Abstract
Background
Males with pancreatic cancer have decreased survival compared with females. Interestingly, perioperative blood transfusions have been shown to reduce survival in patients with pancreatic adenocarcinoma. Recent evidence incriminates blood transfusions from female donors as a causative factor in acute lung injury. We therefore hypothesize thatmalemice with pancreatic cancer will have greater tumor progression than female mice in response to transfusion.
Methods
Mice previously inoculated with pancreatic cancer cells received an intravenous injection of acellular plasma collected from single donor erythrocytes from either male or female donors. Control mice received an equal volume of intravenous saline. Necropsy to determine metastasis was performed in female mice at 4 wk status post-transfusion. The male group necessitated sacrifice at 3 wk post-transfusion due to clinical deterioration.
Results
Male mice developed more metastatic events than female mice, and this was accentuated when receiving blood from female donors. Male mice experienced weight loss within 2 wk of tail vein injection, and three mice in the male transfused groups died secondary to malignancy. Female mice did not manifest substantial weight loss, and did not die in the study time period.
Conclusion
Male mice, compared with female, had significantly more metastatic events following transfusion of plasma from stored erythrocytes in an immuno-competent murine model of pancreatic adenocarcinoma. Moreover, the adverse effect of transfusion was augmented with female donor blood. These data are consistent with clinical outcomes from centers of excellence in treating pancreatic cancer and warrant further investigation.
Keywords: transfusion, pancreatic cancer, metastasis, proliferation, erythrocytes, immunomodulation, gender difference
INTRODUCTION
Transfusion of autologous packed red blood cells (pRBCs) has been invoked in the pathogenesis of adverse outcome in multiple clinical settings, including following resection of malignancies [1–3]. In 1973, it was first observed that kidney transplant recipients who received autologous pRBCs had improved graft survival, which was presumed to be due to an immunosuppressive effect [4]. Ultimately this immune suppression was termed transfusion related immunomodulation (TRIM) [5]. In 1981, Gantt hypothesized that TRIM in cancer patients undergoing tumor resection could cause an increase in cancer growth [6]. Whether due to immune modulation or direct effects of other mediators in pRBCs on tumor growth and progression, it is generally believed that blood transfusions hasten cancer related death.
More than 150 studies have been conducted to determine the effect of blood transfusion on cancer progression [7]. Retrospective studies matching clinically important prognostic factors, such as stage and operative difficulty, have shown that patients receiving blood transfusion have more cancer recurrence and worse survival than those who do not receive blood [1, 2, 8]. Furthermore, it has been shown that transfusion requirement is not simply a surrogate marker of stage [1].
When specifically examining pancreatic adenocarcinoma, it has been shown that patients undergoing pancreaticoduodenectomy have better outcomes when they do not receive blood transfusion [9]. Similar deleterious effects of transfusions were seen with resection of periampullary cancers [10]. In these studies, blood transfusion was an independent negative prognostic indicator.
Despite many studies, however, it has been difficult to elucidate the precise role of transfusion in cancer recurrence possibly due to diversity of tumors, patient specific factors, transfusion requirements, and blood storage practices. Our laboratory has utilized an immunocompetent murine metastatic pancreatic cancer model [11] that controls for tumor burden, genetic diversity within the host, operative trauma, and the amount of blood transfused. We have previously shown with that blood products promote tumor cell migration and metastasis using an immunocompetent murine model [12, 13].
Provocatively, two seminal reports have recognized gender differences in long-term outcome following curative resection of pancreatic cancer. In a study of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas, the survival duration was greater for women than men [14]. Similarly, in a study of long-term survival of patients with pancreatic cancer, nine of the 12 patients who survived 5 y or longer were female [15].
Gender differences in donor blood have been proposed to play a role in the pathogenesis of transfusion related acute lung injury (TRALI) [16]. Patients receiving female donor fresh frozen plasma (FFP) have been observed to have a higher incidence of TRALI [17]. Although the exact mechanism is unclear, differences are speculated to be due to anti-white blood cell (WBC) antibodies and anti-human leukocyte antigen (HLA) antibodies produced from alloimmunization of multiparous female donors [18–20]. In fact, a policy change in which 90% of FFP was taken from male donors in Britain resulted in a reduction from 15.5 cases of TRALI per million units of FFP to 3.2 cases per million units [21]. Congruent results were seen in patients undergoing repair of ruptured abdominal aortic aneurysms in which the incidence of TRALI decreased after female plasma was excluded [22].
Based on these data, we hypothesize that in a murine model of metastatic pancreatic cancer, there will be gender differences in cancer progression, and that these differences will be accentuated by gender-specific donor pRBCs transfusion.
