Abstract
The epothilone B analogue, ixabepilone, binds to β-tubulin, is effective for taxane-refractory metastatic breast cancer (MBC), and may be given every 3 weeks or weekly. We evaluated the efficacy of weekly ixabepilone (I) plus trastuzumab (T) and carboplatin (C) as first line therapy in HER2 + MBC. Patients with HER2+ (3+ by IHC or FISH amplified) MBC received I (15 mg/m2 IV) and C (area under the curve, AUC = 2 IV) on days 1, 8, and 15 of a 28-day cycle for a maximum of 6 cycles, plus weekly T (4 mg/kg loading dose then 2 mg/kg IV) during chemotherapy then every 3 weeks (6 mg/kg IV) until disease progression. The primary objective was to determine whether the combination was associated with a response rate (RR) of at least 75%. Fifty-nine patients were treated, and 39 had HER2 overexpression confirmed in a central lab (cHER2+). For all treated patients, objective response occurred in 26 patients (44%; 95% CI 31–58%), median time to progression was 8.2 months (95% CI 6.3–9.9), and median overall survival was 34.7 months (95% CI 25.7 to [not reached]). Results were comparable for cHer2+ cancers. Grade 3–4 adverse events included neutropenia (49%), thrombocytopenia (14%), fatigue (12%), nausea (7%), diarrhea (7%), and neuropathy (7%). One patient died from treatment complications during cycle 1. Weekly ixabepilone and carboplatin plus trastuzumab have an acceptable toxicity profile, but are not likely to be associated with an RR of 75% in HER2+ MBC. Efficacy appears comparable to paclitaxel, carboplatin, and trastuzumab.
Keywords: Weekly ixabepilone, Carboplatin, Trastuzumab, Breast cancer
Introduction
When added to chemotherapy, the monoclonal antibody trastuzumab has been shown to improve clinical outcome compared with chemotherapy alone for the treatment of metastatic breast cancer (MBC) patients whose tumors overexpress human epidermal growth factor receptor 2 (HER2+) [1]. The addition of trastuzumab to paclitaxel was associated with improvement in response rate (RR) (38 vs. 16%, P <0.001), time to progression (TTP) (6.9 vs. 3.0 months, P <0.001), and overall survival (OS) (22.1 vs. 18.4 months, P = 0.17). When the AC–trastuzumab combination proved to be prohibitively cardiotoxic, paclitaxel-trastuzumab emerged as the standard therapy.
The addition of carboplatin to paclitaxel and trastuzumab also demonstrated higher RRs of 50–80% in multiple single arm phase II trials [2–5]. A larger phase III trial which evaluated the role of carboplatin included 196 women with HER2+ MBC who were randomized to receive weekly trastuzumab plus paclitaxel (175 mg/m2 IV every 3 weeks) given alone or in combination with carboplatin (area under the curve, AUC = 6) IV every 3 weeks [6.] The addition of carboplatin was associated with significantly higher RR (57 vs. 36%, P = 0.03) and progression-free survival (PFS, 10.7 vs. 7 months, P = 0.03) with a trend toward improvement in OS (35.7 vs. 32.2 months) that did not reach statistical significance.
Most recently, the use of weekly paclitaxel has shown improvement in clinical outcome for the treatment of breast cancer patients in the adjuvant, neoadjuvant, and metastatic settings [7–11.] In a phase III randomized trial for the treatment of patients with MBC, the CALGB demonstrated that the use of weekly paclitaxel (80 mg/m2 weekly) was associated with higher response (42 vs. 29%, P = 0.0004), improved TTP (9 vs. 5 months, P <0.0001), and OS (24 vs. 12 months, P = 0.0092) compared to every 3 week administration (175 mg/m2); albeit with higher rates of peripheral neuropathy (24 vs. 12%, P = 0.0003) [8]. This study also contained a smaller subset of HER2+ patients (N = 174) who received trastuzumab in combination with paclitaxel, which demonstrated no difference in response between weekly paclitaxel (RR = 55%) and Q-3 week paclitaxel (RR = 58%) in this group of patients.
The North Central Cancer Treatment Group (NCCTG) investigated both a weekly dosing schedule and an every 3 week dosing schedule of paclitaxel and carboplatin in combination with trastuzumab [5]. Although patients were not randomly assigned to treatment, the weekly treatment regimen appeared to demonstrate additional benefit in response (RR 81 vs. 65%), TTP (13.8 vs. 9.9 months), and OS (3.2 vs. 2.3 years) when compared to the every 3 week regimen. The weekly dosing regimen was also associated with less myelosuppression (grade 3–4 neutropenia: 52 vs. 88%, grade 3 thrombocytopenia: 4 vs. 30%) and peripheral neuropathy (grade 3: 2 vs. 19%).
Epothilones, such as ixabepilone, bind β-tubulin at or near the paclitaxel binding site with greater potency, result in similar mitotic block at lower mean inhibitory concentrations, and demonstrate cytotoxicity in taxane resistant cells that overexpress P-glycoprotein or harbor mutations at the paclitaxel binding site of β-tubulin [12, 13].
Several phase II clinical trials have demonstrated activity for Q-3 week dosing schedules of ixabepilone in patients with MBC with RR of 11.5–22% in patients previously treated with taxanes for MBC and 42–57% when used as first line therapy for MBC [14–18]. Additionally, phase I trials have demonstrated tolerability for the weekly administration of ixabepilone as well ixabepilone given in combination with carboplatin [19, 20]. Unpublished data have also established that weekly ixabepilone (15 mg/m2) and carboplatin (AUC = 2) could be safely administered on days 1, 8, and 15 of a 28-day cycle (Daniel Sullivan, personal communication).
