Abstract
OBJECTIVE
To examine the association of childbearing with weight and waist circumference (WC) changes, we compared women with and without pregnancies or births during follow-up.
STUDY DESIGN
A multicenter, longitudinal observational study over 10 years. Comparison groups defined by the number of pregnancies and births during follow-up: P0 (0 pregnancies; nongravid), P1 (1+ miscarriages or abortions; ‘short’ pregnancies), B1 (1 birth), and B2 (2+ births). Mean changes in weight and WC for P1, B1 and B2 groups vs P0 were examined separately by race (black and white), baseline parity (nulliparous and parous) and baseline weight status (normal weight; BMI <25 kg/m2 and overweight; BMI ≥25 kg/m2).
SUBJECTS
A population-based sample of 2070 women aged 18–30 y at baseline (1053 black subjects and 1017 white subjects) from Birmingham, Alabama, Chicago, Illinois, Minneapolis, Minnesota, and Oakland, California were examined five times between 1985–1986 and 1995–1996.
MEASURMENTS
Weight and WC measurements were obtained using standardized protocol at baseline and examinations at years 2, 5, 7 and 10. Sociodemographic, reproductive, and behavioral attributes were assessed at baseline and follow-up examinations.
RESULTS
Gains in weight and WC associated with pregnancy and childbearing varied by race (P<0.001), baseline parity (P<0.05) and overweight status (P<0.001). Among overweight nulliparas, excess gains in weight (black subjects: 3–5 kg, white subjects: 5–6 kg) and WC (black subjects: 3–4 cm, white subjects: 5–6 cm) were associated with ‘short’ pregnancies and one or more birth(s) during follow-up compared to no pregnancies (P<0.01 and 0.001). Among normal weight nulliparas, excess gains in weight (about 1 kg) and WC (2–3 cm) were associated with follow-up birth(s) (P<0.05). Among women parous at baseline, no excess weight gains were found, but excess WC gains (2–4 cm) were associated with follow-up births.
CONCLUSION
Substantial excess weight gain is associated with both short pregnancies and a first birth in women overweight prior to initiation of childbearing. Excess weight gain was not associated with higher order births. Increases in waist girth were cumulative with both first and higher order births among overweight as well as normal weight women. Interventions to prevent obesity should be targeted at women who are overweight prior to initiation of childbearing. The impact of excess WC gains associated with childbearing on women’s future health risk should be evaluated further.
Keywords: medical subject headings, central adiposity, obesity, parity, pregnancy, weight gain, waist circumference
Introduction
Pregnancy has been associated with excess weight gain and development of overweight, with such changes persisting beyond 1 y postpartum.1-5 Estimates of pregnancy-related weight gain from cohort studies of pregnant women range from 0.5 to 1.5 kg for white subjects, and up to 3 kg for black subjects by 6–18 months postpartum.6-10 These estimates may be inflated by under-reporting error of one or more kg based on self-reported pregravid weight 11,12 and weight gain unrelated to pregnancy from secular trends and age-related changes. Also, most studies,8,13-18 but not all,19,20 have found higher pregnancy-related weight gains for women already overweight before pregnancy. Prospective studies including nonparous comparison groups that obtained weight measurements before and after pregnancy have corrected for the biases identified above.3,5 In these studies, excess weight gain associated with a single birth has been estimated at 2–3 kg.3,5 While our previous 5-y analysis found that excess gain was associated with the first birth but not subsequent births,3 another study of 10-y weight change found no excess weight gain after the first birth, but a gain of 1.7 kg per additional birth among multiparas.5 Inconsistent findings from these two prospective studies with nonparous comparison groups may be related to their insufficient power to examine pregravid body size and parity as effect modifiers in the association of childbearing with long-term weight changes.
Even less is known about changes in measures of central adiposity associated with childbearing. A positive correlation of waist–hip ratio (WHR) with parity has been reported in large cross-sectional studies.21-23 In the only prospective study to our knowledge, we reported increases in WHR among women who gave birth during follow-up compared to those who did not.3 Change in waist circumference (WC), another measure of central adiposity, has not been reported in previous studies.
Based on evidence that pregnancy may adversely affect body weight and central adiposity, we explored these relationships further in the larger, biracial sample of women with and without pregnancies and births over a 10-y follow-up period from the Coronary Artery Risk Development in Young Adults Study (CARDIA). The purposes of this study are to:
Estimate weight and WC changes associated with pregnancy and childbearing independent of secular trends and aging, and
Determine whether the associations of pregnancy and childbearing with weight and WC changes vary by race, parity and body size prior to pregnancy, and;
Determine whether any associations are solely a consequence of the first birth.
Materials and methods
Study population
The CARDIA Study is a multicenter, longitudinal observational study designed to describe the development of risk factors for coronary heart disease in young black and white men and women. The study population was recruited from four geographic areas by community-based sampling in Birmingham, Alabama, Chicago, Illinois, and Minneapolis, Minnesota, and by sampling from the Kaiser Permanente Health Plan membership in Oakland, California. Details of the study design, methodology, and cohort characteristics are reported elsewhere.24-26
Baseline data were collected between 1985 and 1986 on 2787 women, aged 18–30 y, of which 53 and 47 percent were black and white, respectively. Follow-up examinations conducted at 2, 5, 7, and 10 y after baseline had high overall retention rates of approximately 91, 86, 81, and 79 percent of surviving participants.27 The five examinations included a variety of physiologic and self-report measures that have been described previously.24,26,27 The Institutional Review Board of each participating center approved the study.
Selection criteria
Among 2192 women with anthropometric measurements available at baseline and at one or more follow-up examinations, we excluded those at baseline who had a hysterectomy or removal of both ovaries (n=20), pregnancy within the past 3 months (n=46), currently pregnant (n=3), currently breastfeeding (n=40), or missing other covariables (n=13). We included all pregnancies and births subsequent to baseline that ended at least 12 months prior to the year-10 examination. Measurements for women who were currently pregnant at follow-up examinations were excluded. The final sample included 2070 women (n=1053 black subjects, and n=1017 white subjects) with baseline and one or more follow-up measurements. For both races, women excluded did not differ in baseline demographic and anthropometric characteristics from those included in the analysis, except white subjects excluded were more likely to be current or past smokers, have 12 or fewer years of education, and higher parity at baseline.
Data collection
Measurements of weight, height, and WC were obtained according to standardized protocol described previously.24 WC was selected due to the stronger correlation with central adiposity than WHR.28 Body mass index (BMI) was computed as weight in kilograms divided by squared height in meters. Baseline BMI groups were defined as overweight (BMI>or=25 kg/m2) and normal weight (BMI <25 kg/m2).
Sociodemographic and behavioral characteristics were measured by self- and interviewer-administered questionnaires at baseline and follow-up examinations. Dieting and weight variables collected at baseline included: history of weight cycling (self-reported as number of times gained or lost 10 pounds since age 12 y) categorized as zero, one to two, or two or more episodes, and dieting to lose weight categorized as never, past, or current. A quantitative food frequency (CARDIA Dietary History) was administered by a trained interviewer at baseline 29 and used to calculate the percentages of kilocalories from fat, protein, and carbohydrate.30 Physical activity score was calculated from the interviewer-based CARDIA Physical Activity History at each examination.31 Other attributes assessed at baseline and follow-up examinations were categorized into groups: cigarette smoking; never, former (past), or current, education; 12 or less, 13–15, and 16 or more years, and oral contraceptive (OC) use; never, past, or current. Months of OC use were calculated from an interviewer-administered OC use history at year 10.
