Abstract
Human autoimmune diseases are characterized by systemic T cell dysfunction, resulting in chronically activated Th1 and Th17 cells that are inadequately suppressed by regulatory T cells (Tregs). IL-6, which is overexpressed in tissue and serum of patients with autoimmune diseases, inhibits human Treg function. We sought to determine the mechanism for the antitolerogenic properties of IL-6 by examining the signaling pathways downstream of IL-6R in primary human T cells. Inhibition of Stat3 signaling in MLCs containing IL-6 restores Treg-mediated suppression, demonstrating that IL-6–mediated loss of Treg suppression requires phosphorylation of Stat3. Cultures in which either effector T cells (Teffs) or Tregs were pretreated with Stat3 inhibitors indicate that phosphorylated (p)Stat3 is required in both T cell populations for IL-6–mediated reversal of Treg function. IL-21, which signals preferentially through pStat3, also reverses Treg suppression, in contrast to IL-27 and IFN-γ, which signal preferentially through Stat1 and do not inhibit Treg function. Interestingly, both Teffs and Tregs respond to IL-6 stimulation through strong Stat3 phosphorylation with minimal MAPK/Erk activation and moderate Stat1 phosphorylation. Finally, Teffs stimulated strongly through the TCR are also resistant to suppression by Tregs and show concurrent Stat3 phosphorylation. In these cultures, inhibition of pStat3 restores functional suppression by Tregs. Taken together, our findings suggest that an early dominance of Stat3 signaling, prior to subsequent T cell activation, is required for the loss of functional Treg suppression and that kinase-specific inhibitors may hold therapeutic promise in the treatment of autoimmune and chronic inflammatory diseases.
Human autoimmune and chronic inflammatory diseases are characterized by dysregulated T cell responses in which the activity of memory/effector T cells (Tmem/Teff) is inadequately controlled by naturally occurring, CD4+Foxp3+ CD25high regulatory T cells (Tregs) (1, 2). This loss of tolerance derives from functional defects in both Tmem/Teff and Treg populations; Tmem/Teffs are hyperproliferative and differentiate to pathogenic Th1 and Th17 cells (3, 4), whereas Tregs are deficient in suppressing not only autoimmune Teffs but also normal (healthy) Teffs (5). Lymphocytes residing in inflamed, autoimmune tissues are exposed to a barrage of proinflammatory stimuli not ordinarily encountered in a healthy tissue microenvironment (6). One such proinflammatory signal is the cytokine IL-6, which is highly expressed in psoriatic lesions (7) as well as other autoimmune target tissues (reviewed in Ref. 8). We recently reported that signaling by IL-6 can reverse the function of human Tregs (7), as previously shown in the mouse (9). IL-6 signaling therefore represents a significant mechanism by which tolerance is broken in the context of autoimmune and inflammatory disease.
IL-6 signals through a heterodimeric receptor complex consisting of two molecules each of gp130 and either the membrane-bound IL-6Rα (classical signaling (10)) or the soluble receptor (s) IL-6Rα (trans-signaling (11)). IL-6 trans-signaling induces distinct antiproliferative effects relative to classical IL-6 signaling (12). Stimulation of the IL-6R leads to activation of several transcription factors, most notably Stat3 (13). Cytokines activating Stat3, including IL-6, IL-21, IL-23, and IL-27, are overexpressed in autoimmune target tissue (3, 7, 14, 15), as is phosphorylated (p) Stat3 itself (16). Activation of Stat3 is critical for the differentiation of pathogenic Th17 cells via expression of RORγt and IL-23R, both of which stabilize the Th17 lineage (17). Persistent Stat3 phosphorylation and the induction of Th17 responses are associated with the development of autoimmune diseases (reviewed in Ref. 18). Accordingly, autoimmunity fails to develop in the absence of Stat3 (17). Although Stat3 activation contributes to the development of pathogenic Th17 cells, expression of pStat3 within mature, CD4+Foxp3+ Tregs is protective against the development of fatal colitis in mice through the physical association of pStat3 with Foxp3 (19), underscoring the complexity of transcription factor regulation of an immune response.
In addition to Stat3 activation, Stat1 (13) and the MAPK proteins Erk1/2, p38, and JNK (20–22) can also be phosphorylated upon IL-6R ligation. Contextual stimuli from the local tissue microenvironment likely dictate the relative activation of these pathways, although the mode of IL-6 signaling (binding its cognate receptor versus trans-signaling) is also critical for determining the outcome of a functional immune response (23). Importantly, coordinated signaling by multiple pathways downstream of gp130 is required for transcriptional control of target genes. For example, recent work has shown that activation of p38 controls Stat3-dependent expression of suppressor of cytokine signaling 3 (20). Cross-talk among pathways not directly downstream of gp130 also control IL-6–mediated gene expression, as activation of NF-κB prevents IL-6–mediated expression of α2-macroglobulin (24).
Because IL-6 reverses the suppressive function of human Tregs (7) and thereby contributes to the loss of tolerance in human chronic inflammatory disease, the current study sought to determine the molecular mechanism by which IL-6 exerts its anti-suppressive effects. Our results indicate that phosphorylation of Stat3 contributes to the loss of Treg-mediated functional suppression. Importantly, the relative levels of pStat3 and pStat1 contribute to determining whether Treg suppression remains intact; strong Stat3 phosphorylation relative to pStat1 and pErk results in the release of T cell proliferation from suppression, whereas pStat3 in the presence of strongly pStat1 results in sustained Treg suppression. In situations where Treg suppression is ineffective, the inhibition of Stat3 phosphorylation restores functional suppression, regardless of the presence of high concentrations of IL-6. We observed that inhibition of Stat3 phosphorylation in either the effector or Treg population could restore Treg suppression, whereas APCs do not respond by pStat3, despite representing a major source of IL-6 protein in vitro and in vivo. Strong activation of Stat3, such as by IL-6 in inflamed tissue, results in a release from Treg suppression, whereas functional suppression is restored upon concurrent activation of Stat1. Contextual signaling in tissue microenvironments that modify the relative activation of Stat3 and Stat1 therefore alters the effectiveness of Treg mechanisms of restraining inflammation.
Materials and Methods
Isolation of purified CD4+CD25– Teffs and CD4+CD25high Tregs
All studies involving human subjects were approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH). Peripheral blood samples were obtained from healthy adult volunteers following informed consent. PBMCs were prepared as previously described (5), and CD4+ lymphocytes were isolated by negative selection (Miltenyi Biotec, Auburn, CA). Cells were cultured overnight in complete RPMI 1640 medium containing 10% FBS (Cambrex, East Rutherford, NJ), l-glutamine, penicillin, and streptomycin (all from Cellgro, Manassas, VA). After overnight rest, CD4+ lymphocytes were stained with anti–CD4-APC (Invitrogen, Carlsbad, CA) and anti–CD25-PE (BD Biosciences, San Jose, CA). Cells were sorted by high-speed flow cytometry using a FACSAria (BD Biosciences). CD4+CD25– T cells were gated using an isotype control Ab, and CD4+CD25high Tregs were defined as the top 1.5% of CD25-expressing cells within the CD4low gate (25). Cell viability was determined by trypan blue exclusion.