METHODS
Plasma Preparation from Stored pRBCs
Plasma was obtained from stored packed red cells as previously described [12]. After obtaining informed consent under a protocol approved by the Colorado Multi-Institutional Review Board, 450 mL of whole blood was collected from healthy male and female donors per American Association of Blood Banks (AABB) criteria [23]. The whole blood was separated into components and the packed red blood cells underwent prestorage leuko-reduced (LR) by filtration using a Pall BPF4TM leuko-reduction filter. These units were stored per AABB Guidelines for 42 d [24]. Samples from these pRBCs units were obtained via sterile couplers, and the plasma was separated by centrifugation [23]. Following plasma isolation a second spin (12,500 g for 5 min) was performed to remove acellular debris [25]. Plasma was aliquoted and stored at −80°C.
Metastatic Immunocompetent Murine Model of Pancreatic Cancer
Animal experiments were performed using the acellular plasma portion of leukoreduced (LR) pRBCs on day 42 (D.42) of storage from male and female donors. All murine studies were carried out according to the guidelines of the American Association for Accreditation of Laboratory Care and had IACUC approval at the University of Colorado at Denver. Mice underwent splenic injection of 2.5 × 105 GFP expressing Pan02 [26] cells as previously described [11], wherein a small left upper quadrant incision is made and the spleen is gently exteriorized such that Pan02 cells can be injected subcapsularly into the spleen using a 30 gauge needle. Mice (n = 4 or 5 per group) were randomized 1 wk post-tumor injection to receive a lateral tail vein (LTV) injection of saline, D.42 LR male donor plasma extract, or D.42 LR female donor plasma extract. Based on volume analysis of pRBCs, it is estimated that a 70 kg patient receives approximately 1 mL/kg of acellular plasma extract per 1 U blood transfusion; thus all animals were given a dose of 1 ml/kg of this acellular plasma diluted into 100 μL of saline. Mice were clinically followed, weighed 3 times per week and sacrificed 5 wk after the splenic injection of tumor cells. The male mice began to clinically decline and several died approximately 3 wk after the receipt of blood product, which necessitated their early sacrifice of males 1 wk prior to the planned sacrifice at 4 wk post-tumor cell injection. Necropsy was performed and extent of disease was quantified noting, visible metastatic disease, tumor size, and sequelae. Necropsy was performed by two individuals blinded to randomization (DB, CCB). Lesions were examined under blue light (485 nm) to confirm presence of GFP tumor cells. Visceral obstruction was assessed by of dilated viscera proximal to GFP positive implants.
Statistical Analysis
The data are expressed as mean ± the standard error of the mean. One-way ANOVA testing was performed to determine the significance of observed differences. Fisher’s PLSD was performed for post hoc comparisons. Statistical significance was determined at P < 0.05.
RESULTS
Animal Weight Change
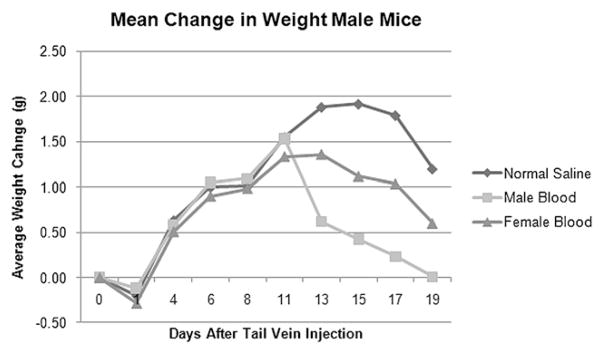
Mice were weighed three times per week following orthotopic injection of tumor cells. Male mice initially had weight gain, and then all groups began to lose weight at approximately 1.5 to 2 wk status post-tail vein injection (Fig. 1). Female mice gained weight for 2 wk following blood product transfusion and, in contrast, did not have subsequent weight loss (Fig. 2).
FIG. 1.
Male mice previously inoculated with pancreatic cancer cells that receiving normal saline, male donor blood, or female donor blood transfusions initially showed weight gain, but began to lose weight and clinically decline around d 11–15.
FIG. 2.