Based on the number of pregnancies and births reported prior to baseline, baseline parity groups were defined as nulliparous (no live births) and parous (one or more live births), and baseline gravidity groups were defined as zero, one, and two or more pregnancies. Reproductive history was updated at each follow-up examination; women reported if they were currently pregnant, number of times they had been pregnant including abortions, miscarriages, stillbirths, and live births since the previous examination, and length of gestation and dates of delivery. Pregnancies during follow-up ending in a miscarriage, abortion and/or less than 20 weeks gestation were counted as ‘short’ pregnancies, and those lasting longer than 20 weeks gestation were counted as births.
Repeated measures of change in outcome measures: four time intervals
Weight and WC measurements were available at all five examinations (0, 2, 5, 7, 10) for 70 percent (n=1449) and at four of five examinations for an additional 22 percent (n=446) of the sample. Four time intervals were defined between the baseline examination and each follow-up examination as year 0–2, 0–5, 0–7, and 0–10. Changes in weight and WC were calculated by subtraction of the baseline measurement from each follow-up measurement for a given interval.
Time-dependent follow-up pregnancy and birth groups
Pregnancies and births during follow-up occurred at various time intervals after baseline and prior to follow-up examinations. Time-dependent follow-up pregnancy and birth groups were based on the number of pregnancies and births reported during each follow-up interval and defined as: P0 (0 pregnancies; nongravid), P1 (one or more miscarriages or abortions; ‘short’ pregnancies), B1 (one birth), and B2 (two or more births). The P0 group had no pregnancies during the current or any prior follow-up intervals, and the P1 group had one or more ‘short’ pregnancies, but no births during current or prior follow-up intervals. Women in B1 had only one birth and any number of ‘short’ pregnancies during prior or current intervals, and women in B2 had two or more births and any number of ‘short’ pregnancies during the current or prior interval although one or more birth(s) may have occurred during prior intervals. Assignment to one of these four groups was time dependent (eg, varied for each specific follow-up time interval) and a woman’s group assignment continued into subsequent intervals unless she reported new pregnancies since her last CARDIA examination. For example, a woman reporting a miscarriage between years 0 and 2 would be classified in P1 for that interval, and if she reported one birth between years 2 and 5 she would classified in B1 for the year 0–5 interval. The woman would remain in B1 for subsequent intervals (0–7 and 0–10) if no subsequent births were reported.
Other time-dependent covariates included age, number of short pregnancies, change in physical activity score (baseline score subtracted from each follow-up score), duration of OC use, and categorical variables; education and smoking status.
Statistical analysis
Descriptive
Preliminary analyses involved description of the cohort’s baseline characteristics among P0, P1, B1, and B2 groups at the end of follow-up stratified by race and baseline parity (nulliparous and parous) groups. The χ2 tests were used to assess associations of follow-up groups with baseline demographic (education, age), and behavioral characteristics (smoking status, OC use, history of weight cycling, dieting to lose weight), BMI groups, and reproductive history (parity and gravidity at baseline). Multiple linear regression methods were used to assess differences in baseline characteristics (height, weight, BMI, WC, dietary intake, and physical activity) among follow-up groups within race and baseline parity groups. All P-values presented are for two-sided tests; significance level <0.05.
Longitudinal changes in anthropometric measurements
Weight and WC measurements from years 0, 2, 5, 7, and 10 were assembled along with fixed variables, race and center, and time-dependent follow-up pregnancy and births groups (P0, P1, B1, and B2) over the four intervals. Repeated measures linear regression methods (SAS 8.2, PROC MIXED) were used to estimate mean changes in weight and WC during follow-up. Follow-up groups were modeled as time-dependent categories to estimate changes associated with childbearing using data for all women as they moved through the relevant time periods. Two-way interactions for covariables of interest were examined simultaneously in models with races combined. Three-way interaction terms (baseline BMI, baseline parity, and follow-up groups) were examined in race-specific models to assess heterogeneity in the associations of weight change and WC change. Based on these analyses, models regressing follow-up groups on weight and WC change outcomes were stratified by race and baseline parity, and an interaction term for BMI groups by follow-up groups was included. Race-specific estimates are given for baseline parity and BMI groups: overweight nulliparous, normal weight nulliparous, overweight parous, and normal weight parous.
In minimally adjusted repeated measures models, mean weight and WC change estimates were adjusted for height, age, center, and time. Fully adjusted repeated measures models included baseline covariates (percent of kcal from protein, physical activity, gravidity, education, smoking status, OC use) and time-dependent follow-up covariates (education, short pregnancies, smoking status, months of OC use, and change in physical activity) in addition to those in minimally adjusted models. Both baseline and time-dependent covariables included as confounders were selected based on associations with outcome measures independent of their association with follow-up groups. For WC change estimates, both minimally and fully adjusted models also included baseline weight as a covariate. The fully adjusted plus weight gain model to estimate WC change included time-dependent weight change as a covariate. The covariance structure in all repeated regression models was compound symmetry; analyses based on the assumption of unstructured variance–covariance matrix yielded similar results.
Pair-wise comparisons of mean weight and WC changes during follow-up were examined for P1, B1, and B2 groups vs P0 (referent), and for B1 and B2 groups. Differences in means between follow-up groups and the referent group were calculated. Positive differences were termed ‘excess’ gains. Weight and WC change estimates for white subjects parous at baseline were not precise due to small sample size; B2 group (n=1) estimates are omitted in the overweight stratum.
Results
Overall, 845 women (448 black and 397 white subjects) had one or more births at least 12 months prior to the year 10 examination, 249 had one or more ‘short’ pregnancies but no births, and 976 had no pregnancies during follow-up. Fewer black subjects (549; 52 percent) than white subjects (834; 82 percent) were nulliparous at baseline. For each race and baseline parity group (Figure 1: Parts A–D), the cumulative distribution of women among the follow-up groups (P0, P1, B1, and B2) is shown over the four time intervals. Among black and white subjects nulliparous at baseline, more than half of the follow-up births occurred between baseline and year 5, and almost 80 percent by year 7. In black subjects parous at baseline, over 70 percent of follow-up births occurred by year 5, and about 90 percent by year 7.
Figure 1.
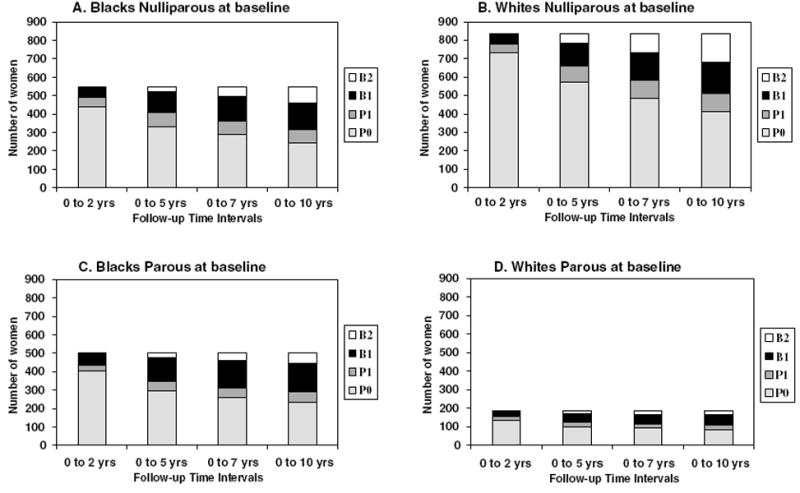
(A–D): Number of women distributed among follow-up pregnancy and birth groups (P0=0 pregnancies, P1=one or more short pregnancies, B1=one birth, and B2=two or more births) within the four time intervals by race and baseline parity groups.