Immunoblots
Cells were serum starved for 30 min and then stimulated with recombinant human (rh)IL-6 (10 ng/ml) and sIL-6Rα (25 ng/ml), rhIL-27 (10 ng/ml), rhIL-21 (10 ng/ml), or rhIFN-γ (10 ng/ml) (all from R&D Systems, Minneapolis, MN). Protein lysates were prepared using 2× Laemmli buffer and were immunoblotted as previously described (26) using polyclonal Abs specific for pY705-Stat3, total Stat3, pT202/p-Y204-MAPK/Erk, total MAPK/Erk, pY701–Stat1, total Stat1, or β-actin (all from Cell Signaling Technology, Danvers, MA), followed by goat anti-rabbit HRP (Santa Cruz Biotechnology, Santa Cruz, CA). Chemiluminescent signals were developed using SuperSignal West Substrate (Thermo Fisher Scientific, Rockford, IL) and detected on Kodak BioMax MR film (Kodak, Rochester, NY). Densitometry was performed using a VersaDoc imaging system and QuantityOne software (both Bio-Rad, Hercules, CA). Mean adjusted volume was compared in all cases.
T cell proliferation assays
Purified populations of effector (CD4+CD25–) and regulatory (CD4+CD25high) T cells were cocultured in 96-well, round-bottom plates in complete RPMI 1640 medium. T cells (2 × 104) were added to each well, with or without equal numbers of CD25high Tregs. In cultures stimulated with allogeneic APCs, 1 × 105 APCs were added to each well. APCs were prepared from total PBMCs by 1-h plastic adherence (to deplete T cells), and the adherent fraction was irradiated with 3000 rad (30 Gy) immediately prior to use in the coculture. Cocultures stimulated with anti-CD3/anti-CD28 Abs were cultured on flat-bottom, anti–CD3-precoated plates (BD “Biocoat”; BD Biosciences). Soluble anti-CD28 (BD Biosciences) was added to these cultures at 1 μg/ml. Where indicated, rhIL-6 (50-100 ng/ml), rhIL-27 (50 ng/ml), rhIL-21 (50 ng/ml), or rhIFN-γ (50 ng/ml) (all from R&D Systems) were added to the cocultures on day 0. Cultures containing rhIL-6 also contained 25 ng/ml sIL-6Rα (R&D Systems). All cocultures were pulsed with 1 μCi/well tritiated thymidine [3H] for the final 16 h of a 7-d culture and harvested using a FilterMate Univeral 96-well cell harvester (PerkinElmer, Waltham, MA). Proliferation was determined using a TopCount scintillation counter (PerkinElmer).
Pharmacological inhibitors of Stat3 and Stat1
Where indicated, small-molecule phosphorylation inhibitors of Stat3 or Stat1 were added to T cell cultures on day 0. Stat3 inhibitor V (“Stattic V”; Santa Cruz Biotechnology) was used between 1 and 50 ng/ml, as indicated; Stat3 inhibitor peptide, the inactive peptide control, and Stat inhibitor VII (all from Calbiochem/EMD Biosciences, San Diego, CA) were used at 50 ng/ml. The Stat1 inhibitor, epigallocatechin-3-gallate (EGCG) (Sigma-Aldrich, St. Louis, MO), was used at 10 ng/ml (27, 28). In experiments involving pretreatment, inhibitors were incubated for 16 h with T cells and then washed out of the T cell cultures.
ELISAs
CD4+CD25– T cells and CD4+CD25high Tregs were cultured alone or at a 1:1 ratio, as described above, and stimulated with either allogeneic APCs or with anti-CD3/anti-CD28 Abs. Supernatants were removed from cultures at 12-, 24-, or 48-h time points and assayed for IL-6, IL-2, and IFN-γ content by ELISA (Quantikine kit; R&D Systems), according to the manufacturer's instructions. Where indicated, neutralizing Abs to IL-6Rα and gp130 (both at 1 μg/ml; R&D Systems) were added to cultures on day 0.
Statistical analysis
Statistical analysis was performed using a two-tailed Student paired t test, for which values of p ≤ 0.05 were considered significant. For experiments involving multiple comparisons, Student t tests were corrected using the Bonferroni method.
Results
Stat3 signaling is necessary for IL-6–mediated reversal of Treg function
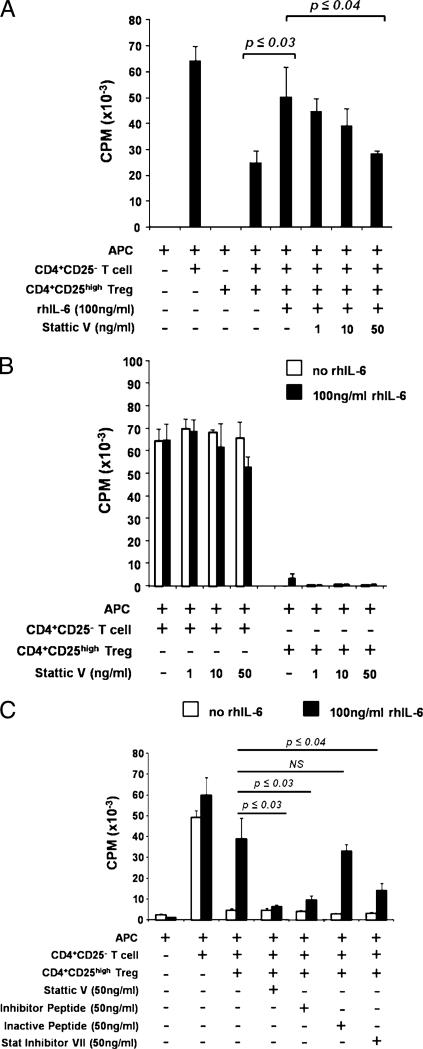
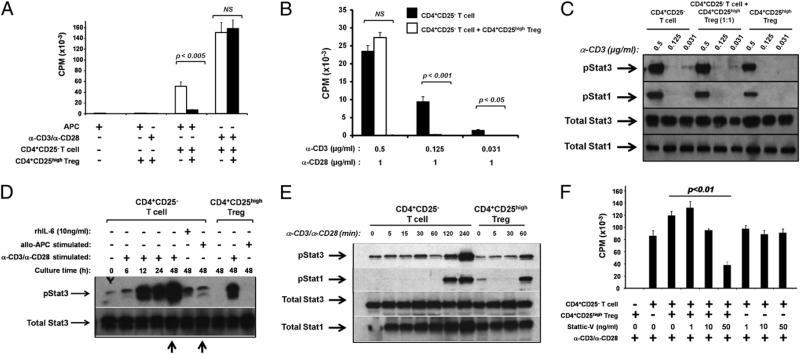
Human T cells have been reported to respond to IL-6 stimulation through a variety of intracellular signaling pathways (reviewed in Ref. 10). As we previously reported, addition of rhIL-6 and sIL-6Rα (to model a strong inflammatory tissue milieu) to T cell cocultures reverses the ability of CD4+CD25high Tregs to suppress the proliferation of Teffs (7). Purified CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood and cultured together with irradiated APCs and 10 ng/ml rhIL-6 and 25 ng/ml sIL-6Rα (which is a critical component of IL-6 trans-signaling and is constitutively present in human serum (29)), in the presence or absence of Stat3 inhibitors. As previously reported (7), rhIL-6/sIL-6Rα resulted in a significant loss of Treg suppression (Fig. 1A). Addition of the small-molecule inhibitor of Stat3 phosphorylation Stattic V restored Treg-mediated suppression in the presence of IL-6 in a concentration-dependent manner (Fig. 1A). The biological effects of Stattic V were not due to inhibition of the baseline proliferation of Teffs or Tregs (Fig. 1B). To confirm specificity of Stattic V for Stat3 signaling, CD4+CD25– T cells were purified and stimulated with rhIL-6/sIL-6Rα, in the presence or absence of Stattic V. Addition of Stattic V at 50 ng/ml reduced phosphorylation of Stat3 and did not affect phosphorylation of Stat1 (Supplemental Fig. 1).