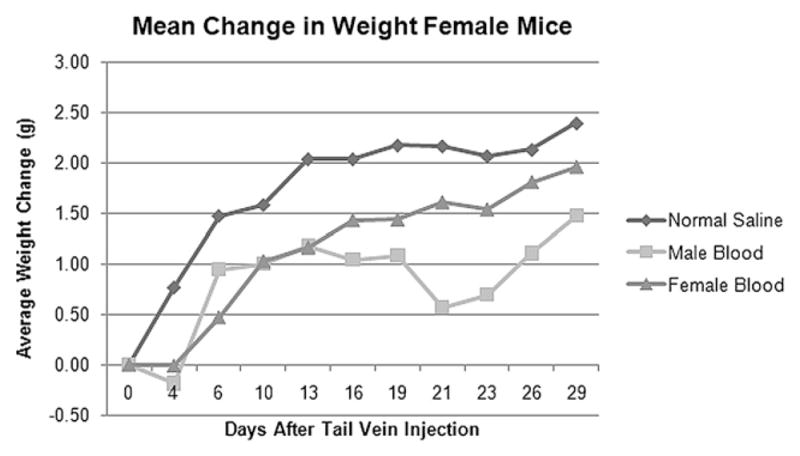
Female mice previously inoculated with pancreatic cancer cells experienced overall weight gain after transfusion of male donor blood, female donor blood, and normal saline.
Mortality
The male mice that received transfusion of the acellular portion of pRBCs from either female or male donors began to deteriorate 3 wk after transfusion. Two male mice that received male donor blood products died from bowel obstruction secondary to tumor metastasis on d 15 and d 19 post-transfusion. One male mouse that received female donor blood died on d 17 post-transfusion from the same cause. By contrast, there were no deaths in the female mice or the male mice receiving normal saline.
Metastatic Events
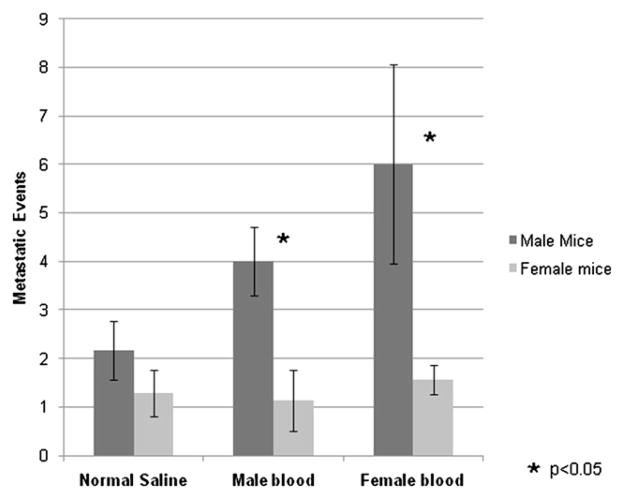
Metastatic events were determined by necropsy at 4 weeks post tail vein injection for female mice and 3 weeks post tail vein injection for male mice due to early mortality (Fig. 3). Male mice had significantly more metastatic events than did female mice in the two experimental groups that received the acellular plasma fraction from male or female donors (P <0.05). The augmented tumor progression appeared to be accentuated by transfusion of female blood, although this was not statistically significant. There was no difference seen between male and female mice that received normal saline (P = 0.5).
FIG. 3.
Male mice developed more metastatic events than female mice following transfusion of either male or female donor blood. No significant difference was observed observed in the control groups that received normal saline injections.
DISCUSSION
Although several large clinical studies suggest a negative impact of transfusion on mortality for patients with pancreatic cancer, the exact mechanism by which perioperative blood transfusion affects the progression of pancreatic cancer remains unclear [9,10]. Clinically, it is difficult to separate the effects of transfusion requirement from tumor burden, and patients display heterogeneity in both tumor biology and stage that can confound results. Furthermore, designing a randomized trial to assess the effects of blood transfusion on cancer progression is ethically problematic.
Using a model that controls for tumor burden, genetic variability, operative stress, and transfusion quantity, this study demonstrates that the transfusion of stored pRBCs promotes gender-specific augmentation of murine pancreatic cancer metastasis. Male mice experienced more extensive cancer progression compared with female mice following transfusion of blood products. Clinically, male mice appeared to deteriorate more rapidly and demonstrated considerable weight loss. Additionally, three male mice receiving pRBCs transfusions died secondary to cancer-related bowel obstruction while no female mice died. The observed worsened outcome in male mice mirrors the phenomenon seen in human pancreatic cancer patients, in which females appear to survive longer than males [14, 15]. Additionally, transfusion of female plasma appears to accentuate the observed discrepancy in metastasis between the male and female mice. Although this was not a statistically significant finding in this study, it merits further investigation, as this could affect clinical guidelines on blood procurement and transfusion.