Differences in baseline characteristics are shown for P0, P1, B1, and B2 groups at the end of follow-up by race and baseline parity groups (Tables 1 and 2). Among white subjects nulliparous at baseline, significant differences in gravidity, OC, education, and smoking history were found, and weight, BMI, and WC reached borderline significance across follow-up groups. Among black subjects nulliparous at baseline, borderline differences in WC and significant differences in gravidity, OC use, age, percent of kcal from fat, and dieting to lose weight were found. Among women parous at baseline, significant differences in age, gravidity, parity, OC use, and dietary intake were found in black subjects, and significant differences in age, gravidity, parity, education, BMI, weight, and WC in white subjects. No other significant differences in other baseline covariates (height, BMI, physical activity, and history of weight cycling; data not shown) were found across follow-up groups, except for borderline differences in OC use among parous white subjects.
Table 1.
Women nulliparous at baseline: baseline characteristics for follow-up pregnancy and birth groups by race
| Nulliparous at baseline | Black women n (%) Follow-up pregnancy and birth groups (end of follow-up) |
White women n (%) Follow-up pregnancy and birth groups (end of follow-up) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | P0: no pregnancies | P1: 1+ short pregnancies | B1: one birth | B2: two or more births | P | P0: no pregnancies | P1: 1 + short pregnancies | B1: one birth | B2: two or more births | P |
| n | 245 | 69 | 147 | 88 | 416 | 96 | 170 | 152 | ||
| Age group | <0.001 | 0.15 | ||||||||
| 18–24 y | 142 (58.0) | 51 (73.9) | 101 (68.7) | 72 (81.8) | 168 (40.4) | 47 (49.0) | 62 (36.5) | 54 (35.5) | ||
| 25–30 y | 103 (42.0) | 18 (26.1) | 46 (31.3) | 16 (18.2) | 248 (59.6) | 49 (51.0) | 108 (63.5) | 98 (64.5) | ||
| Weight status | 0.46 | 0.20 | ||||||||
| BMI <25 | 141 (57.5) | 40 (58.0) | 96 (65.3) | 51 (58.0) | 325 (78.1) | 77 (80.2) | 137 (80.6) | 131 (86.2) | ||
| BMI ≥25 | 104 (42.5) | 29 (42.0) | 51 (34.7) | 37 (42.0) | 91 (21.9) | 19 (19.8) | 33 (19.4) | 21 (13.8) | ||
| Education | 0.16 | <0.001 | ||||||||
| High school or less | 86 (35.1) | 21 (30.4) | 68 (46.2) | 35 (39.8) | 75 (18.0) | 24 (25.0) | 38 (22.4) | 18 (11.8) | ||
| Some college | 106 (43.3) | 37 (53.6) | 57 (38.8) | 38 (43.2) | 135 (32.5) | 33 (34.4) | 49 (28.8) | 30 (19.7) | ||
| College graduate | 53 (21.6) | 11 (15.9) | 22 (15.0) | 15 (17.0) | 206 (49.5) | 39 (40.6) | 83 (48.8) | 104 (68.4) | ||
| Smoking history | 0.92 | 0.02 | ||||||||
| Never | 175 (71.4) | 47 (68.1) | 106 (72.1) | 60 (68.2) | 250 (60.1) | 40 (41.7) | 96 (56.5) | 99 (65.1) | ||
| Past | 13 (5.3) | 5 (7.3) | 5 (3.4) | 5 (5.7) | 75 (18.0) | 24 (25.0) | 35 (20.6) | 25 (16.5) | ||
| Current | 57 (23.3) | 17 (24.6) | 36 (24.5) | 23 (26.1) | 91 (21.9) | 32 (33.3) | 39 (22.9) | 28 (18.4) | ||
| Gravidity | 0.02 | 0.002 | ||||||||
| None | 191 (78.0) | 42 (60.9) | 107 (72.8) | 59 (67.0) | 338 (81.3) | 61 (63.5) | 127 (74.7) | 117 (77.0) | ||
| One or more | 54 (22.0) | 27 (39.1) | 40 (27.2) | 29 (33.0) | 78 (18.7) | 35 (36.5) | 43 (25.3) | 35 (23.0) | ||
| Parity (0) | 245 (100) | 69 (100) | 147 (100) | 88 (100) | — | 416 (100) | 96 (100) | 170 (100) | 152 (100) | — |
| OC use | 0.02 | <0.001 | ||||||||
| Never | 85 (34.7) | 16 (23.2) | 38 (25.9) | 13 (14.8) | 157 (37.7) | 23 (24.0) | 31 (18.2) | 32 (21.1) | ||
| Past | 81 (33.1) | 29 (42.0) | 55 (37.4) | 36 (40.9) | 154 (37.0) | 54 (56.2) | 72 (42.4) | 59 (38.8) | ||
| Current | 79 (32.2) | 24 (34.8) | 54 (36.7) | 39 (44.3) | 105 (25.3) | 19 (19.8) | 67 (39.4) | 61 (40.1) | ||
| Mean (s.d.) | ||||||||||
| Weight (kg) | 69.3 (18.8) | 67.2 (18.7) | 66.5 (16.7) | 66.5 (13.7) | 0.37 | 63.3 (13.2) | 62.8 (11.7) | 61.1 (10.8) | 60.7 (9.7) | 0.05 |
| Waist circumference (cm) | 76.4 (14.4) | 73.5 (12.7) | 73.2 (10.5) | 74.1 (10.4) | 0.06 | 71.7 (9.4) | 71.5 (9.0) | 70.4 (7.7) | 69.8 (7.4) | 0.08 |
| Dietary fat (% kcal) | 37.0 (5.7) | 38.7 (5.3) | 37.9 (5.5) | 38.5 (5.3) | 0.04 | 35.8 (6.2) | 35.9 (6.5) | 36.7 (6.8) | 36.0 (7.5) | 0.19 |
| Dietary CHO (% kcal) | 48.3 (7.1) | 46.5 (7.4) | 47.2 (7.2) | 47.2 (7.0) | 0.17 | 47.6 (7.2) | 46.8 (7.5) | 46.2 (7.6) | 46.6 (7.5) | 0.49 |
| Dietary protein (% kcal) | 14.1 (2.3) | 14.5 (2.2) | 14.5 (2.6) | 14.1 (2.5) | 0.39 | 15.4 (2.9) | 15.5 (3.0) | 15.5 (3.0) | 15.6 (2.6) | 0.81 |
Table 2.
