Abstract
Introduction
Perioperative blood transfusion has been linked to decreased survival for pancreas cancer. Noting clinical data associating female blood products with increased morbidity, our lab has demonstrated that transfusion of female blood augments metastatic events compared to male blood in an immunocompetent murine pancreatic cancer model. It has been suggested that tumor-associated macrophages correlate with tumor progression by promoting angiogenesis. More recently, tumor-associated neutrophils have been implicated in aggressive tumor behavior. We hypothesize that differences in gender-specific transfusion-mediated pancreatic cancer progression are due to microenvironmental changes within the tumor. To test this hypothesis, we examined tumor-associated neutrophils and macrophage ratios in male and female mice with pancreatic cancer receiving blood transfusion from male or female donors.
Methods
C57/BL6 mice, age 7–9 weeks, underwent splenic inoculation with 2.5×105 PanO2 murine pancreatic adenocarcinoma cells. Mice were transfused on post-op day 7 with 1 ml/kg supernatant from day 42 male or female packed red cells. Necropsy was performed at 5 weeks or earlier for clinical deterioration, and tumors harvested. Frozen sections (5 μm) were stained for neutrophils and macrophages by immunofluorescence. Data were analyzed using ANOVA; p≤0.05 was used to determine significance; N≥3 per group.
Results
Clinically, male mice had greater morbidity and mortality than female mice when receiving female blood products, with roughened hair coat, development of ascites and death due to bowel obstruction. In evaluating the tumor microenvironment from mice receiving female blood products, male mice were noted to have a greater neutrophil to macrophage ratio than female mice, 0.176±0.028 vs. 0.073±0.012, p=0.03. When examining neutrophil to macrophage ratio in mice receiving male blood products, no difference was noted (p=0.48).
Conclusions
Male mice with pancreas cancer have greater morbidity than female mice when receiving female blood products. Furthermore, the difference in neutrophil to macrophage ratio suggests that gender-specific blood transfusion promotes aggressive tumor behavior in male mice via microenvironmental changes. These data warrant further study to delineate sex-related differences in pancreatic cancer progression.
Keywords: Transfusion, Pancreas cancer, Metastasis, Erythrocytes, Immunomodulation, Neutrophil to macrophage ratio
Introduction
Perioperative blood transfusion has been linked to negative outcomes in patients undergoing pancreaticoduodenectomy for cancer.1–4 However, a causal mechanism has not been elucidated. Allogeneic blood transfusions expose patients to foreign cells, antigens, and cytokines and lipid mediators that may promote tumor growth or affect the host immune system. This phenomenon is termed transfusion-related immunomodulation (TRIM).5 The affect of blood transfusion on immune function was first described in 1973 wherein it was noted that renal allografts had prolonged survival in patients who received transfusions preoperatively.6 Gantt later proposed that transfusion may increase tumor progression.7 Since the 1980s, many trials have examined TRIM effects on cancer progression, with similar outcomes.
When specifically examining pancreatic cancer, there is evidence that males perform more poorly than females.3,8,9 Our laboratory has recently shown in an immunocompetent murine pancreatic cancer–transfusion model that male mice perform worse than females and have more metastatic events.10 This was particularly noted in males who received a transfusion from a female donor. Previous work has demonstrated gender-related dimorphism in the area of transfusion-related acute lung injury (TRALI) with female blood products being deleterious.11–16 Although the exact mechanism is unclear, differences may be due to anti-white blood cell (WBC) antibodies or anti-human leukocyte antigen (HLA) antibodies produced from alloimmunization of multiparous female donors.13–15 Based on these data, we hypothesize that differences in gender-specific transfusion-mediated pancreatic cancer progression are due to microenvironmental changes in the inflammatory cells within the tumor.
Recent work has shown that increasing tumor-associated macrophages and tumor-associated neutrophils (TAMs and TANs, respectively) promote tumor growth and invasiveness.17–19 Neutrophils in particular have been implicated in being effector cells in the tumor microenvironment and have been shown to be integral in the initial angiogenic switch, which preceded the metastatic phenotype.19 Queen et al. have demonstrated that TANs correlate with tumor progression by promoting angiogenesis and cell detachment in an in vitro model of breast cancer.20 Our laboratory has also shown that neutrophils aggregate at the leading edge of tumors, likely playing a role in tumor metastasis.21 We propose that TAMs and TANs may be sensitive to the effects of TRIM with TANs acting as the principle effector cell.
