIK3R1.
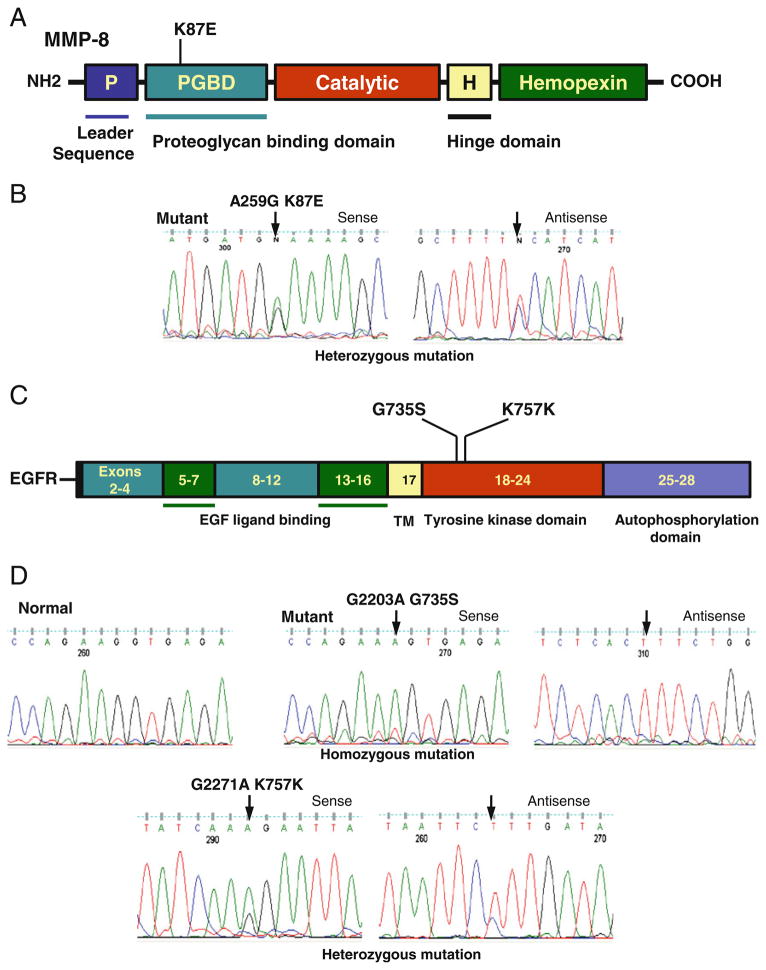
Our sequencing results showed no mutation in and around the hot spot codon 209 in the GNAQ gene in 12 thyroid cancer cell lines and 40 thyroid cancer samples (including 20 FTC and 20 ATC). We did not examine PTC as this cancer was found to harbor no GNAQ mutation previously [11]. The normal amino acid, glutamine, encoded by codon 209 of the GNAQ gene lies within the RAS-like domain of GNAQ (corresponding to residue 61 of Ras) and is essential for GTP hydrolysis. In members of RAS family, mutations at this site and at codon 12 cause loss of GTPase activity with constitutive activation of Ras. Given this similarity of GNAQQ209L mutation with Ras mutations and the fact that melanoma and colon cancer are similar to thyroid cancer in terms of their high prevalence of Ras mutations, we additionally analyzed 20 cutaneous melanoma and 20 colon cancer samples for the GNAQ mutation and found that none of them harbored this mutation. We did not find any novel MMP8 mutation in 12 thyroid cancer cell lines and 31 PTC, 20 FTC, and 20 ATC tumor samples. As illustrated in Fig. 1, we observed a frequent homozygous/heterozygous A>G transition at nucleotide position 259, resulting in codon 87 switch between AAA and GAA and amino acid 87 switch between lysine and glutamic acid (K87E) in exon 2 of MMP8. This represents a single nucleotide polymorphism (SNP) (rs1940475) reported in the SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). We found the G259 pattern in 25 of 31 (80.6%) PTC, 14 of 19 (73.6%) FTC, and 8 of 9 (88.8%) ATC. Conversely, the A259 pattern was found in 19.4%, 26.4%, and 11.2% of these tumors, respectively. We did not find any AKT3 mutation in 12 thyroid cancer cell lines and 20 PTC, 20 FTC, and 20 ATC tumor samples. We also did not find any PIK3R1 mutation in 12 thyroid cancer cell lines and 20 PTC, 32 FTC, and 32 ATC samples. However, we found an EGFR mutation in 1 of 21 (5%) PTC tumor samples. This mutation was not found in 12 thyroid cancer cell lines. As illustrated in Fig. 1, this mutation is a homozygous missense mutation resulting in G>A transition at nucleotide position 2203 of the EGFR gene. This mutation caused codon 735 to change from GGT>AGT, resulting in the amino acid change G735S in the EGFR protein. We also found a rare and novel silent mutation resulting in G>A transition at the nucleotide position 2271 and it has not been reported in the SNP data base (Fig. 1). Figure 1 shows the mutations and SNP identified and their related protein domain.