FIGURE 1.
IL-6–mediated reversal of Treg function requires activation of pStat3. CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood of healthy donors. Cells were cultured alone or together for 7 d at a 1:1 ratio with irradiated, allogeneic APCs in MLRs. Proliferation was assessed by titrated thymidine [3H] incorporation during the final 16 h of the 7-d assay. A and B, Cells were cocultured with or without 100 ng/ml rhIL-6 and indicated concentrations of Stattic V, an inhibitor of activated Stat3. C, Cells were cocultured with or without 100 ng/ml rhIL-6 and 50 ng/ml Stattic V, a peptide inhibitor of Stat3, an inactive peptide control, or Stat inhibitor VII. All experiments (A–C) were supplemented with 25 ng/ml sIL-6Rα. Three separate experiments were performed for each of A, B, and C, and data from one representative experiment are shown. Each individual experiment was internally significant where indicated (six cell culture replicates for each condition ± SEM), and p values were adjusted using the Bonferroni method.
To confirm that Stat3 phosphorylation is indeed a critical signaling event required for IL-6–mediated reversal of Treg suppression, we sought alternate approaches to inhibit pStat3. Although the use of small interfering RNA silencing of pStat3 was a desirable approach to study the role of pStat3 in this system, the exceedingly low transfection efficiency of primary human T cells prevented the use of this technology. Instead, alternate pharmacological and peptide inhibitors were used in allogeneic MLR cocultures containing IL-6. Coculture of CD4+CD25– T cells, CD4+CD25high Tregs, APCs, and rhIL-6/sIL-6Rα with a peptide targeting Stat3 restored functional Treg-mediated suppression, whereas coculture with a control (scrambled) peptide did not (Fig. 1C). Stat inhibitor VII, another pharmacologic inhibitor of Stat3, which acts through a similar molecular mechanism to Stattic V, also restored suppression in the presence of IL-6 (Fig. 1C). Neither inhibitor nor the scrambled peptide control resulted in any change to CD4+CD25– T cell or CD4+CD25high Treg proliferation (Supplemental Fig. 2).
Relative phosphorylation levels of Stat3 and Stat1 in Teffs control suppressive potential of Tregs in allogeneic MLR coculture
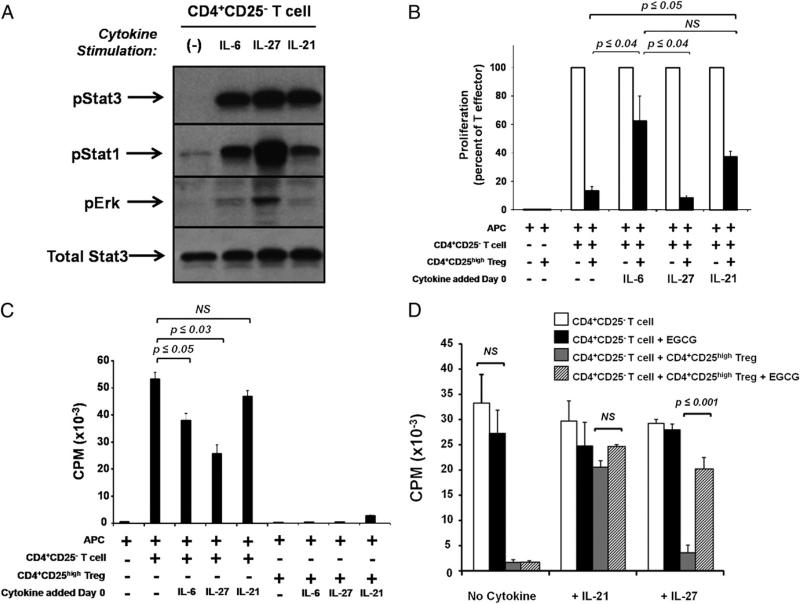
The dependence of Treg functional effectiveness in the presence of IL-6 or pStat3 inhibition raised the question of whether other cytokines that induce Stat3 phosphorylation also reverse functional suppression by Tregs. To characterize Stat3 signaling in response to cytokines other than IL-6, CD4+CD25– T cells were purified from peripheral blood and stimulated with 10 ng/ml rhIL-6, rhIL-27, or rhIL-21, cytokines for which specific receptor chains are expressed on primary human T cells (30–32). IL-6–, IL-27–, and IL-21–treated T cells each were characterized by strong and sustained Stat3 phosphorylation and minimal MAPK/Erk phosphorylation (Fig. 2A). However, IL-27 differed from IL-6 and IL-21 in that IL-27–treated T cells demonstrated a more robust induction of pStat1 relative to cells treated with either IL-6 or IL-21 (Fig. 2A).
FIGURE 2.
Stat3 phosphorylation in response to IL-21, but not IL-27, also inhibits Treg function. CD4+CD25– and CD4+CD25high Tregs were isolated from peripheral blood of healthy donors. A, CD4+CD25– T cells were stimulated for 30 min with 10 ng/ml of indicated cytokines. Protein lysates were prepared and probed with Abs specific for phosphorylated (p)-Y705 (activated) Stat3, p-Y701 (activated) Stat1, p-T202/p-Y204 (activated) Erk1/2, and total Stat3. The blot shown is representative of three separate experiments. B–D, Cells were cocultured for 7 d in MLRs with allogeneic APCs, and 50 ng/ml of indicated cytokines was added on day 0. Proliferation was assessed by [3H] incorporation for the final 16 h of culture. D, Where indicated, EGCG was added at 10 ng/ml on day 0 of coculture. Results shown are the mean ± SEM of two (D) or three (B, C) separate experiments, and p values were adjusted using the Bonferroni method.
Each of the three pStat3-activating cytokines was then added to CD4+CD25– T cells and CD4+CD25high Treg in allogeneic MLR cocultures. Addition of IL-6 and IL-21 to allogeneic MLR cocultures mediated a significant loss of Treg-suppressive function (p ≤ 0.02; n = 3), whereas IL-27 treatment allowed full Treg functional suppression despite inducing similar levels of pStat3 to those observed in IL-6– and IL-21–treated cells (Fig. 2B). Baseline proliferation of CD4+CD25– T cells was reduced upon culture with IL-6 and IL-27 (Fig. 2C), as has been previously reported (12, 33), but not sufficiently to explain the full suppression in the presence of Tregs (Fig. 2B). IL-21 had no effect on T cell proliferation (Fig. 2C).
Because stimulation of T cells with IL-27 did not reverse Treg suppression despite robust phosphorylation of Stat3 (Fig. 2B) and IL-27 signaling resulted in elevated phosphorylation of Stat1 compared with signaling by IL-6 or IL-21 (Fig. 2A), we hypothesized that Stat1 phosphorylation may protect Treg suppression, even in the presence of pStat3. EGCG, an inhibitor of Stat1 phosphorylation (27, 28), was added to T cell cocultures on day 0 at 10 ng/ml. As expected, addition of EGCG did not restore Treg-mediated suppression in the presence of IL-6 (data not shown) but did, however, lead to a loss of suppression in cocultures treated with IL-27 (Fig. 2D).
