Abstract
Allopregnanolone (ALLO) is a neurosteroid that has many functions in the brain, most notably neuroprotection and modulation of gamma-amino butyric acid (GABA) neurotransmission. Using a mouse model of cardiac arrest and cardiopulmonary resuscitation, we have previously demonstrated that ALLO protects cerebellar Purkinje cells (PCs) from ischemia in a GABAA receptor-dependent manner. In this study we examined the effect of sex on ALLO neuroprotection, observing that low dose ALLO (2 mg/kg) provided greater neuroprotection in females compared to males. At a higher dose of ALLO (8 mg/kg), both sexes were significantly protected from ischemic damage. Using an acute cerebellar slice preparation, whole cell voltage clamp recordings were made from PCs. Spontaneous inhibitory postsynaptic currents (IPSCs) were analyzed and the response to physiological ALLO (10 nM) was significantly greater in female PCs compared to male. In contrast, recordings of miniature IPSCs, did not exhibit a sex difference in response to ALLO, suggesting that ALLO affects males and females differentially through a mechanism other than binding postsynaptic GABAA receptors. We conclude that the female brain has greater sensitivity to ALLO mediated potentiation of GABAergic neurotransmission, contributing to increased neuroprotection.
Keywords: allopregnanolone, GABAA receptor, Purkinje cell, sex difference, ischemia, neuroprotection
1. Introduction
Cardiac arrest requiring cardiopulmonary resuscitation (CA/CPR) is a leading cause of death and disability in the United States, affecting over 300,000 adults and children in the United States each year (Roger et al., 2010). Gender is a significant factor influencing incidence and possibly outcome, with women being relatively protected compared to men (Kim et al., 2001; Rosamond et al., 2008; Vukmir, 2003; Wigginton et al., 2002). Animal models of cerebral ischemia, including CA/CPR, mimic the human epidemiology, exhibiting sex-specific differences in tissue damage and long-term behavioral recovery (For Review See Herson and Hurn, 2010). Innate protection from ischemic damage in the female is due in large part to endogenous levels of sex steroids, the estrogens and progesterone. In addition to the estrogens and progesterone, there is emerging evidence that metabolites of these hormones have important physiological and pathophysiological roles in the brain (For review see Liu et al., 2010).
The neurosteroid allopregnanolone (ALLO) is a metabolite of progesterone that is neuroprotective in several animal models of neurodegenerative diseases including Alzheimer’s (Brinton and Wang, 2006), traumatic brain injury (TBI) (Djebaili et al., 2004), stroke (Sayeed et al., 2006), and recently cardiac arrest (Kelley et al., 2008). Cerebral ischemia, as experienced following CA/CPR results in selective damage to vulnerable neuronal populations, including hippocampal CA1 neurons (Pulsinelli et al., 1982) and cerebellar Purkinje cells (Ardeshiri et al., 2006; Brasko et al., 1995; Fonnum and Lock, 2000; Horn and Schlote, 1992). We have previously demonstrated that ALLO can protect cultured PCs from in vitro ischemia, oxygen-glucose deprivation, and global ischemia in vivo, in part by potentiating GABAA receptor activity (Ardeshiri et al., 2006) and also by preserving GABAA receptor protein and function (Kelley et al., 2008). To date, the interaction between animal sex and ALLO has not been examined in the context of neuroprotection. In the current study, we tested whether there is a sex difference in ALLO neuroprotection of PCs and we examined the ability of ALLO to enhance GABAergic neurotransmission in the cerebellum.
2. Methods
2.1. Cerebellar Slice Preparation
All animal experiments were performed in accordance with the National Institutes of Health guidelines for care and use of Laboratory animals and approved by the Oregon Health and Science University Animal Care and Use Committee. Adult (8–16 wk old) C57/Bl6 male and female mice (Charles River) were anesthetized by i.p. injection of a ketmine/xylazine (80/12 mg/ml, Sigma Aldrich) cocktail and transcardially perfused with ice cold oxygenated cutting solution (see 2.3). Mice were decapitated, cerebellum removed, and sagittal slices (400 μm thick) were cut from the vermis of the cerebellum with a vibroslicer (Vibratome or Leica). Slices were incubated at 37°C in warm oxygenated artificial cerebrospinal fluid (ACSF, see 2.3) for 30 minutes then stored at room temperature. All experiments were performed at room temperature and completed within 4–5 hours of slicing to ensure cell viability.