Fig. 1.
Detection of MMP8 and EGFR mutations. a Schematic diagram of domains of MMP8 protein showing a single nucleotide polymorphism (K87E) identified in thyroid cancer. The MMP8 gene is located on chromosome 11q22.3 contains 10 exons and intervening sequences. b The sequencing results were shown with a representative sense and antisense sequence profile of a single nucleotide polymorphisms (A259G) found in exon 2 of MMP8 gene. c Schematic diagram of EGFR showing a mutation (G735S) and a single nucleotide polymorphism (K757K) identified in thyroid cancer. The EGFR gene is located on chromosome 7p11.2 contains 28 exons and intervening sequences. d The sequencing results were shown with sense and antisense sequence profiles of a mutation (G2203A) and a single nucleotide polymorphism (G2271A) found in exon 19 of EGFR gene. Arrow indicates mutated nucleotide. The nucleotide and amino acid alterations are indicated above the arrow. Nucleotide numbers refers to the position within coding sequence, where position 1 corresponds to the first position of the initiation codon. All the samples were sequenced in two repeated examinations with independent PCR by forward and reverse primers
Discussion
We examined the MMP8, AKT3, and PIK3R1 genes for their mutation status in various thyroid cancers and GNAQ in anaplastic thyroid cancer and found no mutation in them. A positive finding in the present study is the discovery for the first time a G735S EGFR mutation in thyroid cancer although it is an infrequent event. This mutation was first identified in lung cancer and subsequently in prostate cancer [18, 19]. The G735 residue is located on the beta-strand of the N-terminal lobe. Three-D rendering of the tyrosine kinase domain has suggested that a possible mechanism for EGFR deregulation by the G735S mutation is a conformational change of the kinase domain, leading to its activation [19]. Functional analyses demonstrated that the G735S EGFR mutant was a gain-of-function mutation with increased tyrosine kinase activity associated with increased signaling activities of the STAT, MAPK, and PI3K/Akt pathways, as reflected by the phosphorylation of STAT, AKT, and ERK as well as increased cell proliferation, anchorage-independent colony formation and invasion [20]. EGFR mutations of other types have been also reported in PTC of Greek [15] and Japanese patients [9] and no mutation of this gene was found in FTC and ATC of American patients [4]. The prevalence of the EGFR mutation in the PTC patients in the present study was 5% (1/21), same to the prevalence (5%, 2/43) of EGFR mutations in Greek PTC patients [15], but lower than that (30%, 7/23) in Japanese PTC patients [9]. Different ethnic backgrounds may explain this variation in prevalence. It is likely that PTC patients harboring mutations in the EGFR gene may respond to therapeutic targeting using specific EGFR inhibitors or dually targeting the PI3K/Akt and MAPK pathways using MEK and AKT inhibitors.