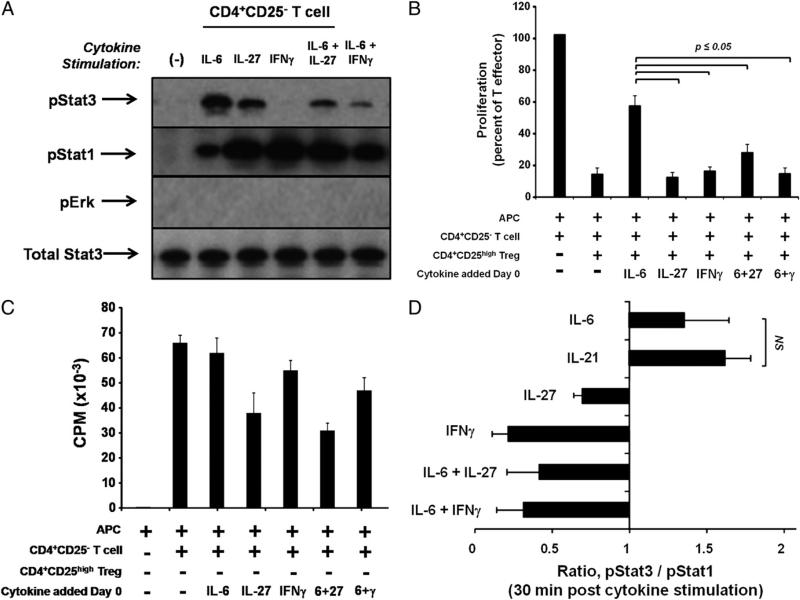
To more thoroughly address the question of whether Stat1 phosphorylation is protective of Treg function, we examined the effect of IFN-γ, a cytokine that phosphorylates Stat1 but not Stat3, in T cell functional assays. First, purified populations of CD4+ CD25– T cells from healthy donors were stimulated with 10 ng/ ml IL-6, IL-27, IFN-γ, or combinations of these cytokines (Fig. 3A). As has been previously described (34), IFN-γ stimulation of Teffs resulted in strong phosphorylation of Stat1 in the absence of Stat3 phosphorylation. Simultaneous stimulation with both IL-6 and IFN-γ, or with both IL-6 and IL-27, resulted in an increase in pStat1 relative to stimulation with IL-6 alone, as well as diminished phosphorylation of Stat3 compared with the pStat3 level upon IL-6 stimulation alone (Fig. 3A).
FIGURE 3.
Strong activation of pStat1 counteracts pStat3-mediated inhibition of Treg function. CD4+CD25– and CD4+CD25high T cell populations were isolated from peripheral blood of healthy donors. A, CD4+CD25– T cells were stimulated for 30 min with 10 ng/ml of indicated cytokines. Protein lysates were prepared and probed with Abs as in Fig. 2, and the blot shown is representative of two separate experiments. B and C, T cells were cocultured for 7 d in MLRs with allogeneic APCs. Indicated cytokines were added on day 0 (all at 50 ng/ml), and proliferation was assessed by [3H] incorporation. Data shown are the mean ± SEM of two separate experiments, and p values were adjusted using the Bonferroni method. D, Levels of pStat3, total Stat3, pStat1, and total Stat1 were calculated from immunoblots using densitometry (see Materials and Methods). The mean ratio of normalized pStat3/pStat1 following 30 min of cytokine stimulation is expressed ± SEM, and each bar represents the mean of at least three healthy human donors.
Next, the functional consequences of increasing the relative level of pStat1 to pStat3 were tested. CD4+CD25– T cells, CD4+CD25high Tregs, and allogeneic APCs were cocultured in MLRs together with rhIFN-γ. Treg suppression remained fully intact upon addition of IFN-γ, compared with cultures containing rhIL-6/sIL-6Rα (p ≤ 0.05) (Fig. 3B). Interestingly, addition of either IFN-γ or IL-27 together with IL-6 to T cell cocultures blocked the effect of IL-6 alone and resulted in sustained Treg functional suppression (p ≤ 0.05) (Fig. 3B). Teffs proliferated robustly in the presence of all cytokine combinations (Fig. 3C), although cultures containing IL-27 exhibited slightly lower proliferation rates, similar to results shown previously (Fig. 2C).
Using a densitometric approach, we quantified the intensity of Stat3 and Stat1 phosphorylation in CD4+CD25– T cells stimulated with IL-6, IL-21, IL-27, IFN-γ, or combinations of these cytokines. A ratio of normalized Stat3 (pStat3/total Stat3) to normalized Stat1 (pStat1/total Stat1) was calculated for cells treated with each cytokine combination (Fig. 3D). Cells treated with IL-6 or IL-21 alone showed an induction of pStat3 relative to pStat1, and interestingly, these were the only cytokine conditions that mediated a loss of Treg suppression in functional assays. Taken together, these data suggest that despite the dependence of IL-6 on Stat3 phosphorylation to reverse Treg suppression, concomitant strong induction of pStat1 overcomes the antisuppressive effects of IL-6 or IL-21 and restores functional Treg suppression.
Stat3 and Stat1 are differentially phosphorylated in human CD4+CD25– Teffs and CD4+CD25high Tregs following IL-6R stimulation
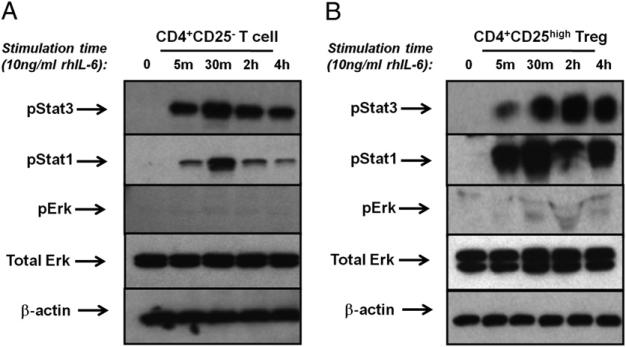
Because both Teffs and Tregs are present in the functional suppression assays that were exposed to immunomodulatory cytokines and pStat3 inhibitors and may differentially respond to IL-6 signaling, we characterized phosphorylation of Stat3, Stat1, and MAPK/Erk in purified Teffs (CD4+CD25–) and Tregs (CD4+ CD25high). Flow cytometry-sorted cell populations were stimulated with 10 ng/ml rhIL-6 protein and 25 ng/ml of the sIL-6Rα. Protein lysates were electrophoretically separated, transferred to nitrocellulose, and analyzed for phosphorylation of Stat3, Stat1, and Erk/MAPK by an immunoblot. MAPK/Erk signaling was minimally activated by IL-6 signaling in CD4+CD25– T cells and CD4+CD25high Tregs. CD4+CD25high Tregs (Fig. 4B), similar to CD4+CD25– T cells (Fig. 4A), showed rapid induction of Stat3 phosphorylation, which was sustained over several hours. Phosphorylation of Stat1 was also evident, reaching maximum levels at 30 min poststimulation and quickly diminishing in CD25– T cells while remaining elevated for several hours in CD25high Tregs. Both Stat1 and Stat3 are phosphorylated in response to very low concentrations of IL-6 (2.5 ng/ml) following a 30-min stimulation of CD4+CD25– T cells (Supplemental Fig. 3). Similar phosphorylation was observed for CD4+CD25high Tregs (data now shown). Despite Stat3 phosphorylation in response to low concentrations of IL-6, higher levels of IL-6 (50–100 ng/ml) are required for the reversal of Treg suppression.
FIGURE 4.
Primary human T cells phosphorylate Stat3 and Stat1 in response to IL-6. CD4+CD25– T cells (A) and CD4+CD25high Tregs (B) were isolated from peripheral blood of healthy donors and stimulated with or without 10 ng/ml rhIL-6 and 25 ng/ml sIL-6R. Protein lysates were prepared and probed with Abs specific for pStat3, pStat1, pErk1/2, total Erk1/2, and β-actin. The immunoblots shown are from the same healthy donor and are representative of three individual experiments.