2.2. Electrophysiology
Whole cell voltage clamp recordings were made from the soma of PCs using an Axopatch 200B (Axon Instruments, Union City, CA) amplifier interfaced to a Dell computer (Dell, Round Rock, TX). Data was collected using pCLAMP9 (Molecular Devices, Sunnyvale, CA) at a sample frequency of 20 kHz, with lowpass filtering at 2 kHz. Electrodes pulled from borosilicate glass capillaries with inner filaments using a Flaming Brown electrode puller (Sutter Instrument Co, Novato, CA) had resistances of 2–4 MΩ when filled with a CsCl internal pipette solution (see 2.3). Whole cell capacitance and resistance were electronically compensated. Adequate whole cell access (Ra < 30 MΩ) was verified at the beginning of the recording, before recording in the presence of ALLO, and at the end of recording. Slices were continuously perfused with aerated ACSF using a gravity fed perfusion system with a flow rate of 1–2 ml/minute.
Purkinje cells were voltage-clamped at a membrane potential of −60 mV and GABA-mediated spontaneous inhibitory post synaptic currents (sIPSCs) were recorded as inward currents (Herson et al., 2003; Konnerth et al., 1990). IPSCs were recorded in 3 minute sweeps. TTX (250 nM) was bath applied for 6 minutes prior to recording baseline miniature IPSCs (mIPSCs) and TTX remained present during ALLO application. ALLO (10 nM) was bath applied for 20 minutes prior to recording its effect on IPSCs. Individual cells were used separately to test the effect of ALLO on sIPSCs or mIPSCs. Recordings were analyzed using Clampfit (Axon Instruments), and Igor Pro software (WaveMetrics, Lake Oswego, OR). Analysis of IPSC amplitude, frequency and kinetics were analyzed as described previously (Herson et al., 2003). Briefly, kinetic analysis was performed by detecting individual IPSCs using a sliding variable amplitude template. 20–30 events from each cell per condition were chosen at random, event baseline was adjusted by subtracting the mean baseline of the events, events were aligned by rise time, and event traces were averaged (Clampfit). Using a sum of exponentials function, decay constants were calculated (Igor). Event detection and fitting were confirmed by eye. The total number of events observed in 3 minute recordings from each cell was used to calculate frequency (Igor). The amplitude of all events detected for each cell during a 3 minute recording was used to calculate average IPSC amplitude and cumulative probability of amplitude for that cell, and data are reported as mean ± SEM. Unless otherwise noted, n represents the number of recordings of individual cells from separate animals. Additionally, recordings were only made from slices that were not previously exposed to exogenous ALLO.
2.3. Solutions and Drugs
Cutting solution was composed of (in mM) 110 choline chloride, 2.5 KCl, 7 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 Dextrose, 11.6 Na-ascorbate, 3.1 Na-pyruvate. The composition of the ACSF solution was (in mM): 119 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 1.3 MgCl2, 2.5 CaCl2, and 10 Dextrose, aerated with 95% O2/5% CO2. Internal pipette solution was (in mM): 140 CsCl, 1 EGTA, 10 HEPES, 1 MgCl2, 5 MgATP, pH of 7.3 with CsOH. TTX was dissolved in DMSO to make a stock concentration of 1 mM, working concentration was 250 nM. For electrophysiology recordings, ALLO was dissolved in DMSO at a stock concentration of 10 mM for storage. Fresh working solution of ALLO (10 nM) was made by serial dilution in ACSF daily. ALLO for in vivo CA/CPR experiments was dissolved in 20% β-cyclodextran in 0.9% saline. ALLO was obtained from Calbiochem/EMD Chemicals (Gibbstown, NJ). TTX was obtained from Tocris (Ellisville, MO).