Women parous at baseline: baseline characteristics for follow-up pregnancy and birth groups by race
| Parous at baseline | Black women n (%) Follow-up pregnancy and birth groups (end of follow-up) |
White women n (%) Follow-up pregnancy and birth groups (end of follow-up) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | P0: no pregnancies | P1: 1+ short pregnancies | B1: one birth | B2: two or more births | P | P0: no pregnancies | P1: 1+ short pregnancies | B1: one birth | B2: two or more births | P |
| n | 233 | 58 | 154 | 59 | <0.001 | 82 | 26 | 54 | 21 | 0.015 |
| Age group | ||||||||||
| 18–24 y | 45 (19.3) | 19 (32.8) | 69 (44.8) | 37 (62.7) | 9 (11.0) | 8 (30.8) | 12 (22.2) | 8 (38.1) | ||
| 25–30 y | 188 (80.7) | 39 (67.2) | 85 (55.2) | 22 (37.3) | 73 (89.0) | 18 (69.2) | 42 (77.8) | 13 (61.9) | ||
| Weight status | 0.20 | <0.001 | ||||||||
| BMI <25 | 110 (47.2) | 33 (56.9) | 88 (57.1) | 33 (55.9) | 42 (51.2) | 18 (69.2) | 42 (77.8) | 20 (95.2) | ||
| BMI ≥25 | 123 (52.8) | 25 (43.1) | 66 (42.9) | 26 (44.1) | 40 (48.8) | 8 (30.8) | 12 (22.2) | 1 (4.8) | ||
| Education | 0.058 | 0.001 | ||||||||
| High school or less | 143 (61.4) | 28 (48.3) | 71 (46.1) | 35 (59.3) | 49 (59.8) | 16 (61.5) | 23 (42.6) | 8 (38.1) | ||
| Some college | 75 (32.2) | 23 (39.7) | 65 (42.2) | 17 (28.8) | 26 (31.7) | 8 (30.8) | 18 (33.3) | 3 (14.3) | ||
| College graduate | 15 (6.4) | 7 (12.0) | 18 (11.7) | 7 (11.9) | 7 (8.5) | 2 (7.7) | 13 (24.1) | 10 (47.6) | ||
| Smoking history | 0.22 | 0.29 | ||||||||
| Never | 112 (48.1) | 27 (46.6) | 85 (55.2) | 34 (57.6) | 36 (43.9) | 6 (23.1) | 28 (51.9) | 10 (47.6) | ||
| Past | 29 (12.5) | 6 (10.3) | 21 (13.6) | 2 (3.4) | 16 (19.5) | 5 (19.2) | 10 (18.5) | 4 (19.1) | ||
| Current | 92 (39.5) | 25 (43.1) | 48 (31.2) | 23 (39.0) | 30 (36.6) | 15 (57.7) | 16 (29.6) | 7 (33.3) | ||
| Gravidity | 0.02 | 0.004 | ||||||||
| One | 57 (24.5) | 14 (24.1) | 52 (33.8) | 25 (42.4) | 17 (20.7) | 8 (30.8) | 23 (42.6) | 12 (57.1) | ||
| Two or more | 176 (75.4) | 44 (75.9) | 102 (66.2) | 34 (57.6) | 65 (79.3) | 18 (69.2) | 31 (57.4) | 9 (42.9) | ||
| Parity | <0.001 | <0.001 | ||||||||
| One | 87 (37.3) | 31 (53.5) | 100 (64.9) | 44 (74.6) | 33 (40.2) | 16 (61.5) | 42 (77.8) | 18 (85.7) | ||
| Two or more | 146 (62.7) | 27 (46.5) | 54 (35.1) | 15 (25.4) | 49 (59.8) | 10 (38.5) | 12 (22.2) | 3 (14.3) | ||
| OC use | <0.001 | 0.09 | ||||||||
| Never | 39 (16.7) | 7 (12.1) | 13 (8.4) | 4 (6.8) | 10 (12.2) | 1 (3.9) | 7 (13.0) | 7 (33.3) | ||
| Past | 146 (62.7) | 29 (50.0) | 69 (44.8) | 24 (40.7) | 57 (69.5) | 20 (76.9) | 33 (61.1) | 10 (47.6) | ||
| Current | 48 (20.6) | 22 (37.9) | 72 (46.8) | 31 (52.5) | 15 (18.3) | 5 (19.2) | 14 (25.9) | 4 (19.1) | ||
| Mean (s.d.) | ||||||||||
| Weight (kg) | 72.2 (19.3) | 67.7 (18.8) | 68.3 (16.9) | 70.0 (21.1) | 0.15 | 68.3 (15.5) | 66.9 (16.0) | 62.0 (14.6) | 57.7 (6.8) | 0.008 |
| Waist circumference (cm) | 79.6 (13.1) | 77.4 (14.4) | 76.2 (11.8) | 77.7 (15.2) | 0.09 | 78.5 (11.7) | 75.5 (14.6) | 72.4 (9.9) | 69.1 (5.2) | 0.001 |
| Dietary fat (% kcal) | 36.9 (6.5) | 38.5 (5.2) | 38.4 (5.5) | 39.2 (6.6) | 0.002 | 36.8 (6.6) | 37.0 (6.6) | 36.7 (5.5) | 38.4 (5.5) | 0.75 |
| Dietary CHO (% kcal) | 49.0 (8.0) | 46.6 (6.8) | 46.7 (7.5) | 45.3 (8.7) | 0.01 | 47.6 (8.6) | 46.3 (7.9) | 46.9 (6.4) | 45.7 (4.5) | 0.70 |
| Dietary protein (% kcal) | 13.7 (2.8) | 14.2 (2.3) | 14.7 (2.8) | 14.8 (3.1) | 0.004 | 15.1 (2.9) | 14.7 (3.1) | 16.3 (4.6) | 15.0 (2.2) | 0.17 |
In repeated measures models, evidence for important two-way interactions were found within the association between time-dependent follow-up groups and weight change (race; P<0.001, baseline parity; P=0.03, baseline BMI; P<0.001) and WC change (race; P<0.001, baseline parity; P<0.06, baseline BMI; P<0.001). In race-specific models, evidence for significant three-way interactions (baseline BMI, baseline parity, follow-up pregnancy, and birth groups) was found in black subjects and white subjects, respectively; weight change (P<0.02 and <0.001), and WC change (P<0.04 and <0.001). Race-specific mean weight and WC gains are presented stratified by baseline parity and BMI groups.
In both minimally and fully adjusted models (Table 3), women who were overweight and nulliparous at baseline had the highest mean weight gain associated with short pregnancies and births (P<0.001) within each race. The fully adjusted estimates for women who were overweight and nulliparous at baseline (Figure 2) were greater for P1, B1, and B2 groups, respectively, compared to the P0 group (black subjects; +3.0, 2.7, and 4.8 kg, P<0.01, and white subjects; +5.7, 4.7, and 6.0 kg, P<0.001). The mean weight gain for B2 did not differ from B1 except among nulliparous black subjects (P<0.05).
Table 3.