T cells stimulated strongly through the TCR are resistant to Treg-mediated suppression and express high levels of pStat3
It has previously been suggested that strong stimulation through the TCR is sufficient to break immune tolerance by Tregs (35). To determine whether this is a pStat3-dependent response, CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood and stimulated in coculture with either allogeneic APCs or anti-CD3/anti-CD28 mAbs. T cells stimulated with anti-CD3/anti-CD28 exhibited higher proliferation than those stimulated with allogeneic APCs (Fig. 5A) and were resistant to suppression by Tregs at a 1:1 ratio (Fig. 5A). The proliferation of CD4+CD25– T cells was directly proportional to the concentration of anti-CD3 Ab used (compare proliferation in Fig. 5A, using 1 μg/ml anti-CD3, with that of Fig. 5B, using 0.5 μg/ml anti-CD3). Tregs were unable to suppress proliferation of Teffs stimulated with either 1 or 0.5 μg/ml anti-CD3 (Fig. 5A, 5B) and did not themselves proliferate in response to any concentration of anti-CD3 tested (data not shown). However, the use of lower concentrations of anti-CD3 (0.125 and 0.021 mg/ml) allowed Treg suppression of albeit lower levels of CD4+CD25– T cell proliferation. Interestingly, phosphorylation of Stat1 and Stat3 among CD4+CD25– T cells and CD4+CD25high Tregs could not be titrated with varying concentrations of anti-CD3 mAb. When stimulated alone or in coculture, T cells phosphorylated both transcription factors in nearly an “on/off” manner where phosphorylation occurred only in response to the highest concentration of anti-CD3 tested (0.5 μg/ml) (Fig. 5C).
FIGURE 5.
T cells stimulated strongly through the TCR are resistant to Treg-mediated suppression, and this lack of suppression correlates with phosphorylation of Stat3. CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood of healthy donors. A, Cells were cultured with allogeneic APCs in a MLR or with anti-CD3/anti-CD28 Abs (both at 1 μg/ml) for 6 d, and proliferation is indicated by incorporation of [3H] during the final 16 h of culture. B and C, Cells were cultured for 6 d with 1 μg/ml anti-CD28 and varying concentrations of anti-CD3 Abs, as indicated. B, Proliferation was assessed by [3H] incorporation during the final 16 h of culture. Results show the mean of eight (A) or three (B) separate experiments ± SEM. p Values have been adjusted using the Bonferroni method. C, Protein extracts were prepared on day 6 of coculture and probed with Abs specific for pStat1, pStat3, total Stat1, or total Stat3. The immunoblot shown is representative of three separate experiments. D, Cells were stimulated with either allogeneic APCs, anti-CD3/anti-CD28 Abs (both at 1 μg/ml), or rhIL-6 (10 ng/ml) as indicated. Cells cultured with APCs were back-sorted from the coculture at 48 h. Cells were lysed at indicated time points and probed with Abs specific for pStat3 or total Stat3. Arrows indicate differential pStat3 levels in cells cultured with anti-CD3/anti-CD28, compared with those stimulated with allogeneic APCs. The immunoblot shown is representative of two separate experiments. E, Cells were stimulated with 1 μg/ml anti-CD3/anti-CD28 and lysed at indicated time points and then probed with Abs specific for pStat3, pStat1, total Stat3, or total Stat1. The immunoblot shown is representative of three separate experiments. F, Cells were cultured with anti-CD3/anti-CD28 Abs (both at 1 μg/ml) and indicated concentrations of Stattic V. Proliferation was assessed by [3H] incorporation during the final 16 h of a 7-d culture. Results show the mean of three separate experiments ± SEM.
We next asked whether pStat3 is significantly induced in T cells stimulated with anti-CD3/anti-CD28 mAbs, compared with those stimulated more weakly through the TCR with allogeneic APCs. T cells were stimulated with either Abs or APCs, then back-sorted from APC cocultures after 48 h. T cell lysates were separated by electrophoresis, transferred to nitrocellulose membranes, and probed for phosphorylated and total Stat3. Phosphorylation of Stat3 increased in T cells stimulated with anti-CD3/anti-CD28 mAbs over 48 h, reaching levels in CD4+CD25– T cells far greater than in cells stimulated through allogeneic APC interaction or cells stimulated in the presence of rhIL-6 (Fig. 5D). Arrows compare robust 48-h phosphorylation of Stat3 in T cells stimulated with anti-CD3/anti-CD28 mAbs to weak pStat3 in T cells stimulated by allogeneic APCs (Fig. 5D). Similarly, CD4+CD25high Tregs show high levels of pStat3 upon stimulation with anti-CD3/anti-CD28 mAbs but not with allogeneic APC stimulation (Fig. 5D). Phosphorylation of Stat1 in T cells stimulated with anti-CD3/anti-CD28 mAbs was also more robust than in T cells stimulated by allogeneic APCs, although pStat1 levels in anti–CD3/anti–CD28-treated cells were less than pStat3 levels in the same cells (data not shown).
To ask whether the Stat3 phosphorylation observed in primary T cells was in response to TCR stimulation, rather than a secondary response to secreted factors, we stimulated CD4+CD25– T cells and CD4+CD25high Tregs with anti-CD3/anti-CD28 mAbs over a short time course. CD25– T cells and CD25high Treg phosphorylated Stat3 nearly immediately and Stat1 within 120 min (Fig. 5E), demonstrating an activation response to TCR engagement.
We next determined whether inhibition of robust pStat3 induction in anti–CD3/anti–CD28-stimulated cultures would restore responsiveness to functional Treg immunosuppression. CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood and stimulated with αCD3/αCD28 mAbs, in the presence or absence of Stattic V. Confirming the criticality of Stat3 phosphorylation for escape from Treg suppression, addition of Stattic V to anti–CD3/anti–CD28-activated T cell cocultures led to a concentration-dependent restoration of functional Treg suppression, without affecting the proliferation of CD4+CD25- T cells (Fig. 5F).
Allogenic culture of T cells produces high levels of IL-6, whereas stimulation of T cells with anti-CD3/anti-CD28 Abs does not
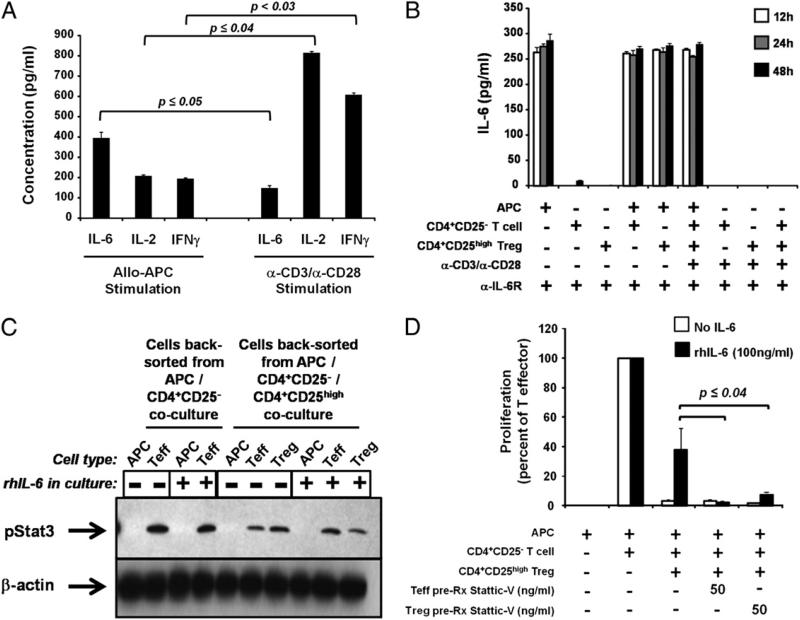
Because levels of pStat3 were high in CD4+CD25– T cells stimulated with anti-CD3/anti-CD28 Abs (Fig. 5D), we hypothesized that stimulated cells may release significant levels of IL-6, which may in turn be responsible for the high levels of pStat3. To address this, purified CD4+CD25– T cells or CD4+CD25high Tregs were cultured with either allogeneic APCs or with anti-CD3/anti-CD28 Abs, and IL-6 production was determined in culture supernatants at 48 h. Interestingly, cells cultured with allogeneic APCs produced significantly more IL-6 protein than those stimulated with anti-CD3/anti-CD28 (p ≤ 0.05; n = 3) (Fig. 6A). In contrast, cells stimulated with anti-CD3/anti-CD28 produced higher levels of IL-2 and IFN-γ than those stimulated with allogeneic APC (Fig. 6A).