2.4. Cardiac Arrest & Cardiopulmonary Resuscitation (CA/CPR)
CA/CPR was performed on adult (20–25g) male and female C57BL/6 mice to simulate global cerebral ischemia as described by our group previously (Kelley et al., 2008; Kofler et al., 2004). Anesthesia was induced with 3% isoflurane and maintained with 1.5–2% isoflurane in O2 enriched air via face mask. Temperature probes were inserted into left temporalis muscle and rectum to monitor head and body temperature simultaneously. For drug administration, a PE-10 catheter was inserted into the right internal jugular vein and flushed with heparinized 0.9% saline. Animals were then endotracheally intubated, connected to a mouse ventilator (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) and set to a respiratory rate of 160 min-1. Tidal volume was adjusted according to body weight to maintain arterial CO2 tension within physiological range (35–45 mm Hg). CA was induced by injection of 50 μl KCl (0.5 M, 4°C) via the jugular catheter, and confirmed by EKG. The endotracheal tube was disconnected from the ventilator and anesthesia stopped. During CA, body temperature was cooled to assure ease of resuscitation by placing the mouse on an ice water filled pad. However, mouse head temperature was maintained at 38.5 C, to assure reproducible injury, by a heated water-filled coil. CPR began 10 min. after induction of CA by injecting 0.5 ml warm epinephrine solution (16 μg/ml), chest compressions at a rate of 300 min-1, ventilation with 100% O2 at a rate of 190 min-1, and 25% increased tidal volume. At initiation of CPR, head temperature was cooled to 37°C and the body re-warmed using a heat lamp and pad. Cardiac compressions were stopped when spontaneous circulation was restored. Catheters and temperature probes were removed and skin wounds closed. CPR was stopped and animal excluded from experiment if circulation was not restored within 2.5 minutes. CA survivors received post-operative care and survived 48 hr post CA/CPR.. ALLO was administered by ip injection (either 2 mg/kg or 8 mg/kg) 30 minutes prior to CA/CPR, with two subsequent boosting injections (at 6 hr & 24 hr), as described previously (Kelley et al., 2008). Vehicle (see 2.3) was administered at the same time points used for ALLO.
2.5. Histology
Following CA/CPR and 48 hr recovery, mice were euthanized with isoflurane, perfused through the left ventricle with saline, followed by cold 4% formaldehyde, then brains were removed and post-fixed in formaldehyde for an additional 12 hr. After removal, brains were coded with randomly assigned numbers. Brains were dehydrated, cleared, and embedded in paraffin. Coronal sections were made through the cerebellum at approximately −5.8 mm from Bregma at a thickness of 6 μm. FluoroJade B staining was used to label injured neurons (Schmued and Hopkins, 2000) as previously described by Kelley et al., 2008. Experimenter performing analysis was blinded to treatment. PC damage was expressed as percentage of FluoroJade positive PCs in the vermis of each cerebellum. N represents the number of mice examined under each condition. For each n, all PCs in a single 6 μM section (approximately 300 cells) were analyzed to determine the percentage of FluoroJade positive PCs.
2.6. Statistical Analysis
All data is presented as mean ± SEM. Each n represents an individual cell for electrophysiology experiments and an individual animal for in vivo experiments. Statistical significance was determined using students t-test (unpaired, 2-tailed, if P<0.05) or two way analysis of variance (ANOVA) with Neuman-Keuls post hoc analysis, P<0.05 to assess sex versus drug interaction.