Weight gain from repeated measures linear regression models for time-dependent follow-up pregnancy and birth groups stratified by race and baseline groups
| Black women: weight gain (kg); mean (95% CI) time-dependent follow-up pregnancy and birth groups |
White women: weight gain (kg); mean (95% CI) time-dependent follow-up pregnancy and birth groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline groups | P0: no pregnancies (referent group) | P1: one or more short pregnancies | B1: one birth | B2: two or more births | P0: no pregnancies (referent group) | P1: one or more short pregnancies | B1: one birth | B2: two or more births |
| Overweight Nulliparous, n | 104 | 29 | 51 | 37 | 91 | 19 | 33 | 21 |
| Minimally adjusted | 8.7 (7.5, 9.9) | 11.2* (9.2, 13.3) | 11.5*** (9.9, 13.1) | 14.2*** (12.1, 16.4) | 4.5 (3.5, 5.5) | 10.3*** (8.3, 12.3) | 10.1*** (8.6, 11.6) | 11.0*** (8.9, 13.1) |
| Fully adjusted | 8.7 (7.5, 9.9) | 11.7** (9.5, 13.8) | 11.4** (9.8,13.0) | 13.5*** (11.3, 15.7) | 4.2 (3.2, 5.1) | 9.8*** (7.8, 11.8) | 8.9*** (7.3, 10.4) | 10.2*** (8.1, 12.4) |
| Normal weight Nulliparous, n | 141 | 40 | 96 | 51 | 325 | 77 | 137 | 131 |
| Minimally adjusted | 6.0 (5.0, 7.0) | 5.4 (3.7, 7.0) | 7.6* (6.4, 8.9) | 7.7 (5.9, 9.6) | 3.0 (2.5, 3.5) | 3.4 (2.4, 4.4) | 4.3*** (3.6, 5.1) | 3.1 (2.2, 4.1) |
| Fully adjusted | 6.0 (5.0, 6.9) | 5.6 (3.9, 7.3) | 7.4* (6.2, 8.7) | 7.9 (6.0, 9.8) | 3.2 (2.7, 3.7) | 3.6 (2.4, 4.9) | 4.1* (3.3, 4.8) | 2.9 (2.0, 3.9) |
| Overweight Parous, n | 123 | 25 | 66 | 26 | 40 | 8 | 12 | 1 |
| Minimally adjusted | 7.4 (6.3, 8.5) | 6.2 (3.8, 8.6) | 8.1 (6.6, 9.6) | 10.3* (7.6, 13.1) | 6.4 (4.5, 8.3) | 5.7 (1.6, 9.8) | 7.0 (3.0, 11.1) | NR |
| Fully adjusted | 7.2 (6.1, 8.3) | 6.1 (3.6, 8.6) | 8.1 (6.6, 9.6) | 9.3 (6.6, 12.0) | 7.2 (5.1, 9.2) | 3.8 (−0.7, 8.3) | 7.1 (2.9, 11.3) | NR |
| Normal weight parous, n | 110 | 33 | 88 | 33 | 42 | 18 | 42 | 20 |
| Minimally adjusted | 6.1 (5.0, 7.1) | 4.8 (2.9, 6.7) | 7.9* (6.6, 9.2) | 7.3 (5.1, 9.6) | 6.0 (4.3, 7.6) | 3.3 (0.7, 5.9) | 5.0 (3.1, 6.8) | 3.9 (1.0, 6.9) |
| Fully adjusted | 6.2 (5.1, 7.2) | 4.2 (2.2, 6.1) | 7.6 (6.3, 8.9) | 7.1 (4.9, 9.3) | 6.3 (4.6, 7.9) | 2.6* (−0.4, 5.6) | 4.7 (2.8, 6.6) | 2.9 (−0.1, 6.0) |
Pairwise comparisons to referent group (P0): P<0.05;
P<0.01;
P< 0.001. n=number of women within groups at the end of follow-up.
Minimally adjusted models include height, age, center, and time. Fully adjusted models include relevant baseline characteristics (height, percent of kcal from protein, OC use, gravidity, education level, smoking status, and physical activity score) and time-dependent follow-up characteristics (number of short pregnancies, smoking status, age, education, duration of OC use, and change in physical activity), center, and time.
NR=not reported due to small sample size.
Figure 2.
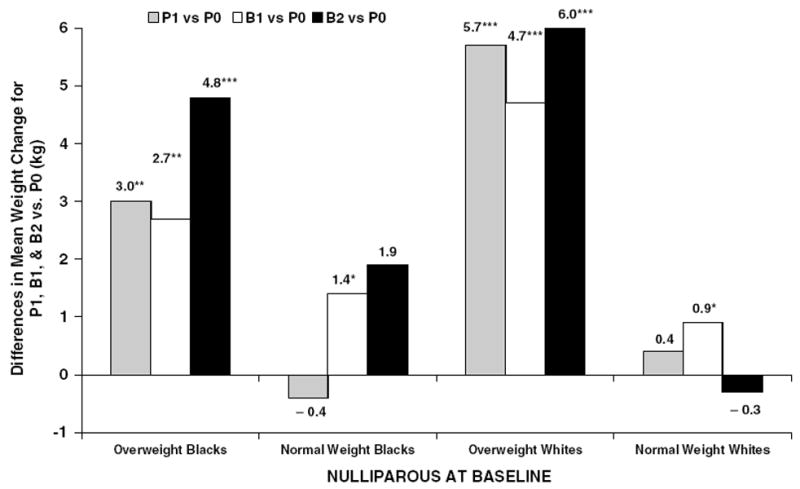
Fully adjusted differences in mean weight gain (kg) between follow-up pregnancy and birth groups ( P1, B1, and B2) compared to no pregnancies ( P0) among nulliparous baseline groups by race and BMI groups (*P-value <0.05; **P-value <0.01; ***P-value <0.001).
Among women who were normal weight and nulliparous at baseline, both minimally and fully adjusted weight gains were greater for the B1 group compared to the P0 group (Table 3). The fully adjusted differences in mean weight gain (Figure 2) were attenuated to +1.4 and +0.9 kg for B1 vs P0 groups among black and white subjects, respectively (P<0.05). Among women parous at baseline, weight gain did not differ among follow-up pregnancy and birth groups regardless of race or baseline BMI group in fully adjusted models.
The mean WC gains (Table 4) from both minimally and fully adjusted models were greatest among women who were overweight and nullliparous at baseline. Fully adjusted estimates of WC change were greater for P1, B1, and B2 groups compared to the P0 group, respectively (black subjects; +3–4 cm, and white subjects; +5–6 cm, P<0.001), among women who were overweight and nulliparous at baseline (Figure 3). Among women who were normal weight and nulliparous at baseline, greater WC gains were found for B1 and B2 groups vs the P0 group (P<0.001) ranging from + 3–4cm for black subjects and +2–3cm for white subjects (Figure 3). Among women parous at baseline, greater mean WC gains were also found for B1 and B2 groups vs P0 in both races (Table 4). Addition of time-dependent weight change to fully adjusted models (Table 4; fully adjusted+weight gain) generally resulted in attenuation of differences in WC changes, but greater gains in WC remained for B1 and B2 groups compared to the P0 group. The mean WC increases for the P1 vs P0 group were no longer significant after adjustment for weight gain during follow-up.
Table 4.