Methods
Plasma Preparation from Stored pRBCs
With institutional review board-approved informed consent, seven healthy donors donated 450 ml of whole blood as per American Association of Blood Banks criteria.22 Preparation of the acellular portion of packed red cells has been described previously.23
Murine Pancreatic Adenocarcinoma Culture
GFP-expressing PanO2 cells were maintained at 37°C in a mixture of 5% CO2 and 95% air in RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin–streptomycin (Invitrogen). Subculture was performed when cells reached 70–90% confluence. Cells were harvested after washing with HEPES-buffered saline solution (Lonza, Walkersville, MD), using trypsin–EDTA (Lonza) for 5 min, and adding trypsin-neutralizing solution (Lonza). Cells were centrifuged at 1,000×g for 10 min, counted, and reconstituted in saline at a concentration of 5×106 cells per milliliter to provide a tumor inoculation of 2.5×105 cells per 50 μl.
Immunocompetent Murine Metastatic Model of Pancreatic Adenocarcinoma
Experiments were performed in groups of male and female C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME) using the acellular component (plasma) of pRBCs from male and female donors. All studies were performed under the guidelines of the University of Colorado at Denver Institutional Animal Care and Use Committee (IACUC). After being allowed to acclimate, mice underwent general anesthesia and were inoculated by a subcapsular splenic injection of 2.5×105 GFP-expressing PanO2 cells. Mice were then randomized 1 week post-tumor inoculation and received a lateral tail vein (LTV) injection of saline control, blood product from male donors, or blood product from female donors. All animals were given a dose of 1 ml/kg acellular plasma diluted into 50 μl of saline. Mice were clinically followed up,24 weighed three times weekly, and sacrificed 5 weeks after injection of tumor cells (or 4 weeks after transfusion) or earlier if there was clinical deterioration per IACUC regulations. Non-tumor-bearing mice were maintained for 4 weeks after LTV injection of plasma extract to observe for any adverse effects from injection of human plasma.
Necropsy was performed and extent of disease was quantified noting the number of gross metastatic events and clinical tumor sequelae such as ascites, bowel obstruction, jaundice, and biliary obstruction. Mice were randomized at the time of necropsy to limit interpreter bias. Organs were harvested for tissue staining, preserved in tissue freezing media (Fischer Scientific, Pittsburgh, PA), frozen, and stored at −80°C.
Tissue Immunofluorescence
Tumors from all groups of animals were examined for the presence of neutrophils and macrophages using immunofluorescence (IF). Sectioning and staining was performed in 5-μm serial sections of frozen liver segments. Slides were fixed in a solution of 30% acetone and 70% methanol for 10 min and air dried for 2 min. After three washes with phosphate buffer solution (PBS), they were again fixed in 4% paraformaldehyde. After three washes in PBS, slides were blocked with 10% donkey serum for 30 min. They were then incubated in primary antibody, either rat anti-mouse PMN (clone 7/4; ABD Serotec, Oxford, UK) or rat anti-mouse CD68 (ABD Serotec) overnight at 4°C. They were then washed in PBS three times and incubated at room temperature in a dark environment with secondary antibodies of a donkey anti-rat CY3 IgG (imaged on the red channel), Alexa Fluor 488-labeled conjugate wheat germ agglutinin (Molecular Probes, Invitrogen), and bisBenimide H33342 trihydrochloride (Sigma-Aldrich, St. Louis, MO) for 60 min. Slides were then washed with PBS three times, rinsed with distilled water, and air dried. They were mounted with quench medium and sealed with nail polish. Slides were then examined with a Leica DMRXA digital microscope (Leica Mikroskopie und Syteme, Wetzlar, Germany), and three random pictures were taken of both areas of tumor as well as the normal surrounding liver parenchyma using the SlideBook 2.6 software (I.I.I., Denver, CO). Neutrophil and macrophage signaling was determined by two different methods. First, signals were counted by hand, with number of cells providing signal per high-power field. Antibody signal was then calculated by SlideBook using the area of the stain signal for neutrophils and macrophages compared with the total area of the picture.
Statistical Analysis
The data are expressed as the mean ± the standard error of the mean. One-way ANOVA testing was performed to determine the significance of observed differences with Fisher’s exact testing for post hoc comparisons. Statistical significance was determined as p<0.05; n≥10 per group for clinical data, n≥3 for tissue IF groups with three sections per mouse.