3. Results
3.1. ALLO neuroprotection following CA/CPR
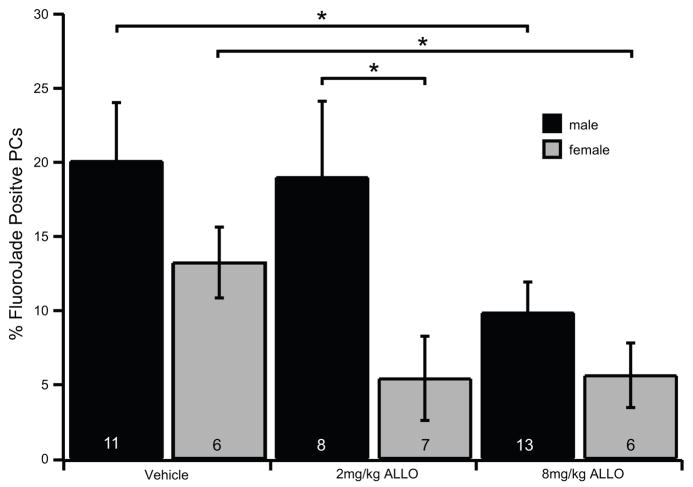
We have previously demonstrated that ALLO can protect PCs from global ischemia in male mice at a dose of 8 mg/kg (Kelley et al., 2008). However, ALLO’s neuroprotective effects have not been examined in female mice. Using our mouse CA/CPR model we tested whether ALLO is neuroprotective in females and if the dose dependence is similar to males. Two doses of ALLO were tested, either 2 mg/kg or 8 mg/kg, administered by ip injection 30 minutes prior to CA/CPR followed by two boosting injections of the same dose at 6 hr & 24 hr recovery. We found that both males and females were significantly protected with the 8 mg/kg dose of ALLO. The percentage of FluoroJade positive PCs for males decreased from 20.09 ± 3.94% with vehicle treatment (n=11) to 9.87 ± 2.06% with 8 mg/kg ALLO (n=13, Fig. 1). The percentage of FluoroJade positive PCs for females decreased from 13.25±2.39% with vehicle (n=6) to 5.64±2.18% with 8 mg/kg ALLO (n=6, Fig. 1). With a 2 mg/kg dose of ALLO males showed almost no protection (19.00±5.12%, n=8), while females were strongly protected (5.43±2.84% n=7; P= 0.06 when compared to female vehicle; Fig. 1). Post-hoc analysis revealed that response to 2 mg/kg was significantly affected by sex (P> 0.05, 2-way ANOVA). While not significant, our data indicates that vehicle treated females suffered less PC damage compared to males, consistent with female neuroprotection attributed to estrogen and progesterone (Herson et al., 2009; Herson and Hurn, 2010; Liu et al., 2010).
Fig. 1.
ALLO reduces Purkinje cell damage following CA/CPR in male and female mice.
Quantification of Purkinje cell damage in mice 48 hr after 10 min CA/CPR. Male and female mice were treated with either vehicle (20% β-cyclodextran in 0.9% saline), 2 mg/kg ALLO, or 8 mg/kg ALLO via i.p. injection given 30 min prior to CA and following 6 hr and 24 hr recovery. FluoroJade positive PCs were counted and expressed as a percentage of total PCs counted in sagittal sections of cerebellum at −5.8 bregma. The data demonstrates that male mice (black bars) were significantly protected compared to vehicle with 8 mg/kg ALLO. Female mice (gray bars) were significantly protected compared to males with 2 mg/kg ALLO, and compared to vehicle with 8 mg/kg ALLO. The number of mice analyzed in each condition is indicated in each bar. Statistical significance was determined by 2-way ANOVA with Neuman-Keuls post hoc analysis *= P≤ 0.05.