Although a mechanism is yet to be uncovered, the observed gender differences in pancreatic cancer progression may be due in part or in whole to hormonal differences between male and female mice; alternatively, this may be due to protein differences in female blood or differences in Micro chimerism between male and female blood products [27, 28]. Previous authors have demonstrated that pancreatic parenchyma is gender steroid-dependent [29], expresses multiple androgen receptors [30], and that androgen blockade may potentially play a role in pancreas cancer therapy [31]. Based on these data, various clinical changes could enhance survival in male pancreatic cancer patients who require perioperative transfusions. If in fact estrogen levels are involved in the protective effect seen in women, then pharmacologic interventions could potentially be implemented in male patients such as estrogen replacement therapy. Conversely, antiandrogen therapy with either flutamide or LHRH antagonists might be effective if testosterone is playing a role in pathogenesis of tumor progression [31].
Error Discussion
These studies represent initial investigations of sexual dimorphism in transfusion-related pancreas cancer progression and despite the ability of the model to control for several important clinical factors, including genetic variability of patients, tumor inoculum, transfusion volume, and operative stress, there are multiple issues that will need to be further examined. The use of interspecies transfusion is problematic, although previous work by Silliman et al. examining the role of this acellular plasma fraction on promoting TRALI [32] has not demonstrated untoward inflammatory effects. It should be noted that very recently a model has been developed to store mouse blood that will potentially allow murine–murine transfusion, although a downside of this model is the significant number of mice that must be sacrificed in order to obtain the stored blood [33]. Although sex hormone differences appear to be a cogent explanation for the observed differences in these experiments, there is a paucity of data regarding sex hormones within stored blood. Clearly, there have been differences in observed outcomes (TRALI) in patients receiving male versus female blood product as mentioned [21, 22], and these mere observations were enough to institute transfusion policy changes in England regarding FFP with significant clinical benefits. Beyond sex hormones, there is the possibility that protein differences [27], or even microchimerism from transfusion [28], may play a significant role in the differences in tumor progression that we observed. Finally, these experiments were performed using a single cancer cell line, which may reflect idiopathic peculiarities of these cells. Despite the difficulties with the model, it is not the first preclinical model to demonstrate untoward effects of blood transfusion- previous work has been done using a metastatic murine fibrosarcoma model [34], and this model of pancreas cancer metastasis has been well established [35–37]. In sum, we believe these results reflect the current clinical situation of patients with pancreatic adenocarcinoma receiving perioperative transfusion, and warrant further investigation.
Acknowledgments
This work was supported in part by American Cancer Society grant no. MSRG-09-034-01 CCE (CCB)
References
- 1.Blumberg N, Chuang-Stein C, Heal JM. The relationship of blood transfusion, tumor staging, and cancer recurrence. Transfusion. 1990;30:291. doi: 10.1046/j.1537-2995.1990.30490273432.x. [DOI] [PubMed] [Google Scholar]
- 2.Wobbes T, Joosen KHG, Kuypers JHC, et al. The effect of packed cells and whole blood transfusions of survival after curative resection for colorectal carcinoma. Dis Colon Rectum. 1989;32:743. doi: 10.1007/BF02562121. [DOI] [PubMed] [Google Scholar]
- 3.Shirwadkar S, Blajchman MA, Frame B, et al. Effect of allogeneic blood transfusion on solid tumor growth and pulmonary metastases in mice. J Cancer Res Clin Oncol. 1992;118:176. doi: 10.1007/BF01410130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opelz G, Teraski P. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 5.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): An update. Blood Rev. 2007;21:327. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Gantt C. Red blood cells for cancer patients. Lancet. 1981;2:363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 7.Dionigi G, Rovera F, Boni L, et al. The impact of perioperative blood transfusion on clinical outcomes in colorectal surgery. Surg Oncol Colorectal Cancer Biol Diagn and Ther. 2007;16(Suppl 1):177. doi: 10.1016/j.suronc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Vamvakas EC. Perioperative blood transfusion and cancer recurrence: Meta-analysis for explanation. Transfusion. 1995;35:760. doi: 10.1046/j.1537-2995.1995.35996029162.x. [DOI] [PubMed] [Google Scholar]
- 9.Yeh JJ, Gonen M, Tomlinson JS, et al. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumor of the pancreas. Br J Surg. 2007;94:466. doi: 10.1002/bjs.5488. [DOI] [PubMed] [Google Scholar]