Waist circumference (WC) gain from repeated measures linear regression models for time-dependent follow-up pregnancy and birth groups stratified by race and baseline groups
| Black women: WC gain (cm); mean (95% CI) time-dependent follow-up pregnancy and birth groups |
White women: WC gain (cm); mean (95% CI) time-dependent follow-up pregnancy and birth groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline groups: | P0: no pregnancies (referent group) | P1: one or more short pregnancies | B1: one birth | B2: two or more births | P0: no pregnancies (referent group) | P1: one or more short pregnancies | B1: one birth | B2: two or more births |
| Overweight Nulliparous, n | 104 | 29 | 51 | 37 | 91 | 19 | 33 | 21 |
| Minimally adjusted | 6.9 (5.6, 8.2) | 9.3** (7.4, 11.2) | 10.6*** (9.1, 12.2) | 11.6*** (9.6, 13.6) | 2.5 (1.3, 3.7) | 7.1*** (5.2, 9.0) | 8.8*** (7.2, 10.3) | 9.2*** (7.2, 11.2) |
| Fully adjusted | 6.8 (5.6, 8.1) | 9.7** (7.8, 11.7) | 10.6*** (9.0, 12.1) | 11.1*** (9.1, 13.1) | 2.4 (1.2, 3.5) | 7.1*** (5.2, 9.0) | 8.0*** (6.5, 9.5) | 8.6*** (6.6, 10.6) |
| Fully adjusted+weight gain | 6.2 (5.6, 6.9) | 7.1 (6.0, 8.2) | 8.3*** (7.4, 9.1) | 7.3 (6.2, 8.4) | 2.7 (2.1, 3.3) | 3.1 (2.1, 4.0) | 4.5*** (3.7, 5.3) | 4.2** (3.1, 5.2) |
| Normal weight Nulliparous, n | 141 | 40 | 96 | 51 | 325 | 77 | 137 | 131 |
| Minimally adjusted | 4.7 (3.8, 5.7) | 4.3 (2.8, 5.9) | 7.9*** (6.7, 9.0) | 8.1*** (6.4, 9.8) | 2.6 (2.2, 3.1) | 2.7 (1.8, 3.6) | 5.6*** (4.9, 6.3) | 4.9*** (4.0, 5.7) |
| Fully adjusted | 4.7 (3.7, 5.7) | 4.6 (3.0, 6.2) | 7.7*** (6.6, 8.9) | 8.3*** (6.5, 10.0) | 2.7 (2.2, 3.2) | 3.1 (2.0, 4.2) | 5.3*** (4.6, 6.0) | 4.6*** (3.8, 5.5) |
| Fully adjusted + weight gain | 5.8 (5.3, 6.2) | 6.0 (5.1, 6.9) | 7.6*** (7.0, 8.2) | 8.0*** (7.0, 9.0) | 3.0 (2.8, 3.2) | 3.0 (2.5, 3.6) | 4.9*** (4.5, 5.2) | 5.2*** (4.7, 5.6) |
| Overweight parous, n | 123 | 25 | 66 | 26 | 40 | 8 | 12 | 1 |
| Minimally adjusted | 6.4 (5.2, 7.6) | 4.8 (2.6, 7.0) | 7.5 (6.0, 9.0) | 10.8*** (8.3, 13.3) | 3.8 (1.5, 6.1) | 5.0 (1.1, 8.8) | 7.7* (3.8. 11.6) | NR |
| Fully adjusted | 6.1 (4.9, 7.3) | 4.7 (2.4, 7.0) | 7.7* (6.2, 9.2) | 10.3** (7.8, 12.8) | 4.6 (2.3, 7.0) | 3.9 (−0.2, 8.0) | 7.9 (3.8, 12.0) | NR |
| Fully adjusted+weight gain | 5.9 (5.1, 6.6) | 5.3 (4.0, 6.7) | 6.7* (5.8, 7.6) | 8.3** (6.8, 9.8) | 3.0 (1.6, 4.3) | 4.2 (1.7, 6.6) | 5.7* (3.3, 8.1) | NR |
| Normal weight Parous n | 110 | 33 | 88 | 33 | 42 | 18 | 42 | 20 |
| Minimally adjusted | 4.9 (3.7, 6.0) | 4.6 (2.8, 6.4) | 7.2*** (5.9, 8.5) | 7.4* (5.4, 9.5) | 4.4 (2.8, 6.0) | 2.3 (0.1, 4.6) | 4.3 (2.5, 6.0) | 3.9 (1.3, 6.5) |
| Fully adjusted | 4.8 (3.7, 5.9) | 4.1 (2.3, 6.0) | 7.0*** (5.7, 8.3) | 7.3* (5.2, 9.4) | 4.5 (2.9, 6.1) | 1.4* (−1.2, 4.0) | 3.8 (2.0, 5.7) | 2.8 (0.1, 5.5) |
| Fully adjusted+weight gain | 5.4 (4.7, 6.1) | 6.2 (5.1, 7.3) | 6.6** (5.8, 7.4) | 7.3** (6.0, 8.5) | 4.5 (3.6, 5.5) | 3.7 (2.1, 5.3) | 4.9 (3.8, 6.0) | 5.1 (3.5, 6.7) |
Pairwise comparisons to referent group (P0): P<0.05;
P<0.01;
P<0.001. n=number of subjects within groups at the end of follow-up.
Minimally adjusted models include height, weight, age, center, and time. Fully adjusted models include relevant baseline characteristics (height, weight, percent of kcal from protein, OC use, gravidity, education level, smoking status, and physical activity score) and time-dependent follow-up characteristics (number of short pregnancies, smoking status, duration of OC use, age, education, and change in physical activity), center, and time. Fully adjusted+weight gain models include time-dependent weight change during follow-up.
Figure 3.
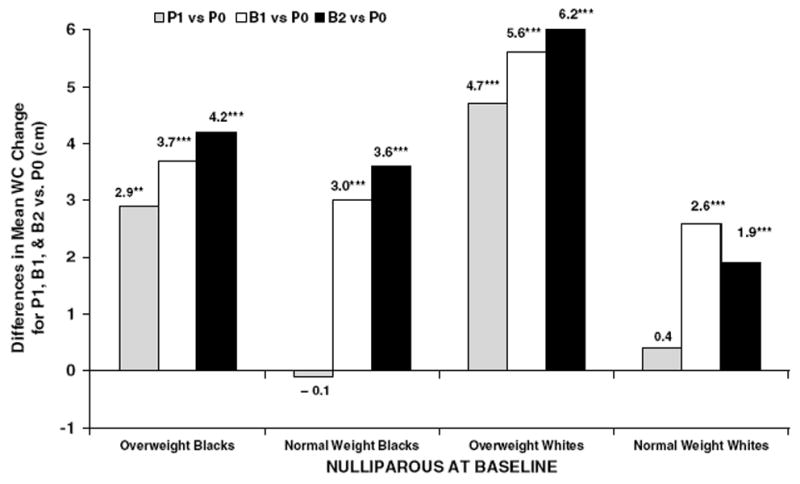
Fully adjusted differences in mean WC changes (cm) between follow-up pregnancy and birth groups (P1, B1, and B2) compared to no pregnancies (P0) among nulliparous baseline groups by race and BMI groups (**P-value <0.01; ***P-value <0.001).
Discussion
To our knowledge, this is the first prospective study to examine both weight and WC changes associated with childbearing in comparison to nongravid women that evaluates the role of pregravid body size. An excess weight gain of 3–6 kg was associated with having one or more ‘short’ pregnancies, or a first birth during 10 y of follow-up among overweight women who had previously never given birth. Gains for one vs two or more births during follow-up were similar among white subjects but not black subjects. An excess weight gain of 0.9–1.4 kg was associated with a first birth during follow-up among women normal weight at baseline. No association with higher order births was found among women parous at baseline. Excess WC gains were cumulative for the first as well as subsequent births. Overweight women had the greatest excess WC gains associated with one or more births. Adjustment for lifestyle changes including physical activity, smoking status, OC use, and other covariates had little effect on the estimates.
The finding that excess weight gain occurs after a first birth, but not subsequent births is consistent with the 5-y CARDIA study3 where a 2–3 kg higher weight gain was found after a first birth, but not subsequent births compared to never giving birth (nulliparous). By contrast, the NHANES I Epidemiologic Follow-Up Study (NHEFS) found a 1.7 kg gain per live birth among women with higher order births, but no greater gain after a first birth compared to never giving birth.5 Differences between the CARDIA and NHEF studies include the age distributions (18–30 y vs 25–45 y at baseline), length of follow-up (5 vs 10 years), and exclusion of women with ‘short pregnancies’ in CARDIA but not NHEFS.3,5 NHEFS included a high proportion of women who had completed childbearing at baseline, thus peri- and postmenopausal women may have been included in the nonparous group.5 Both studies had small sample sizes of primiparas (<50), only two weight measurements, and insufficient power to evaluate effect modification by pregravid maternal body size and baseline parity.3,5 Our analysis included over 380 births for each race, and multiple repeated measurements over the 10-y follow-up period before and after both short pregnancies and births. No association between higher order births and excess weight gain in practically all groups in our study may mean that an initial adaptive response to pregnancy reaches a threshold for adipose tissue deposition after a first birth for susceptible individuals (eg, overweight women) or major adjustments to lifestyle changes occur mainly with the first birth, such that weight gain for subsequent births is similar to that of nongravid women.