3.2. Effects of ALLO on sIPSCs
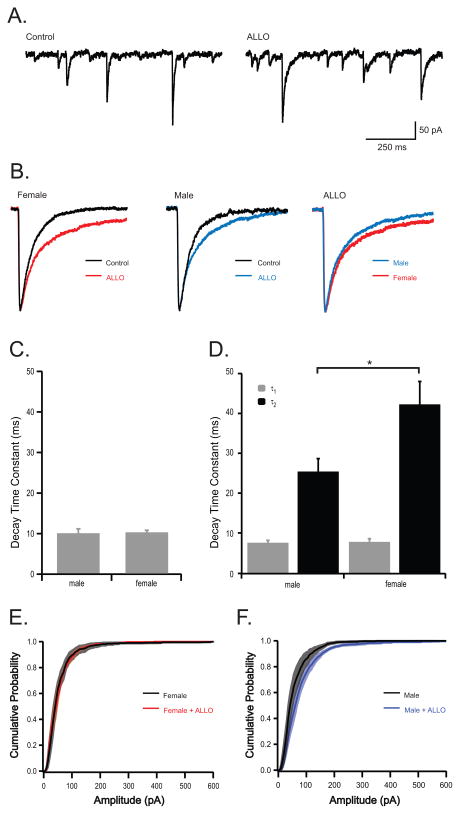
ALLO is a well-characterized GABAA receptor potentiator (For Review see (Belelli and Lambert, 2005)). In acute brain slice recordings, ALLO prolongs IPSC decay kinetics (Cooper et al., 1999; Harney et al., 2003; Koksma et al., 2003; Vicini et al., 2002), resulting in increased overall inhibitory neurotransmission. To test sex-specific sensitivity of cerebellar PCs, a physiological concentration of ALLO (10 nM) {Purdy, 1991 115/id} was applied to PCs recorded from adult male and female mice. Spontaneous IPSCs were recorded from PCs in acute brain slices and individual synaptic events were analyzed to determine decay kinetics. In all recordings, bath application of ALLO slowed the decay kinetics of IPSCs (Fig. 2a), consistent with previous studies. In male PCs, sIPSC decay kinetics were best fit by a single exponential (τ: 9.85 ± 1.08 ms, n=5 cells from 3 mice) and ALLO significantly altered the kinetics of sIPSC decay, resulting in sIPSCs best fit with a double exponential (τ1 = 7.54 ± 0.60 ms and τ2 = 25.42 ± 3.25 ms, n=5 cells from 3 mice). Similarly, under control conditions IPSCs recorded from female PCs had decay kinetics best fit by a single exponential (10.15 ± 0.51 ms, n=5). ALLO significantly slowed the kinetics of sIPSC decay in female PCs, resulting in IPSCs fit by a double exponential (τ1 = 7.76 ± 0.79 ms and τ2 = 42.26 ± 5.75 ms, n=5). While no difference in IPSC kinetics was observed between male and female PCs under control conditions, IPSCs recorded from female PCs had a significantly greater response to ALLO; the slow component of the IPSC (τ2) was significantly different between sexes in the presence of ALLO, where males were 25.42 ± 3.25 ms and females were 42.26 ± 5.75 ms (Fig. 2c). Frequency and amplitude of sIPSCs were not significantly different between males and female PCs under control condition and interestingly, ALLO had no effect on frequency or amplitude of IPSCs (Table 1; Fig 2e, f).
Fig. 2.
ALLO prolongs sIPSC decay kinetics in PCs and has a greater effect in females.
(a) Representative recordings illustrating sIPSCs recorded from PCs in acute brain slices from a male mouse under control conditions and 20 min after exposure to 10 nM ALLO. (b) Representative ipsc kinetics acquired by averaging 20–30 events recorded from one female cell (left), and one male cell (middle) under control conditions (black) and in the presence of 10 nM ALLO (colored). Far right traces illustrate difference in male and female response to ALLO. (c, d) Quantification of decay kinetics in PCs recorded from male (n=5) and female (n=5) mice. Under control conditions sIPSCs were best fit by a single exponential (c). In contrast, following exposure to ALLO, sIPSCs were best fit by a double exponential (τ1 and τ2; d). (d, e) Cummulative probability of sIPSC amplitudes for females (e), males (f), and in the presence of ALLO (red/blue). (The symbol * indicates statistical significance, determined by students t-test, p ≤ 0.05.
Table 1.