We found no mutation in the MMP8 gene. However, we found a common A259G SNP (rs1940475), resulting in a K87E amino acid switch in the proteoglycan binding domain of MMP8. The more common nucleotide pattern is G259, resulting in amino acid glutamic acid at position 87 of MMP8 and seen in about 70–90% of thyroid cancer cases. The less common nucleotide pattern is A259, resulting in amino acid lysine at position 87 and conversely seen in about 10–30% of the cases of thyroid cancer. The biological and pathological relevance of this missense genetic change resulting in the K87E amino acid switch in MMP8 and its particular role in thyroid tumorigenesis remain to be studied. We speculate that the type of amino acid at position 87, i.e., lysine or glutamic acid, could, through certain mechanisms such as affecting the binding with proteoglycans of proteins in the intercellular matrix, significantly affect the function of MMP8 and its role in the invasion, metastasis, and ultimately clinicopathologicl outcomes of human cancers.
Somatic mutations in MMP8 and PIK3R1 have not been investigated previously in thyroid cancer. Akt3 has not been analyzed in follicular and anaplastic thyroid cancer and GNAQ has not been analyzed in anaplastic thyroid cancer. Our mutational analyses in the present study showed absence of somatic mutations in these genes in thyroid cancer. These findings suggest that genetic alterations in these genes may not play a significant role in the tumorigenesis of this cancer. It is probably not surprising that GNAQ, MMP8, AKT3, and PIK3R1 gene mutations are not common in thyroid cancer since many of the upstream effectors such as EGFR, RET/PTC, RAS, BRAF, PTEN, PIK3CA, PIK3CB, and PDK1 are commonly activated via mutations or genetic amplifications that can independently activate the MAPK or the PI3K/Akt pathway in thyroid cancers [4]. Moreover, unlike AKT1 and AKT2, AKT3 may not play a significant role in the tumorigenesis of thyroid cancer [21, 22]. Therefore, genetic alterations in the AKT3 gene might predictably unnecessary for thyroid cancer tumorigenesis (Table 1).
Table 1.
Genetic alteration in GNAQ, MMP8, AKT3, EGFR, and PIK3R1 genes
| Samples | GNAQ
|
MMP8
|
AKT3
|
EGFR
|
PIK3R1
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of samples | Mutations (%) | No of samples | Mutations (%) | No of samples | Mutations (%) | No of samples | Mutations (%) | No of samples | Mutations (%) | |
| Thyroid cancer | ||||||||||
| Cell lines | ||||||||||
| FTC | 2 | 2 | 2 | 2 | 2 | |||||
| ATC | 8 | 8 | 8 | 8 | 8 | |||||
| PTC | 2 | 2 | 2 | 2 | 2 | |||||
| 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | 0 | |
| Tumors | ||||||||||
| PTCa | 31 | 0 | 20 | 0 | 21 | 1 (5) | 20 | 0 | ||
| FTC | 20 | 0 | 20 | 0 | 20 | 0 | 32 | 0 | ||
| ATC | 20 | 0 | 20 | 0 | 20 | 0 | 32 | 0 | ||
| Melanoma | 20 | 0 | ||||||||
| Colon cancer | 20 | 0 | ||||||||
| Total (376) | 92 | 83 | 72 | 33 | 96 | |||||
The 31 PTC for MMP8 analysis included 16 conventional PTC (CPTC), 10 follicular variant PTC (FVPTC), and five tall cell PTC; the 20 PTC for Akt3 analysis included 12 CPTC, four FVPTC, and four TCPTC; the 21 PTC for EGFR analysis included 16 CPTC, three FVPTC, and two TCPTC; and the 20 PTC for PIK3R1 analysis included 16 CPTC and four FVPTC
Acknowledgments
We thank Drs. NE Heldin, KB Ain, N Onoda, M Santoro, D Wynford Thomas, G Brabant, R Schweppe, and B Haugen for kindly providing us the accessibility to the cell lines used in this study.
Funding This study was supported by the NIH grant R01CA134225 to M. Xing.
Footnotes
Declaration of Interest The authors have no conflict of interest to declare.
Contributor Information
Avaniyapuram Kannan Murugan, Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology and Metabolism, The Johns Hopkins University School of Medicine, 1830, East Monument Street, Suite 333, Baltimore, MD 21287, USA.