Secondly, this is the first prospective study with a nongravid comparison group to have adequate power to examine overweight and normal weight women separately. In the NHEFS,Wolfe et al found that parity-associated weight gain varied by baseline weight; white women weighing above 200 lbs at baseline had the largest weight gains for one or more births vs none.32 Higher excess weight gains after a first birth among overweight women in our study is also consistent with pregnancy cohort studies reporting higher postpartum weight gain or retention among overweight compared to normal weight women.8,13-18,33 Since these studies lacked nongravid/nonparous comparison groups, it has been unclear whether higher weight retention in overweight women is related to their own pregravid weight gain trajectory vs pregnancy or its aftermath. Our findings comparing overweight women with short pregnancies and births to nongravid overweight women based on weight measurements before pregnancy provide direct evidence that childbearing is associated with greater excess weight gain in both black and white subjects who are overweight.
Although overweight women have been reported to have more variable and lower gestational weight gains,8,34 and lower or similar gestational fat gains,35-37 they also have greater increases in central adiposity, weight, and fat deposition during the postpartum period compared to lower BMI groups.36 Therefore, women who are overweight prior to pregnancy may have a tendency to accumulate excessive fat stores in response to pregnancy that continues into the postpartum period, or have patterns of postpartum fat deposition that differ from those who are not overweight. Both physiologic and behavioral processes may influence these excess gains among overweight women; however, our analysis cannot distinguish between these mechanisms.
Thirdly, a few studies have suggested that postpartum weight retention may be higher in black than white women.8,17 Ours is the largest prospective study including nongravid women to have sufficient numbers of both black and white women with births to examine whether excess weight gain associated with childbearing varies by race. Excess weight gain after a first birth differed for black and white subjects after controlling for body size, selected behavioral covariates, age-related weight gain, and secular trends. Among women nulliparous at baseline, black subjects gained more weight than white subjects during follow-up. However, the excess weight gain attributed to childbearing was lower among overweight black subjects than white subjects because of greater gain among black women who were nongravid during follow-up. Hence, cultural as well as biologic and behavioral factors may influence the predisposition of overweight women to gain weight associated with childbearing. Similar racial differences were found for excess gains in girth associated with births, but adjustment for weight gain minimized these differences.
Behavioral factors may contribute to gains in both weight and central adiposity associated with pregnancy or its aftermath. For example, heritable predisposition to gain weight, changes in lifestyle during and after pregnancy (smoking cessation, dietary intake, and physical activity) as well as cultural perceptions of women’s body image, employment, and maternal roles have been reported to have an impact on postpartum weight gain or retention.9,38,39 We examined changes in physical activity, education, and smoking status and found only weak influences on weight gain, while dietary intake and history of weight cycling at baseline were not independently associated with long-term weight and WC gains.
Fourthly, excess gains in WC were associated with one or more births among both overweight and normal weight women. A first birth and subsequent births were associated with excess gains in WC compared to nongravid groups, indicating that WC increases are cumulative with successive births. Excess WC gains associated with childbearing were generally attenuated when accounting for weight gain, but remained significant. These findings are consistent with the 5-y CARDIA analysis; in both races, greater increases in WHR were associated with both the first birth and one higher order birth during follow-up compared to nulliparity.3 It has been reported that 68 percent of gestational fat gain is deposited in the trunk 40 and excess gain remaining at 1 y postpartum tends to be located in the trunk.36,40 Continued excess WC gains after first and higher order births may be related to physical changes in body structure such as expansion of the abdominal muscles, ribcage, and uterus during the third trimester of pregnancy to accommodate fetal growth as well as greater adipose tissue deposition. Further investigation is needed to determine whether cumulative excess gains in WC are due to changes in central adiposity.
Lastly, nongravid rather than nonparous reference groups were used because short pregnancies influenced changes in weight and girth; however, previous studies have not evaluated this major confounder. Maternal fat deposition begins early in pregnancy and has been estimated at 1.5 kg by 7 weeks gestation.41 Therefore, early fat gain may be retained long term even after a short pregnancy. Our study found greater increases in both weight and girth associated with one or more short pregnancies compared to no pregnancies during follow-up, but only among overweight nulliparas. No additional WC increase associated with short pregnancies after accounting for weight gain supports the theory that increased girth associated with birth(s) may be partially related to changes in maternal physical structure occurring relatively late in gestation as well as increased central adiposity during early gestation.
Limitations of our study include lack of information on changes in dietary intake and other lifestyle factors associated with parenting, and determinants of reproductive patterns. Nongravid women may differ in other respects that were not measured in this study, and some women who remained nulliparous may be subject to hormonal disorders affecting fertility (eg, polycystic ovary syndrome), which would tend to minimize differences in weight gain associated with childbearing, or increase weight gain associated with miscarriages among overweight women. Gestational gain, lifestyle behaviors, and other postpartum characteristics (eg, delivery interval, lactation) may influence weight changes associated with childbearing as previously reported in studies of pregnant women.7,16,18 Lastly, our analyses were conducted using single-inference, rather than multipleinference, procedures (single association P-values and 95% confidence intervals) as recommended by Greenland and Rothman,42 thus it is possible that some of our estimates achieved statistical significance at the 0.05 level by chance alone. However, our findings are consistent with previous studies showing an association of high BMI with greater pregnancy-related weight gains.4,34
Our findings support the conclusion that maternal body size is an effect modifier in the association of one or more short pregnancies and a first birth with excess gains in weight and girth for both black and white women. Being overweight before a first birth, but not subsequent births was associated with substantial excess weight (3–6 kg) and WC (3–6 cm) gains relative to a nongravid comparison group. Excess gains associated with a first birth among women normal weight at baseline were also found, but were somewhat less than for overweight women. Excess waist girth increases were associated with higher order births as well as a first birth. Evidence from clinical studies of fat gain during pregnancy supports our findings; however, data are needed to identify the risk profile for women susceptible to excessive weight gain. It is uncertain whether excess waist girth associated with births is primarily increased visceral or subcutaneous adiposity and therefore represents a greater risk of chronic disease,43 or other physical changes that may not affect health.
Acknowledgments
Supported by Contracts # N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095, from the National Heart, Lung, and Blood Institute, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant number 1 K12 AR47659 from the Office of Research on Women’s Health, Building Interdisciplinary Research Careers in Women’s Health (BIRCWH), and Career Development Award, Grant number 1 K01 DK59944-01A1 from the National Institute of Diabetes, Digestive and Kidney Diseases.
We acknowledge the consultation provided by Dr David Jacobs, University of Minnesota to develop the multiple linear repeated measures regression models. We also thank Kim Tolan and Heather McCreath for programming support.