Amplitude and Frequency of spontaneous and miniature IPSCs
| Male | Female | |||
|---|---|---|---|---|
| condition | amplitude (pA) | frequency (Hz) | amplitude (pA) | frequency (Hz) |
| sIPSC | 62.00±9.69 (5) | 3.13±1.38 (5) | 59.80±9.53 (5) | 1.48±0.46 (5) |
| sIPSC+ALLO | 63.88±10.34 (5) | 2.19±1.22 (5) | 61.12±6.64 (5) | 1.14±0.37 (5) |
| mIPSC | 39.99±4.59 (8) | 1.68±0.32 (8) | 29.31±2.10 (7) | 1.07±0.25 (7) |
| mIPSC+ALLO | 39.34±2.56 (8) | 1.23±0.20 (8) | 31.89±3.02 (7) | 0.97±0.21 (7) |
Each value is the mean±SEM
The number of neurons studied is in parentheses
3.3. Effects of ALLO on mIPSCs
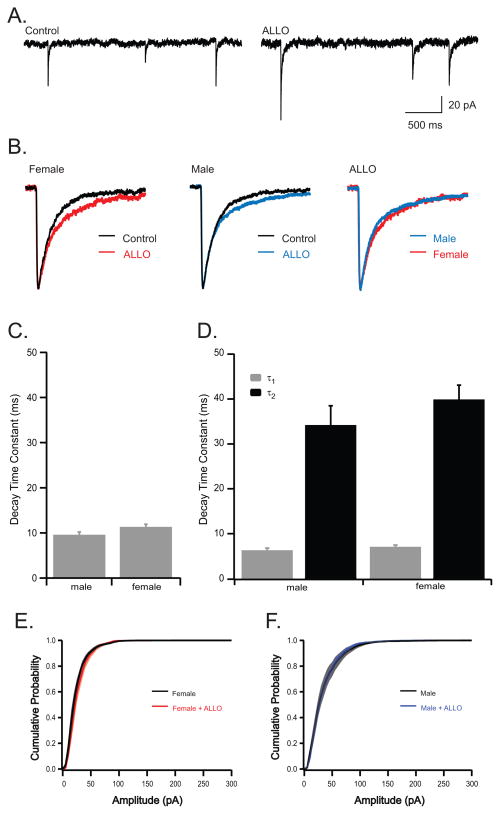
Miniature IPSCs were recorded (250 nM TTX) to assess the role of post-synaptic GABAA receptors in response to ALLO observed in sIPSCs. Under control conditions, decay kinetics of male and female mIPSCs were best fit with a single exponential and were not significantly different at baseline (τ1 was 9.59±0.62 ms, n=8; 11.33±0.58 ms, n=7 for males and females respectively; Fig. 3c). Similar to ALLO’s effect on sIPSCs, ALLO significantly altered the kinetics of mIPSCs in both sexes, resulting in mIPSCs best fit with a double exponential. Interestingly, the effect of ALLO on mIPSC kinetics was not significantly different between sexes (τ1 was 6.31±0.47 ms, 7.09±0.27 ms for male and females respectively; τ2 was 34.21±4.29 ms, 39.91±2.78 for males and females respectively; n=8 for males, n=7 for females; Fig. 3a–c). Frequency and amplitude of mIPSCs were not significantly different between males and females, and as described for sIPSCs, ALLO had no effect on frequency or amplitude of mIPSCs in either sex (Table 1; Fig. 3e.f).
Fig. 3.
ALLO prolongs mIPSC decay kinetics but isolating single synaptic events abolishes the sex difference observed in spontaneous activity.
(a) Representative recordings illustrating mIPSCs recorded in the presence of 250 nM TTX from PCs in acute brain slices from a male mouse under control conditions and 20 min after exposure to 10 nM ALLO. (b) Representative averaged events from one female cell (left), and one male cell (middle) under control conditions (black) and in the presence of 10 nM ALLO (colored). Far right traces illustrate difference in male and female response to ALLO. (c, d) Quantification of decay kinetics in PCs recorded from male (n=8) and female (n=7) mice. Under control conditions mIPSCs are best fit by a single exponential (c). In contrast, following exposure to ALLO, mIPSCs were best fit by a double exponential (d). Cumulative probability of mIPSC amplitudes for females (e), males (f), and in the presence of ALLO (red/blue). The symbol * indicates statistical significance, determined by students t-test, p ≤ 0.05.