Results
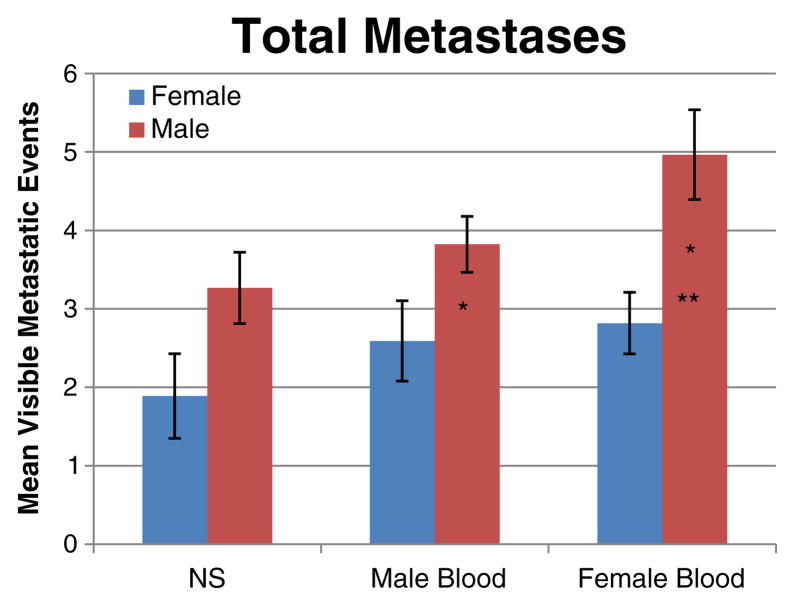
Clinically, male mice had greater morbidity and mortality when receiving female blood product, with roughened hair coat, development of ascites, and death due to bowel obstruction. Seventeen percent of male mice died (when able to examine—this was due to malignant bowel obstruction with perforation). The earliest death due to malignant cause was 9 days post-transfusion. All groups of evaluated male mice were necessarily sacrificed early, between days 21 and 25 post-transfusion due to excessive morbidity (ascites) according to IACUC regulations. Female mice groups were sacrificed at 4 weeks post-transfusion. Control male mice had a trend of more metastatic events than female mice, 3.27±0.45 vs. 1.89± 0.54 (p=0.064). Males had significantly more metastatic events compared with females when receiving male blood product, 3.82±0.36 vs. 2.59±0.51 (p=0.048), and female blood product, 4.96±0.57 vs. 2.82±0.39 (p=0.005; Fig. 1).
Fig. 1.
Total metastatic events in male and female mice receiving male blood, female blood, or saline control. Male mice receiving male or female blood had significantly more metastatic events compared with females (one asterisk). Males who received female blood also had significantly more metastases than males receiving saline (two asterisks). Gender differences in the saline control did not reach significance
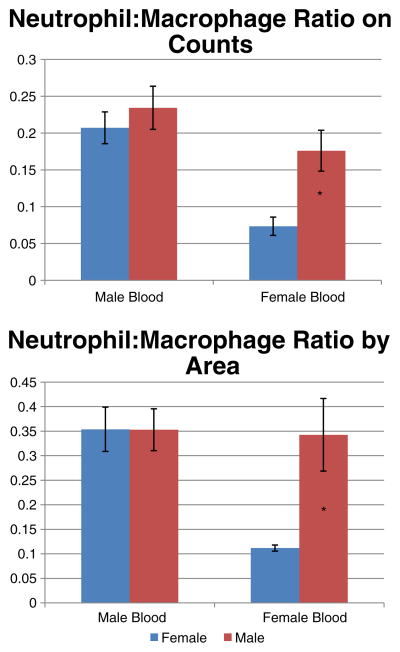
In evaluating the tumor microenvironment, counts of neutrophils and macrophages were examined by tissue IF (Fig. 2). When examining neutrophil to macrophage ratios in male and female mice receiving male blood product, there was no comparative difference. However, in mice receiving female blood product, male mice were noted to have a greater neutrophil to macrophage ratio than females, 0.176±0.028 vs. 0.073±0.012 (p=0.03; Fig. 3), which may correlate with the poorer outcomes observed clinically. The signal area of both neutrophils and macrophages were compared with the total area of the high-power field and confirmed the above findings, as in mice receiving female blood product, male mice again had a greater neutrophil to macrophage ratio than female mice, 0.343±0.074 vs. 0.112±0.006 (p=0.046).