FIGURE 6.
T cells, and not APCs, mediate the primary effects of IL-6 on reversal of Treg-induced suppression. CD4+CD25– T cells and CD4+CD25high Tregs were isolated from peripheral blood of healthy volunteers. Cells were cocultured with either allogeneic APCs or anti-CD3/anti-CD28 Abs as indicated. A and B, Supernatants were removed from cocultures at indicated time points and assayed for cytokine production by ELISA. Neutralizing Abs against IL-6Rα and gp130 were included at 1 μg/ml in B. Results shown are mean of three experiments ± SEM. C, T cells were cultured with allogeneic APCs, 25 ng/ml sIL-6R, and with or without 10 ng/ml rhIL-6 as indicated. Cells were back-sorted from cocultures at 48 h, and protein lysates were probed for pStat3 and β-actin. The immunoblot shown is representative of two separate experiments. D, CD25– T cells (“Teff”) or CD25high Tregs were pretreated for 16 h with Stattic V and then washed and cocultured together with indicated concentrations of rhIL-6 and 25 ng/ml sIL-6R. Results shown are mean of three experiments ± SEM, and p values were adjusted using the Bonferroni method.
We wanted to determine the cellular source of IL-6 in allogeneic APC/T cell cocultures. Supernatants were collected from CD4+CD25– T cells, CD4+CD25high Tregs, and APCs cultured either separately or together for 48 h. All cultures contained anti–IL-6Rα and anti–gp130-neutralizing Abs (each at 1 μg/ml) to prevent possible reuptake of IL-6 during the 48-h culture. IL-6 protein, measured by ELISA, was secreted at ≥250 pg/ml in all cultures containing APCs, including cultures of APCs alone (Fig. 6B), despite APC irradiation at 30 Gy. This high level of IL-6 was detectable as early as 12 h after culture initiation (the earliest time point tested). In agreement with data presented in Fig. 6A, T cells cultured in the absence of allogeneic APCs did not produce significant levels of IL-6 (Fig. 6B). Thus, it appears that APCs act as the major source of IL-6 released upon APC/Teff/Treg coculture.
APCs do not express high levels of IL-6R nor respond functionally to IL-6 stimulation
To determine whether APCs respond to the IL-6 they produce, APCs were prepared from healthy peripheral blood, irradiated (or not), and stained with Abs specific for IL-6Rα and gp130. Flow cytometric analysis demonstrated that regardless of irradiation status, APCs express only low levels of IL-6Rα (0.2 ± 0.1 and 0.7 ± 0.3% of irradiated and nonirradiated cells, respectively; n = 3) and similarly low levels of gp130 (8.7 ± 1.8 and 12.3 ± 1.4%, respectively; n = 3) (Supplemental Table I). Interestingly, the level of pStat3 induction within the cocultures did not vary in the presence or absence of IL-6, suggesting the possibility of an alternative, endogenous activator of pStat3 during T cell activation.
Despite their low expression levels of IL-6R chains, the possibility still exists that APCs could respond to IL-6 in the context of T cell coculture through phosphorylation of Stat3. To address this possibility, CD4+CD25– T cells, CD4+CD25high Tregs, and allogeneic APCs were cocultured with or without addition of rhIL-6/ sIL-6Rα, and purified cell populations were back-sorted at the end of 48 h. Protein lysates were prepared and analyzed for total and pStat3 by an immunoblot. pStat3 was detected in both T cell populations but not in APCs (Fig. 6C). Freshly prepared APCs also failed to demonstrate pStat3 following stimulation with 10 ng/ml rhIL-6 (data not shown).
Phosphorylation of Stat3 must occur in both CD4+CD25– and CD4+CD25high T cells in order for IL-6 to mediate reversal of Treg function
Whether IL-6 is mediating its effects through CD4+CD25– T cells, CD4+CD25high Tregs, or APCs was next addressed. Because APCs express very low levels of IL-6Rα and gp130 (Supplemental Table I) and do not phosphorylate Stat3 in response to direct cytokine stimulation or in the context of T cell coculture (Fig. 6C), we hypothesized that one or both T cell subsets were responsible for the IL-6–mediated loss of suppression observed in these conditions. T cell populations were purified from peripheral blood, pretreated separately with or without Stattic V (50 ng/ml) for 16 h, and then washed. Pretreated cells were cocultured with allogeneic APCs in “criss-cross” functional assays. Interestingly, pretreatment of either T cell subset with small-molecule inhibitors of Stat3 phosphorylation was sufficient to restore Treg-mediated suppression (p ≤ 0.04, n = 3) (Fig. 6D), indicating that early phosphorylation of Stat3 in both CD4+CD25– and CD4+CD25high T cells is required for IL-6 to reverse suppression. When both Teffs and Tregs were preincubated with Stattic V and cocultured together, functional immune suppression was similarly restored (data not shown).
Discussion
Loss of immune tolerance, associated with autoimmunity and chronic inflammatory disease, is mediated in part by the proinflammatory cytokine IL-6, which is expressed in diseased tissue (7). IL-6 signaling in primary human T cells results in robust Stat3 phosphorylation with concurrent but more transient phosphorylation of Stat1 and only minimal activation of MAPK/Erk (Fig. 4). Inhibition of pStat3, but not pStat1, in vitro restored functional immune suppression by Tregs (Figs. 1, 2, 5), demonstrating that activation of Stat3 is necessary for IL-6–mediated loss of immune tolerance (7). Interestingly, Stat3 is also phosphorylated during T cell activation, independently of exogenous addition of IL-6 (Figs. 5, 6), suggesting that the T cell may be “preconditioned” through TCR-dependent phosphorylation of Stat3 and that this may be a determinant of functional responsiveness to Treg-mediated immune suppression.
Our results indicate that although phosphorylation of Stat3 is necessary, it is not sufficient to inhibit Treg-mediated suppression. For instance, stimulation of primary T cells by IL-27, a member of the IL-12 cytokine family, leads to Stat3 phosphorylation (Fig. 2A), yet IL-27 does not reverse Treg function in vitro (Fig. 2B). Immunosuppression associated with IL-27 signaling in vivo has been previously reported (33, 36) and is supported by our data demonstrating antiproliferative effects when T cells are cultured with IL-27 in vitro (Fig. 2C). Although IL-27 leads to a dampening of T cell proliferation, T cells cultured in the presence of IL-27 still proliferate in culture, with cpm values of ~2.5 × 104 (Fig. 2C). Therefore, it seems unlikely that suppression observed in Teff/Treg cocultures is the result of IL-27–induced immunosuppression, but rather, it likely reflects Treg-specific action.