References
- 1.Lewis CE, Funkhouser E, Raczynski JM, Sidney S, Bild DE, Howard BV. Adverse effect of pregnancy on high-density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Am J Epidemiol. 1996;144:247–254. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 2.Ness RB, Schotland HM, Flegal KM, Shofer FS. Reproductive history and coronary heart disease risk in Women. Epidemiol Rev. 1994;16(2):298–314. doi: 10.1093/oxfordjournals.epirev.a036155. [DOI] [PubMed] [Google Scholar]
- 3.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 4.Gunderson EP, Abrams B. The epidemiology of gestational weight gain and body weight change after pregnancy. Epidemiol Rev. 1999;21(2):261–275. doi: 10.1093/oxfordjournals.epirev.a018001. [DOI] [PubMed] [Google Scholar]
- 5.Williamson DF, Madams J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord. 1994;18:561–569. [PubMed] [Google Scholar]
- 6.Schauberger CW, Rooney BL, Brimer LM. Factors that influence weight loss in the puerperium. Obstet Gynecol. 1992;79(3):424–429. doi: 10.1097/00006250-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes Relat Metab Disord. 1990;14:159–173. [PubMed] [Google Scholar]
- 8.Keppel K, Taffel S. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine Guidelines. Am J Pub Health. 1993;83:1100–1103. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene GW, Smiciklas-Wright H, Scholl TO, Karp RJ. Postpartum weight change: how much of the weight gained in pregnancy will be lost after delivery? Obstet Gynecol. 1988;71:701–707. [PubMed] [Google Scholar]
- 10.Rookus MA, Rokebrand P, Burema J, Deurenberg P. The effect of pregnancy on the body mass index 9 months postpartum in 49 women. Int J Obes. 1987;11(6):609–618. [PubMed] [Google Scholar]
- 11.Stevens-Simon C, Roghmann KJ, McAnarney ER. Relationship of self-reported prepregnant weight and weight gain during pregnancy to maternal body habitus and age. J Am Dietetic Assoc. 1992;92(1):85–87. [PubMed] [Google Scholar]
- 12.Rowland ML. Reporting bias in height and weight data. Statist Bull. 1989;70:2–11. [PubMed] [Google Scholar]
- 13.McKeown T, Record RG. The influence of weight and height on weight changes associated with pregnancy in women. J Endocrinol. 1957;15:423–429. doi: 10.1677/joe.0.0150423. [DOI] [PubMed] [Google Scholar]
- 14.Billewicz WZ, Thomson AM. Body weight in parous women. Br J Prevent Soc Med. 1970;24:97–104. doi: 10.1136/jech.24.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris HE, Ellison GT, Holliday M, Lucassen E. The impact of pregnancy on the long-term weight gain of primiparous women in England. Int J Obes Relat Metab Disord. 1997;21:747–755. doi: 10.1038/sj.ijo.0800466. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord. 2001;25(6):853–862. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 17.Boardley DJ, Sargent RG, Coker AL, Hussey JR, Sharpe PA. The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstet Gynecol. 1995;86(5):834–838. doi: 10.1016/0029-7844(95)00283-W. [DOI] [PubMed] [Google Scholar]
- 18.Coitinho DC, Sichieri R, D’Aquino Benicio MH. Obesity and weight change related to parity and breast-feeding among parous women in Brazil. Public Health Nutr. 2001;4(4):865–870. doi: 10.1079/phn2001125. [DOI] [PubMed] [Google Scholar]
- 19.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 20.Linne Y, Rossner S. Interrelationships between weight development and weight retention in subsequent pregnancies: the SPAWN study. Acta Obstetr Gynecol Scand. 2003;82(4):318–325. doi: 10.1080/j.1600-0412.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 21.Troisi RJ, Wolf AM, Mason JE, Klingler KM, Colditz GA. Relation of body fat distribution to reproductive factors in pre- and postmenopausal women. Obes Res. 1995;3(2):143–151. [PubMed] [Google Scholar]
- 22.den Tonkelaar I, Seidell JC, van Noord PA, Baanders-van Halewijn EA, Ouwehand IJ. Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study in 11,825 Dutch women participating in the DOM-project. Int J Obes Relat Metab Disord. 1990;14(9):753–761. [PubMed] [Google Scholar]
- 23.Kaye SA, Folsom AR, Prineas RJ, Potter JD, Gapstur SM. The association of body fat distribution with lifestyle and reproductive factors in a population study of postmenopausal women. Int J Obes Relat Metab Disord. 1990;14(7):583–591. [PubMed] [Google Scholar]
- 24.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, Hulley SB, Jacobs DR, Liu K, Manolio TA, Oberman A, Perkins LL, Savage PJ, Serwitz JR, Sidney S, Wagenknecht L. Cardiovscular risk factors in young adults. The CARDIA baseline monograph. Controlled Clin Trials. 1991;2:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 26.Hughes GH, Cutter G, Donahue R, Friedman, Hulley S, Hunkeler E, Jacobs DR, Liu K, Orden S, Pirie P, Tucker B, Wagenknecht L. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Controlled Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 27.Lewis CE, Jacobs DR, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 28.Weidner MD, Gavigan KE, Tyndall GL, Hickey MS, McCammon MR, Houmard JA. Which anthropometric indices of regional adiposity are related to the insulin resistance of aging? Int J Obes Relat Metab Disord. 1995;19:325–330. [PubMed] [Google Scholar]
- 29.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D, Jr, Liu K, Hubert H, Gernhofer N, Betz E, Havlik D. The CARDIA dietary history. J Am Diet Assoc. 1991;91(9):1104–1112. [PubMed] [Google Scholar]
- 30.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, Jacobs DR. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282:1539–1546. doi: 10.1001/jama.282.16.1539. [DOI] [PubMed] [Google Scholar]
- 31.Andersen N, Jacobs DR, Sidney S, Bild DE, Sternfeld B, Slattery ML, Hannan P. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Epidemiol. 1996;143:351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe WS, Sobal J, Olson C, Frongillo EA, Williamson DF. Parity-associated weight gain and its modification by sociodemographic and behavioral factors: a prospective analysis in US women. Int J Obes Relat Metab Disord. 1997;21:802–810. doi: 10.1038/sj.ijo.0800478. [DOI] [PubMed] [Google Scholar]
- 33.Lederman SA, Alfasi G, Deckelbaum RJ. Pregancy-associated obesity in black women in New York City. Matern Child Health J. 2002;6(1):37–42. doi: 10.1023/a:1014364116513. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine. Nutrition during pregnancy Part I Weight gain. Food and Nutrition Board. National Academy of Sciences; Washington, DC: 1990. [Google Scholar]
- 35.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90(4 Pt 1):483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- 36.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight, and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 37.Sidebottom AC, Brown JE, Jacobs DR. Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reproduct Biol. 2001;94:216–223. doi: 10.1016/s0301-2115(00)00329-8. [DOI] [PubMed] [Google Scholar]
- 38.Harris HE, Ellison GTH, Clement S. Relative importance of heritable characteristics and lifestyle in the development of maternal obesity. J Epidemiol Community Health. 1999;53:66–74. doi: 10.1136/jech.53.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohlin A, Rossner S. Trends in eating patterns, physical activity and sociodemographic factors in relation to postpartum body weight development. Br J Nutr. 1994;71:457–470. doi: 10.1079/bjn19940155. [DOI] [PubMed] [Google Scholar]
- 40.Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- 41.Clapp JF, Seaward BL, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. Am J Obstet Gynecol. 1988;159:1456–1460. doi: 10.1016/0002-9378(88)90574-1. [DOI] [PubMed] [Google Scholar]
- 42.Greenland S, Rothman KJ. Fundamental of epidemiologic data analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. 2. Lippincott-Raven Publishers; Philadelphia: 1998. [Google Scholar]
- 43.Okosun IS. Ethnic differences in the risk of type 2 diabetes attributable to differences in abdominal adiposity in American women. J Cardiovasc Risk. 2000;7:425–430. doi: 10.1177/204748730000700606. [DOI] [PubMed] [Google Scholar]