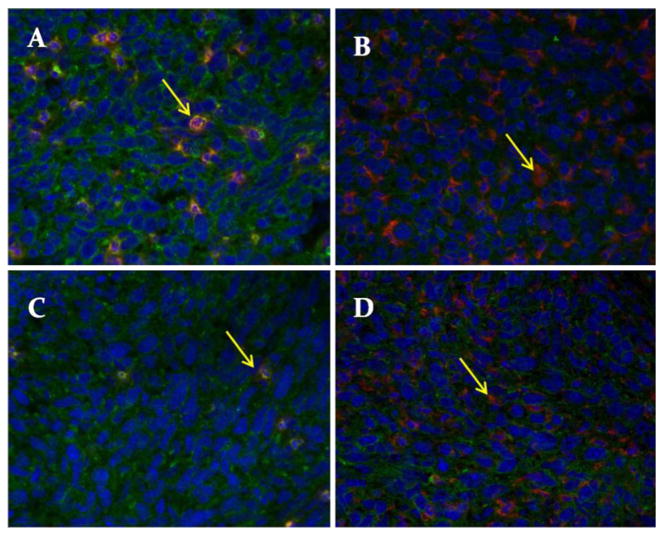
Fig. 2.
Representative photomicrographs. a Neutrophil stain from male mouse. b Macrophage stain from male mouse. c Neutrophil stain from female mouse. d Macrophage stain from female mouse. Arrows indicate cell signal (red), blue stain is the nuclear stain (H33342), and green stain is wheat germ agglutinin
Fig. 3.
Neutrophil to macrophage ratio by counts and by area. Male mice receiving female blood had a significantly higher neutrophil to macrophage ratio than females receiving female mice receiving female blood (*p<0.05 in both). No gender differences were noted in mice receiving male blood product
Discussion
Limitations in clinical trials for pancreatic cancer, overall poor prognosis, small sample sizes, and clinical variation inherent among patients such as stage and operative difficulty make it complicated to elicit prognostic factors that can impact survival. Therefore, preclinical models offer great opportunities to control for many of these variables and study the effects of TRIM on tumor biology in pancreatic adenocarcinoma. Herein, we have developed an immunocompetent murine metastatic pancreatic cancer model21 that allows for control of tumor burden, genetic diversity, operative trauma, and the amount of transfusion. Given the potential for gender differences in pancreatic cancer outcomes, we examined the gender-related dimorphism in blood transfusion.
The results of this pilot study indicate that male recipients of female blood products have the highest comparative tumor neutrophil to macrophage ratios and males receiving female blood had the worst clinical outcomes. This suggests that the immunomodulatory effects of blood transfusion may alter the inflammatory cell makeup of the tumor microenvironment and may be a mechanism behind the gender differences that we have observed.
Previous investigations evaluating the interaction between cancer cells and surrounding inflammatory cells17–19 have shown that increased TAMs correlate with poorer outcomes.18 The cytokines produced by macrophages may lead to a permissive environment via proteases and growth factors.18 Unlike other macrophages, TAMs exhibit an alternatively activated phenotype, which has the ability to produce factors that suppress T-cell proliferation and facilitate tumor growth.25 A recent in vitro model of murine lung adenocarcinoma showed that alternatively activated macrophages have high expression of VEGF-C and that co-incubation with cancer cells enhanced lymphangiogenesis.25 Neutrophils have also been investigated as effector cells in tumor progression and have been shown to be critical in the angiogenic switch.19 Neutrophils are capable modulators in metastatic capability.21,26,27 These two inflammatory cells may act in concert to promote tumor progression, wherein the macrophages recruit the neutrophils as effectors in breaking down the extracellular matrix to cell detachment and invasion via matrix metalloproteases and VEGF. Clinically, the balance of neutrophils and macrophages play crucial roles in the control of pulmonary infections where increased neutrophils in relation to macrophages are necessary to eliminate parasite infection.28 In examining colorectal liver metastases, high ratios of circulating neutrophils to lymphocytes are linked to poorer outcomes,29–31 suggesting that the ratio of effector cells, not gross numbers, play an important role in the tumor metastatic phenotype. Although controversial, ratios between different inflammatory cells have been linked to clinical outcomes and suggest that microenvironmental neutrophil to macrophage ratios are an important area of investigation.