Activation of other signaling pathways may modulate the activities of Stat3. Most cytokines activate multiple pathways, including IL-6– and IL-27–induced Stat3, Stat1, and MAPK/Erk phosphorylation (10), which we also observed in T cells. Interestingly, Stat1 activation is stronger than Stat3 activation in cells stimulated with IL-27, compared with IL-6 (Figs. 2A, 3A, 3D). The differential Stat3 versus Stat1 response elicited by IL-6 compared with IL-27 suggests that the extent of Stat1 phosphor-ylation may dampen pStat3-mediated reversal of suppression. Interestingly, inhibition of Stat1 phosphorylation in T cells cocultured with IL-27 resulted in a loss of Treg function (Fig. 2D), suggesting that Stat1 phosphorylation may be protective of Treg-mediated suppression.
Cytokines similar to IL-6, such as IL-21 (37), exhibit signaling profiles that mirror IL-6, inducing strong Stat3 phosphorylation, moderate activation of Stat1, and minimal MAPK/Erk activation (Fig. 2A). This similar pattern of phosphorylation correlates with the partial loss of Treg function (Fig. 2B) following stimulation with exogenous IL-21, suggesting that this activation signature is important for mediating the loss of immune tolerance. Although IL-21 stimulation results in a signaling pattern similar to IL-6–induced phosphorylation, IL-21 signals through the common γ-chain and not gp130 (38); indeed, a Stat3 consensus motif on the IL-21Rα–chain is responsible for activating Stat3 in IL-21–stimulated T cells (39). The absence of gp130 signaling may explain the partial loss of Treg suppression upon IL-21 stimulation as compared with IL-6, which initiates more robust reversal of Treg suppression (Fig. 2B). It has been reported that the four distinct Stat3-recruitment motifs on gp130 can activate Stat3 with differing potencies (40), suggesting that the specific context of gp130 activation is important for downstream effects. Indeed, IL-27 does signal through gp130 to activate Stat3, similar to IL-6; however, this signal does not result in a loss of Treg suppression (Fig. 2B). Therefore, although gp130 is used by both IL-6 and IL-27 to activate Stat3, divergent downstream effects may occur because of an altered gp130 signaling pathway or potentially altered balance of kinase usage.
Of the four Stat3-activating tyrosine motifs present on gp130, two (Y905LPQ and Y915MPQ) contribute to the activation of Stat1 through interactions with its Src homology domain (41). This Src homology domain of Stat1 is also recruited to unrelated phosphotyrosine motifs on the IFN-γR (42), inducing activation of Stat1 in response to IFN-γ. Although recent data suggest that Stat3 may compete with Stat1 for binding to the IFN-γ phosphotyrosine motifs (43), Stat3 was not phosphorylated in response to IFN-γ stimulation in our system (Fig. 3A). Accordingly, we found that Stat1 activation alone, induced following IFN-γ stimulation, does not affect Treg-mediated suppression, indicating that Stat1 phosphorylation itself does not have an inhibitory role (Fig. 3B).
Activation of Stat3 in T cells by IL-6 or IL-21 has been shown to be critical for the differentiation of human Th17 cells (17). Fully differentiated Th17 cells express the transcription factor RORγt, are dependent on IL-23 for survival, and secrete proinflammatory cytokines including TNF-α, IL-6, IL-22, and IL-17A/F (3). Mature Th17 cells are resistant to Treg-mediated suppression (44) unless Stat3 is concurrently activated in the Treg population (19); this is the first evidence, to our knowledge, that coordinated signaling between effector and regulatory cell populations may act to control the outcome of immune suppression. In our system, we did not find evidence that IL-6 or IL-21 signaling resulted in Th17 differentiation among Teffs in coculture (data not shown). Whereas Stat3 activation in this previously reported model (19) contributed to Treg function, our results indicate a potentially dysfunctional role for Stat3 signaling in both Tregs and Teffs, further suggesting that Stat3 signaling in T cells incorporates numerous simultaneous signals to achieve an integrated and context-dependent immune response.
High levels of Stat3 phosphorylation can be induced in the absence of exogenous cytokine in T cell cocultures through strong activation of the TCR by polyclonal anti-CD3/anti-CD28 mAbs (Fig. 5C–E). Accordingly, these T cells stimulated by anti-CD3/anti-CD28 are resistant to Treg-mediated suppression, consistent with reports indicating that strength of effector cell TCR stimulation inversely correlates with efficiency of suppression by Tregs (35). Indeed, our data demonstrate that high level (≥0.5 μg/ml) anti-CD3 is required for Tregs to lose suppressive function (Fig. 5B), and interestingly, at anti-CD3 concentrations < 0.5 μg/ml, TCR-dependent phosphorylation of Stat3 in T cells is also lost (Fig. 5C). Taken together, these data suggest that high concentrations of anti-CD3 are sufficient to cause acute phosphorylation of Stat3, which is rapidly observed following TCR stimulation of CD4+CD25– T cells (Fig. 5E), and that induction of pStat3 correlates with a functional loss of suppressive function by Treg (Fig. 5B). Stat3 activation is critical for the loss of tolerance associated with strong TCR stimulation in Teff and Treg cocultures (Fig. 5A, 5B), as Treg suppression is restored upon inhibition of Stat3 phosphorylation (Fig. 5F).
It is unclear whether anti–CD3/anti–CD28-mediated phosphorylation of Stat3 involves IL-6 or other cytokines, as we detected low amounts of IL-6 protein in these APC-free cultures (Fig. 6A). In addition, CD3 cross-linking can directly activate Stat3 in human CD4+ T cells through a Src-dependent mechanism, supporting our findings that TCR activation in the absence of exogenous cytokine is sufficient for Stat3 phosphorylation (45). In contrast to anti-CD3/anti-CD28 stimulation of the TCR, cocultures of Teffs and allogeneic APCs do release high levels of IL-6 into the culture medium (Fig. 6B); the primary source of IL-6 in these cocultures are APCs, which are likely to release preformed stores of IL-6 protein upon coculture with T cells (7). Importantly, although IL-6 is produced in T cell/APC cocultures, concentrations are not high enough to mediate loss of Treg suppression.
Our results demonstrate that Stat3 phosphorylation in the absence of strong pStat1 in primary human T cells, following IL-6 stimulation, is sufficient to reverse Treg-mediated immune suppression. Immune suppression can be restored with the application of specific inhibitors of pStat3 (Fig. 1). A similar pStat3/pStat1 pattern is induced by IL-21 and also results in a partial loss of immune tolerance. In contrast, IL-27 stimulation results in high levels of both pStat3 and pStat1, a condition that does not result in a functional reversal of Treg suppression, despite the presence of pStat3. Interestingly, a combination of IL-6 and IFN-γ stimulation also restored Treg suppression in a manner similar to the pStat3 inhibitor or IL-27 treatment. Taken together, these findings suggest that induction of pStat1 is capable of dampening the Stat3 signaling cascade at a functional level.
These observations lead us to propose a model in which T cells accumulating in an autoimmune microenvironment are exposed to high IL-6 and experience a dominant Stat3 phosphorylation early and prior to engagement of APCs and Tregss. Furthermore, the coordinated activation of Stat3 in Teffs and Tregs may result in a localized loss of functional suppression by Tregs. Thus, chronic inflammatory conditions provide contextual signaling to T cells that alters the Stat3/Stat1 phosphorylation pattern, resulting in functional defects in Treg suppression.