Jianli Dong, Department of Pathology, University of Texas Medical Branch, Galveston, TX 77555, USA.
Jingwu Xie, Department of Pediatrics, Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Mingzhao Xing, Email: mxing1@jhmi.edu, Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology and Metabolism, The Johns Hopkins University School of Medicine, 1830, East Monument Street, Suite 333, Baltimore, MD 21287, USA.
References
- 1.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epdemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 2.Sprague BL, Warren Andersen S, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes and Control. 2008;19:585–593. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 3.Hundahl I, Fleming D, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and bluenaevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumors and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutt A, Salvesen HB, Greulich H, Sellers WR, Beroukhim R, Meyerson M. Somatic mutations are present in all members of the AKT family in endometrial carcinoma. Br J Cancer. 2009;101:1218–1219. doi: 10.1038/sj.bjc.6605301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masago K, Asato R, Fujita S, Hirano S, Tamura Y, Kanda T, et al. Epidermal growth factor receptor mutations in papillary thyroid carcinoma. Int J Cancer. 2009;124:2744–2749. doi: 10.1002/ijc.24250. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, et al. Somatic mutations in p85α promote tumorigenesis through class 1A PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuse M, Mitsutake N, Nishihara E, Rogounovitch T, Saenko V, Rumyantsev P, et al. Lack of GNAQ hotspot mutation in papillary thyroid carcinomas. Thyroid. 2009;19:921–922. doi: 10.1089/thy.2009.0059. [DOI] [PubMed] [Google Scholar]
- 12.Lamba S, Felicioni L, Buttitta F, Bleeker FE, Malatesta S, Corbo V, et al. Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009;4:e6833. doi: 10.1371/journal.pone.0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassol CA, Guo M, Ezzat S, Asa SL. GNAq mutations are not identified in papillary thyroid carcinomas and hyperfunctioning thyroid nodules. Endocr Pathol. 2010;21:250–252. doi: 10.1007/s12022-010-9129-4. [DOI] [PubMed] [Google Scholar]
- 14.Sozopoulos E, Litsiou H, Voutsinas G, Mitsiades N, Anagnostakis N, Tseva T, et al. Mutational and immunohistochemical study of the PI3K/Akt pathway in papillary thyroid carcinoma in Greece. Endocr Pathol. 2010;21:90–100. doi: 10.1007/s12022-010-9112-0. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiades CS, Kotoula V, Poulaki V, Sozopoulos E, Negri J, Charalambous E, et al. Epidermal growth factor receptor as a therapeutic target in human thyroid carcinoma: mutational and functional analysis. J Clin Endocrinol Metab. 2006;91:3662–3666. doi: 10.1210/jc.2006-0055. [DOI] [PubMed] [Google Scholar]
- 16.Murugan AK, Dong J, Xie J, Xing M. MEK1 mutations, but not ERK2 mutations, occur in melanomas and colon carcinomas, but none in thyroid carcinomas. Cell Cycle. 2009;8:2122–2124. doi: 10.4161/cc.8.13.8710. [DOI] [PubMed] [Google Scholar]
- 17.Nagai Y, Miyazawa H, Huqun, Tanaka T, Udagawa K, Kato M, et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005;65:7276–7282. doi: 10.1158/0008-5472.CAN-05-0331. [DOI] [PubMed] [Google Scholar]
- 18.Tsao MA, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 19.Douglas DA, Zhong H, Ro JY, Oddoux C, Berger AD, Pincus MR, et al. Novel mutations of epidermal growth factor receptor in localized prostate cancer. Front Biosci. 2006;11:2518–2525. doi: 10.2741/1986. [DOI] [PubMed] [Google Scholar]
- 20.Cai CQ, Peng Y, Buckley MT, Wei J, Chen F, Liebes L, et al. Epidermal growth factor receptor activation in prostate cancer by three novel missense mutations. Oncogene. 2008;27:3201–3210. doi: 10.1038/sj.onc.1210983. [DOI] [PubMed] [Google Scholar]
- 21.Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;6:6105–6111. [PubMed] [Google Scholar]
- 22.Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]