- 10.Park SJKS, Jang JY, Lee KU, et al. Intraoperative Transfusion: Is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002;26:487. doi: 10.1007/s00268-001-0254-6. [DOI] [PubMed] [Google Scholar]
- 11.Roland CL, Dineen SP, Toombs JE, et al. Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic Xenografts. Exp Biol Med. 2010;235:263. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 12.Barnett CC, Beck A, Halloway S, et al. Intravenous delivery of the plasma fraction of stored packed red blood cells promotes pancreatic cancer growth in immunocompetent mice. Cancer. doi: 10.1002/cncr.25140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dineen SP, Roland CL, Toombs JE, et al. The acellular fraction of stored platelets promotes tumor cell invasion. J Surg Res. 2009;153:132. doi: 10.1016/j.jss.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: Treatment variables and survival duration. Ann Surg Oncol. 2001;8:123. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 15.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma: Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palfi M, Berg S, Emerudh J, et al. A randomized controlled trail of transfusion-related acute lung injury: Is plasma from multiparous blood donors dangerous? Transfusion. 2001;41:317. doi: 10.1046/j.1537-2995.2001.41030317.x. [DOI] [PubMed] [Google Scholar]
- 17.Triulzi DJ. Transfusion-related acute lung injury: Current concepts for the clinician. Anesth Analg. 2009;108:770. doi: 10.1213/ane.0b013e31819029b2. [DOI] [PubMed] [Google Scholar]
- 18.Gajic O, Yilmaz M, Iscimen R, et al. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components. Crit Care Med. 2007;35:1645. doi: 10.1097/01.CCM.0000269036.16398.0D. [DOI] [PubMed] [Google Scholar]
- 19.Eder AF, Herron R, Strupp A, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 20.Imoto S, Araki N, Shimada E, et al. Comparison of acute nonhemolytic transfusion reactions in female and male patients receiving female or male blood components. Transfus Med. 2007;17:455. doi: 10.1111/j.1365-3148.2007.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman CE, Stainsby D, Jones H, et al. Serious Hazards of Transfusion Steering Group. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:402. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 22.Wright SE, Snowden CP, Athey SC, Leaver, et al. Acute lung injury after ruptured abdominal aortic aneurysm repair: The effect of excluding donations from females from the production of fresh frozen plasma. Crit Care Med. 2008;36:1796. doi: 10.1097/CCM.0b013e3181743c6e. [DOI] [PubMed] [Google Scholar]
- 23.Technical Manual. American Association of Blood Banks; Bethesda: 2003. [Google Scholar]
- 24.Technical Manual. 14. American Association of Blood Banks; Bethesda: 2002. [Google Scholar]
- 25.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684. [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717. [PubMed] [Google Scholar]
- 27.Skornicka EL, Kiyatkina N, Weber MC, et al. Pregnancy zone protein is a carrier and modulator of placental protein-14 in T-cell growth and cytokine production. Cell Immunol. 2004;232:144. doi: 10.1016/j.cellimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Reed W, Lee TH, Norris PJ, et al. Transfusion-associated microchimerism: A new complication of blood transfusions in severely injured patients. Semin Hematol. 2007;44:24. doi: 10.1053/j.seminhematol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Robles-Diaz G, Duarte-Rojo A. Páncreas: A sex steroiddependent tissue. IMAJ. 2001;3:364. [PubMed] [Google Scholar]
- 30.Konduri S, Schwarz MA, Cafasso D, et al. Androgen receptor blockade in experimental combination therapy of pancreatic cancer. J Surg Res. 2007;142:378. doi: 10.1016/j.jss.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Greenway BA. Effect of flutamide on survival in patients with pancreatic cancer: Results of a prospective, randomized, double blind, placebo-controlled trial. BMJ. 1935;1998:316. doi: 10.1136/bmj.316.7149.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998:1011458. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and post-transfusion in vivo survival. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02173.x. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordin JO, Bardossy L, Blajchman MA. Growth enhancement of established tumors by allogeneic blood transfusion in experimental animals and its amelioration by leukodepletion: The importance of the timing of the leukodepletion. Blood. 1994;84:344. [PubMed] [Google Scholar]
- 35.Dineen SP, Sullivan LA, Beck AW, et al. The anectine CT-322 is a novel VEGF receptor 2 inhibitor that decreases tumor burden in an orthotopic mouse model of pancreatic cancer. BMC Cancer. 2008;27:352. doi: 10.1186/1471-2407-8-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck AW, Luster TA, Miller AF, et al. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639. doi: 10.1002/ijc.21684. [DOI] [PubMed] [Google Scholar]
- 37.Fleming JB, Brekken RA. Functional imaging of angiogenesis in an orthotopic model of pancreatic cancer. J Cell Biochem. 2003;90:492. doi: 10.1002/jcb.10644. [DOI] [PubMed] [Google Scholar]