Supplementary Material
Figure S1: Stattic-V decreases level of Stat3, but not Stat1, phosphorylation in IL-6-stimulated CD4+CD25- T cells. CD4+CD25- T cells were isolated from healthy donors by flow cytometry sorting. Cells were stimulated with or without rhIL-6 (10 ng/ml) and Stattic V (10 ng/ml) for 30 min. Protein lysates were prepared and immunoblotted using antibodies specific for pStat3, total Stat3, pStat1, and total Stat1. Immunoblot shown is representative of six separate experiments.
Figure S2: Inhibition of Stat3 phosphorylation does not alter proliferation of CD4+CD25- T cells or CD4+CD25high Treg cells. CD4+CD25- T cells and CD4+CD25high Treg cells were isolated from the peripheral blood of healthy volunteers by flow cytometry sorting, and cultured with allo-APC and indicated inhibitors of Stat3 phosphorylation. Data shown is representative of three separate experiments ± SEM.
Figure S3: Primary T cells phosphorylate Stat1 and Stat3 in response to very low concentrations of rhIL-6. CD4+CD25- T cells were isolated from peripheral blood of healthy donors. Cells were stimulated for 30 min with indicated concentrations of rhIL-6. Blot shown is representative of three separate experiments.
Table SI. Antigen presenting cells express minimal levels of IL-6Rα and gp130.
Acknowledgments
We thank W.M. Sramkoski and J. Toska for excellent technical support for flow cytometry experiments and B. Schmotzer for assistance with statistical analysis.
This work was supported by National Institutes of Health Grants AR-051498, P30AR39750, and P50AR05508 (to K.D.C.) as well as AI083609 and DK54213 (to A.D.L.), the Flow Cytometry Center of the Case Comprehensive Cancer Center (Grant P30CA43703), and the Murdough Family Center for Psoriasis.
Abbreviations used in this article
- EGCG
epigallocatechin-3-gallate
- p
phosphorylated
- rh
recombinant human
- s
soluble receptor
- Teff
effector T cell
- Tmem
memory T cell
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr. Opin. Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol. Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 4.Weaver CT, Murphy KM. The central role of the Th17 lineage in regulating the inflammatory/autoimmune axis. Semin. Immunol. 2007;19:351–352. doi: 10.1016/j.smim.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano T. Cytokines in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:297–298. doi: 10.1016/s1359-6101(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 7.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 9.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 12.Santer FR, Malinowska K, Culig Z, Cavarretta IT. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr. Relat. Cancer. 2010;17:241–253. doi: 10.1677/ERC-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6–type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, Giustizieri ML, Pacciani V, Mazzotta A, Campione E, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat. Med. 2009;15:1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- 15.Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M, Miyagaki T, Kai H, Saeki H, Shirakata Y, et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J. Invest. Dermatol. 2010;130:1034–1039. doi: 10.1038/jid.2009.349. [DOI] [PubMed] [Google Scholar]
- 16.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 17.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode JG, Ludwig S, Freitas CA, Schaper F, Ruhl M, Melmed S, Heinrich PC, Häussinger D. The MKK6/p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biol. Chem. 2001;382:1447–1453. doi: 10.1515/BC.2001.178. [DOI] [PubMed] [Google Scholar]
- 21.Schiemann WP, Bartoe JL, Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor α- and gp130-mediated stimulation of mitogen-activated protein kinase: evidence for participation of multiple signaling pathways which converge at Ras. J. Biol. Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- 22.Zauberman A, Zipori D, Krupsky M, Ben-Levy R. Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene. 1999;18:3886–3893. doi: 10.1038/sj.onc.1202738. [DOI] [PubMed] [Google Scholar]
- 23.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am. J. Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode JG, Fischer R, Häussinger D, Graeve L, Heinrich PC, Schaper F. The inhibitory effect of IL-1β on IL-6–induced α2-macroglobulin expression is due to activation of NF-κB. J. Immunol. 2001;167:1469–1481. doi: 10.4049/jimmunol.167.3.1469. [DOI] [PubMed] [Google Scholar]
- 25.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 26.Das L, Levine AD. TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J. Immunol. 2008;180:1490–1498. doi: 10.4049/jimmunol.180.3.1490. [DOI] [PubMed] [Google Scholar]
- 27.Menegazzi M, Tedeschi E, Dussin D, De Prati AC, Cavalieri E, Mariotto S, Suzuki H. Anti-interferon γ action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. FASEB J. 2001;15:1309–1311. doi: 10.1096/fj.00-0519fje. [DOI] [PubMed] [Google Scholar]
- 28.Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann. N. Y. Acad. Sci. 2002;973:435–437. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 29.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med. Microbiol. Immunol. (Berl.) 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 30.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 31.Betz UA, Müller W. Regulated expression of gp130 and IL-6 receptor α chain in T cell maturation and activation. Int. Immunol. 1998;10:1175–1184. doi: 10.1093/intimm/10.8.1175. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor β chain. Proc. Natl. Acad. Sci. USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 34.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 35.Tree TI, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the Immunology of Diabetes Society T Cell Workshop. Ann. N. Y. Acad. Sci. 2006;1079:9–18. doi: 10.1196/annals.1375.002. [DOI] [PubMed] [Google Scholar]
- 36.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 37.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 38.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann U, Sommer U, Smyczek T, Hörtner M, Frisch W, Volkmer-Engert R, Heinrich PC, Schaper F, Haan S. Determinants governing the potency of STAT3 activation via the individual STAT3-recruiting motifs of gp130. Cell. Signal. 2006;18:40–49. doi: 10.1016/j.cellsig.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Gerhartz C, Heesel B, Sasse J, Hemmann U, Landgraf C, Schneider-Mergener J, Horn F, Heinrich PC, Graeve L. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J. Biol. Chem. 1996;271:12991–12998. doi: 10.1074/jbc.271.22.12991. [DOI] [PubMed] [Google Scholar]
- 42.Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grötzinger J, Wollmer A, Zhong Z, Darnell JE, Jr., Graeve L, et al. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of stat factor activation. J. Biol. Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 43.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-γ. J. Biol. Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 44.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J. Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerwien J, Nielsen M, Labuda T, Nissen MH, Svejgaard A, Geisler C, Röpke C, Odum N. Cutting edge: TCR stimulation by antibody and bacterial superantigen induces Stat3 activation in human T cells. J. Immunol. 1999;163:1742–1745. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Stattic-V decreases level of Stat3, but not Stat1, phosphorylation in IL-6-stimulated CD4+CD25- T cells. CD4+CD25- T cells were isolated from healthy donors by flow cytometry sorting. Cells were stimulated with or without rhIL-6 (10 ng/ml) and Stattic V (10 ng/ml) for 30 min. Protein lysates were prepared and immunoblotted using antibodies specific for pStat3, total Stat3, pStat1, and total Stat1. Immunoblot shown is representative of six separate experiments.
Figure S2: Inhibition of Stat3 phosphorylation does not alter proliferation of CD4+CD25- T cells or CD4+CD25high Treg cells. CD4+CD25- T cells and CD4+CD25high Treg cells were isolated from the peripheral blood of healthy volunteers by flow cytometry sorting, and cultured with allo-APC and indicated inhibitors of Stat3 phosphorylation. Data shown is representative of three separate experiments ± SEM.
Figure S3: Primary T cells phosphorylate Stat1 and Stat3 in response to very low concentrations of rhIL-6. CD4+CD25- T cells were isolated from peripheral blood of healthy donors. Cells were stimulated for 30 min with indicated concentrations of rhIL-6. Blot shown is representative of three separate experiments.
Table SI. Antigen presenting cells express minimal levels of IL-6Rα and gp130.