4. Discussion
Our experiments demonstrate that sex modifies the response of GABAergic neurotransmission to physiological levels of ALLO in cerebellar Purkinje cells. This finding correlates with our in vivo data demonstrating that females are protected from global ischemia at a dose of ALLO four times lower than that required to protect males. Our mIPSC data were surprising and suggest that the differences we observed, comparing male and female sIPSCs, are likely not at the level of the postsynaptic receptors.
ALLO has been proven to be a strong neuroprotectant against focal ischemia in male rats at a dose of 8 mg/kg (Sayeed et al., 2006). Using the same experimental paradigm, we previously demonstrated that 8 mg/kg ALLO reduces cerebellar Purkinje cell damage following global cerebral ischemia induced by CA/CPR in male mice (Kelley et al., 2008). Data in the current study are consistent with previous reports, where ALLO significantly reduced PC damage in both male and female mice following CA/CPR. Few studies utilize both sexes in the same set of experiments, yet it is of great clinical relevance to do so. Comparison of male and female response to CA/CPR revealed that females exhibit less damage compared to males, consistent with the large body of literature demonstrating that females are relatively protected compared to males due to the well characterized neuroprotection by ovarian hormones, estrogen and progesterone (Herson et al., 2009; Herson and Hurn, 2010; Liu et al., 2010). Despite the less severe damage observed in females, ALLO provided further protection, decreasing damage by over 50%. Our most striking finding was that male and female mice responded differently to a lower dose (2 mg/kg) of ALLO, having no effect in males following CA/CPR, but providing significant protection in females, reducing damage by 60%. This surprising observation suggests that male and female cerebellum respond differently to ALLO, with female being more sensitive.
ALLO can be derived by metabolizing progesterone or may be synthesized de novo in the brain from cholesterol (Mellon and Vaudry, 2001). Therefore, the concentration of ALLO in neural tissue may vary greatly, but plasma levels have been estimated to range between 3–10 nM under normal physiological conditions (Purdy et al., 1991). The current study utilized 10 nM ALLO in physiological recordings to directly assess the relative sensitivity of male and female PCs to ALLO. We tested the effect of a physiological concentration of ALLO on its well documented ability to potentiate GABAergic transmission using acute brain slice recordings from male and female mice. Consistent with many studies in other brain regions, we observed that ALLO potentiated GABAergic neurotransmission, observed as a slowing of the decay kinetics of sIPSCs in cerebellar PCs. Previous studies have used relatively high concentrations of ALLO (100 nM -1 μM), in order to observe large effects on IPSC kinetics (Cooper et al., 1999; Harney et al., 2003; Vicini et al., 2002). The current study demonstrated that low, physiological levels of ALLO potentiate GABAergic neurotransmission, resulting in significant changes in the decay kinetics of sIPSCs. Our data indicates that sIPSCs recorded from male and female PCs respond differently to low concentrations of ALLO, with females having a significantly greater response compared to males.
GABAA receptor sensitivity to neurosteroids, such as ALLO, has been shown to be modulated by several factors, including subunit composition (Lambert et al., 2003) and protein kinases PKC and PKA (Jones and Westbrook, 1997; Poisbeau et al., 1999). Therefore, it is possible that male and female PCs are different either in subunit composition or post-translational modification. To begin to address this issue we recorded GABAergic neurotransmission in the presence of TTX (mIPSCs), to isolate action potential independent neurotransmitter release and observe synaptic activity from release of single vesicles (Collingridge et al., 1984). Therefore, data from mIPSCs supplied information regarding postsynaptic GABA receptors while data from sIPSCs revealed changes in presynaptic release as well as postsynaptic activation of receptors. We expected to observe a sex difference in mIPSC kinetics, suggesting distinct expression of GABAA receptor subunits in male and female PCs. ALLO significantly potentiated mIPSCs, slowing the decay kinetics in PCs from both male and female mice. However, mIPSC kinetics were not significantly different between sexes and the degree of slowing was similar to that observed in male sIPSCs. Therefore, our data suggest that ALLO has an additional effect on female sIPSCs on a site other than the post-synaptic receptor.