There are some limitations of this study. A xenotransfusion of human blood product may affect outcomes and may lead to inflammatory and immunological responses in the innate mouse immune system. However, in studying TRALI, it has been shown that giving fresh human blood product is safe and does not lead to a significant inflammatory injury in rats.32 In this pilot trial examining the microenvironment of immunocompetent mice with pancreas cancer receiving either male or female blood product, there were significant gender-specific differences in clinical outcomes. In particular, male mice receiving female blood had severe clinical sequelae, including ascites, bowel obstruction, and significantly more metastatic events than their female counterparts. Finding a significant comparative difference in the neutrophil to macrophage ratio, we believe this may explain the clinical observations seen. Further examination of the neutrophil to macrophage ratio in males and female mice receiving male blood however showed similar but high ratios. The reason for these findings is unclear as these groups performed clinically better. It is possible that cross-gender transfusion of male blood into females elevated the neutrophil to macrophage ratio in females and males with pancreas cancer may have higher ratios in general. However, male mice receiving female blood performed extremely poorly with one mouse dying as soon as 9 days post-transfusion. It is our belief that, if these animals had been allowed to live longer, the neutrophil ratio would have been even higher in this group. In any event, the significant gender dimorphism observed in male mice receiving female blood and the comparative difference in neutrophil to macrophage ratio in this group warrants further study.
Conclusion
In summary, male mice receiving female blood product have the worst clinical outcomes and the highest comparative neutrophil to macrophage ratio in the tumor when compared with females, with associated higher morbidity. These data suggest that neutrophils are important effector cells in mediating tumor progression, and a threshold between increased neutrophils compared with macrophages may trigger an aggressive metastatic phenotype. These data warrant further study to delineate sex-related differences in pancreatic cancer progression.
Acknowledgments
This work was supported in part by the American Cancer Society, grant number MSRG-09-034-01-CCE.
Contributor Information
Douglas D. Benson, Denver Health Medical Center, 777 Bannock Street, Denver, CO 80204-0206, USA. Department of Surgery, University of Colorado, Aurora, CO, USA
Marguerite R. Kelher, Bonfils Blood Center, Denver, CO, USA
Xianzhong Meng, Department of Surgery, University of Colorado, Aurora, CO, USA.
David A. Fullerton, Department of Surgery, University of Colorado, Aurora, CO, USA
Joon H. Lee, Department of Surgery, University of Colorado, Aurora, CO, USA
Christopher C. Silliman, Department of Surgery, University of Colorado, Aurora, CO, USA. Bonfils Blood Center, Denver, CO, USA
Carlton C. Barnett, Jr., Email: Carlton.Barnett@dhha.org, Denver Health Medical Center, 777 Bannock Street, Denver, CO 80204-0206, USA. Department of Surgery, University of Colorado, Aurora, CO, USA. Bonfils Blood Center, Denver, CO, USA
References
- 1.Yeh J, Gonen M, Tomlinson J, Indrees K, Brennan M, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumor of the pancreas. Br J Surg. 2007;94:466–72. doi: 10.1002/bjs.5488. [DOI] [PubMed] [Google Scholar]
- 2.Park S, Kim S, Jang J, Lee K, Park Y. Intraoperative transfusions: Is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002;26:487–92. doi: 10.1007/s00268-001-0254-6. [DOI] [PubMed] [Google Scholar]
- 3.Cameron J, Crist D, Sitzmann J, Hruben R, Boitnott J, Seidler A, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–5. doi: 10.1016/0002-9610(91)90371-j. [DOI] [PubMed] [Google Scholar]
- 4.Millikan K, Deziel D, Silverstein J, Kanjo T, Christein J, Doolas A, Prinz R. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–24. [PubMed] [Google Scholar]
- 5.Vamvakas E, Blajchman M. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21(6):327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Opelz G, Senger D, Mickey M, Terasaki P. Effect of blood transfusion on subsequent kidney transplants. Transfusion Proc. 1973;5:253–9. [PubMed] [Google Scholar]