One explanation for the extra prolongation of decay kinetics observed in female sIPSCs that is absent in mIPSCs could be that in addition to directly interacting with post-synaptic GABAA receptors, ALLO can alter pre-synaptic release in female PCs. However, ALLO did not affect PC sIPSC amplitude or frequency in either sex, making it unlikely that ALLO alters release probability. Interestingly, Puia et al. observed that reducing endogenous ALLO by inhibiting the 5α-reductase enzyme decreased the sensitivity of sIPSCs to ALLO (Puia et al., 2003). This observation led to the hypothesis of the existence of a “receptor on the receptor,” which allows endogenous levels of ALLO to calibrate the brain’s sensitivity to ALLO. In the context of our current study, we would hypothesize that female cerebellum is exposed to higher levels of ALLO than male. This would sensitize females to consequent exposure to ALLO, as we observed in both our neuroprotection studies as well as sIPSC recordings. Further work is warranted to elucidate the mechanism underlying enhanced sensitivity of female PCs to ALLO potentiation of GABAergic neurotransmission. Nonetheless, our electrophysiological data is consistent with our histological data and indicates that the enhanced sensitivity of GABAergic neurotransmission in female PCs likely contributes to increased neuroprotection observed at low doses of ALLO.
5. Conclusions
The current study provides the first evidence that ALLO protection against global cerebral ischemia is significantly different in male and female mice. Consistent with the neuroprotection results, ALLO potentiates GABAergic neurotransmission in PCs in a sex specific manner. In summary, our data suggests that ALLO is strongly neuroprotective and that sex influences sensitivity to ALLO.
Supplementary Material
Highlights.
ALLO (8 mg/kg) provides significant neuroprotection from global ischemia in male and female mice
Low doses of ALLO (2 mg/kg) provide greater neuroprotection from global ischemia in female mice compared to males.
Spontaneous inhibitory postsynaptic currents are prolonged by ALLO with sex specific sensitivity.
Miniature inhibitory postsynaptic currents are not modulated by ALLO in a sex specific manner.
Acknowledgments
We would like to thank Marjorie Grafe and Xiao-Jing Nie for help with histological analysis. This work was supported by NIH RO1NS058792. Melissa Kelley is a graduate student funded by NIH F31NS060220.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur J Neurosci. 2006;24:2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Brasko J, Rai P, Sabol MK, Patrikios P, Ross DT. The AMPA antagonist NBQX provides partial protection of rat cerebellar Purkinje cells after cardiac arrest and resuscitation. Brain Res. 1995;699:133–138. doi: 10.1016/0006-8993(95)01015-n. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Gage PW, Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EJ, Johnston GA, Edwards FA. Effects of a naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol. 1999;521(Pt 2):437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Lock EA. Cerebellum as a target for toxic substances. Toxicol Lett. 2000;112–113:9–16. doi: 10.1016/s0378-4274(99)00246-5. [DOI] [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Herson PS, Hurn PD. Gender and the injured brain. Prog Brain Res. 2010;186:177–187. doi: 10.1016/B978-0-444-53630-3.00012-9. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. A mouse model of episodic ataxia type-1. Nat Neurosci. 2003;6:378–383. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol (Berl) 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MH, Taguchi N, Ardeshiri A, Kuroiwa M, Hurn PD, Traystman RJ, Herson PS. Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J Neurochem. 2008;107:668–678. doi: 10.1111/j.1471-4159.2008.05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–2703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Koksma JJ, Van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Luddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABA(A) receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35:127–143. [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABA(A) receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2010 doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Vicini S, Losi G, Homanics GE. GABA(A) receptor delta subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology. 2002;43:646–650. doi: 10.1016/s0028-3908(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Vukmir RB. Prehospital cardiac arrest and the adverse effect of male gender, but not age, on outcome. J Womens Health (Larchmt ) 2003;12:667–673. doi: 10.1089/154099903322404311. [DOI] [PubMed] [Google Scholar]
- Wigginton JG, Pepe PE, Bedolla JP, DeTamble LA, Atkins JM. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: a multiyear, prospective, population-based study. Crit Care Med. 2002;30:S131–S136. doi: 10.1097/00003246-200204001-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.