- 7.Gantt C. Red blood cells for cancer patients. Lancet. 1981:363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 8.Breslin T, Hess K, Harbison D, Jean M, Cleary K, Dackiw A, Wolff R, Abbruzzese J, Janjan N, Crane C, Vauthey J, Lee J, Pisters P, Evans D. Neoadjuvant chemotherapy for adenocarcinoma of the pancreas: Treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–32. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 9.Conlon K, Klimstra D, Brennan M. Long-Term Survival After Curative Resection for Pancreatic Ductal Adenocarcinoma: Clinicopathologic Analysis of 5-Year Survivors. Ann Surg. 1996;223(3):273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore P, Benson D, Kelher M, Moore E, Fragoso M, Silliman C, Barnett C. The plasma fraction of stored erythrocytes augments pancreatic cancer metastases in male versus female mice. J Surg Res. doi: 10.1016/j.jss.2010.05.047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palfi M, Berg S, Emerudh J, Berlin G. A randomized Controlled Trail of transfusion-related acute lung injury: is plasma from multiparous blood donors dangerous? Transfusion. 2001;41(3):317–322. doi: 10.1046/j.1537-2995.2001.41030317.x. [DOI] [PubMed] [Google Scholar]
- 12.Triulzi D. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108(3):770–776. doi: 10.1213/ane.0b013e31819029b2. [DOI] [PubMed] [Google Scholar]
- 13.Gajic O, Yilmaz M, Iscimen R, Kor D, Winters J, Moore S, Afessa B. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components. Crit Care Med. 2007;35(7):1645–8. doi: 10.1097/01.CCM.0000269036.16398.0D. [DOI] [PubMed] [Google Scholar]
- 14.Eder A, Herron R, Strupp A, Dy B, Notari E, Chambers L, Dodd R, Benjamin R. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47(4):599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 15.Imoto S, Araki N, Shimada E, Saigo K, Nishimura K, Nose Y, Bouike Y, Hashimoto M, Mito H, Okazaki H. Comparison of acute non-haemolytic transfusion reactions in female and male patients receiving female or male blood components. Transfus Med. 2007;17(6):455–65. doi: 10.1111/j.1365-3148.2007.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman C, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson L Serious Hazards of Transfusion Steering Group. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):402–5. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 17.Coussens L, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard J. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 19.Nozawa H, Chui C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queen M, Ryan R, Holzer R, Keller-Peck C, Jorcyk C. Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res. 2005;65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 21.Roland C, Dineen S, Toombs J, Carbon J, Smith C, Brekken R, Barnett C. Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreas xenografts. Exp Biol Med. 2010;235:263–70. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 22.Technical Manual. American Association of Blood Banks; Bethesda: 2003. [Google Scholar]
- 23.Barnett C, Beck A, Holloway S, Kehler M, Schluterman M, Brekken R, Fleming J, Silliman C. Intravenous delivery of the plasma fraction of stored packed red cells promotes pancreatic cancer growth in immunocompetent mice. Cancer. doi: 10.1002/cncr.25140. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Animal Resources Body Weight Study of 1993. The Jackson Labs; 1993. [Google Scholar]
- 25.Zhang B, Wang J, Gao J, Guo Y, Chen X, Wang B, Gao J, Rao Z, Chen Z. Alternatively activated RAW264.7 macrophages enhance tumor lymphangiogenesis in mouse lung adenocarcinoma. J Cell Bio. 2009;107:134–43. doi: 10.1002/jcb.22110. [DOI] [PubMed] [Google Scholar]
- 26.Roland C, Harken A, Sarr M, Barnett C. ICAM-1 expression determines malignant potential of cancer. Surgery. 2007;141:705–7. doi: 10.1016/j.surg.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Strell C, Lang K, Niggemann B, Zaenker K, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316:138–48. doi: 10.1016/j.yexcr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Novais F, Santiago R, Bafica A, Khouri R, Afonso L, Borges V, Brodskyn C, Barral-Netto M, Barral A, de Oliveira C. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183:8088–98. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 29.Kishi Y, Kopetz S, Chun Y, Palavecino M, Abdalla E, Vauthey J. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–22. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 30.Lui H, Lui G, Bao Q, Sun W, Bao H, Bi L, Wen W, Lui Y, Wang Z, Yin X, Bai Y, Hu X. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in rectal carcinoma. J Gastrointest Cancer. 2010 doi: 10.1007/s12029-009-9125-4. Feb 2 Epub. [DOI] [PubMed] [Google Scholar]
- 31.Halazun K, Aldoori A, Malik H, Al-Mukhtar A, Prasad K, Toogood G, Lodge J. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Silliman C, Bjornsen J, Wyman T, Kelher M, Allard J, Bieber S, Voelkel N. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]