Based upon ixabepilone’s single-agent activity and the validated efficacy of weekly trastuzumab/paclitaxel/carboplatin for the treatment of HER2+ MBC, the Eastern Cooperative Oncology Group (ECOG) performed E2103, a phase II trial of weekly ixabepilone in combination with carboplatin and trastuzumab for the first-line treatment of patients with HER2+ MBC. The primary objective of the study was to determine if the combination would be associated with a ~30% improvement in the 57% RR previously reported for paclitaxel/carboplatin/trastuzumab (target RR = 75%) [6].
Patients and methods
Patient selection
All patients had MBC with biopsy-proven HER2 overexpression (3+ by immunohistochemistry [IHC] or gene amplification by fluorescent in situ hybridization [FISH], HER2/CEP17 ratio ≥ 2.0) in either the primary tumor or a metastatic focus as determine in a local institutional laboratory. Breast cancer samples were confirmed HER2+ (cHER2+) by centralized testing (3+ by IHC using the DAKO HercepTest or a HER2/CEP17 ratio of ≥ 2, Vysis PathVvsion, Abbott Molecular, Abbott Park, IL, USA) in the Eastern Cooperative Oncology Group Pathology Coordinating Office and Reference Laboratory at the Robert H. Lurie Comprehensive Cancer Center, Chicago, IL, USA; however, patients were allowed to initiate therapy prior to confirmation. Other requirements included age at least 18 years, have measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST) criteria [21], an ECOG performance status of ≤1, adequate hematologic (granulocytes ≥ 1500/mm3, platelets ≥ 100000/mm3), hepatic (transaminases ≤ 1.5× upper limit of normal in absence of hepatic metastasis and 2.0× upper limits of normal in the presence of hepatic metastasis) and renal function (creatinine ≤ 1.5 mg/dl), left ventricular ejection fraction at or above the lower institutional limits of normal as measured by echocardiogram or MUGA scan. Exclusion criteria included a history of New York Heart Association class 3 or 4 heart failure, prior hypersensitivity reaction to polyoxyethylated castor oil (Cremophor EL), cumulative dose of >60 mg/m2 of doxorubicin or equivalent dosing of epirubicin, history of brain metastasis, radiation within 2 weeks of registration, patients who were pregnant or breast feeding, or who had prior chemotherapy or trastuzumab for metastatic disease. Adjuvant trastuzumab was allowed; however, patients relapsing within 6 months of adjuvant trastuzumab were not eligible for study participation.
This study was conducted in accordance with the US Food and Drug Administration and the Declaration of Helsinki. The study was approved by the local institutional review board at all participating centers and all patients gave written informed consent prior to enrollment.
Treatment
Patients received induction therapy with trastuzumab given at a loading dose of 4 mg/kg IV over 90 min on cycle 1, day 1 then 2 mg/kg IV over 30 min weekly. After completion of trastuzumab, patients were premedicated with diphenhydramine (50 mg po or IV) and ranitidine (150 mg po or IV). Thirty minutes after premedication, ixabepilone (15 mg/m2 IV over 1 h) was administered followed by carboplatin (AUC = 2 IV over 1 h) on days 1, 8, and 15 of a 28-day cycle. Patients were allowed to receive a maximum of six cycles of chemotherapy. Those with responding or stable disease after six cycles of treatment received maintenance trastuzumab (6 mg/kg IV every 3 weeks) until disease progression or study discontinuation.
Dose reductions were allowed for grade 2 or higher non-hematologic toxicity (excluding alopecia) that was unresponsive to supportive therapy and for hematologic toxicity, including febrile neutropenia or inadequate hematologic recovery prior to scheduled dosing (Table 1). Growth factor support was used at the discretion of the treating physician in patients who developed febrile neutropenia or anemia, and loperamide was recommended for the treatment of chemotherapy-induced diarrhea. All supportive treatments consistent with optimal patient care were allowed while patients received protocol therapy, including antiemetics and bisphosphonate therapy.
Table 1.
Chemotherapy dose reductions for treatment related toxicity
| Dose level | Ixabepilonea | Carboplatinb |
|---|---|---|
| Starting dose | 15 mg/m2 | AUC = 2 |
| First dose reduction | 12.5 mg/m2 | AUC = 1 |
| Second dose reduction | 10 mg/m2 | Discontinue |
| Third dose reduction | Discontinue | Discontinue |
Doses were held for ANC <800, platelets <50,000 or grade 2 non-hematologic toxicity (excluding alopecia) unresponsive to supportive measures. When recovered from toxicity, patients were restarted using the reduced dose of therapy
Carboplatin was not held or dose reduced for grade 2 or higher hepatic toxicity
Response assessment
The primary objective of this study was to determine activity of the combination therapy as measured by tumor response. Tumor response evaluation (by physical exam and/or imaging studies) was performed after cycles 3 and 6 of induction therapy and every 12 weeks while receiving maintenance trastuzumab. Overall response was assessed according to RECIST and was not subject to review by an independent review committee [21]. All patients who received treatment but were not assessed for response were considered non-responders for the purposes of statistical analyses.
Toxicity assessment
Toxicity was continuously evaluated and reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Laboratory parameters were measured on days 1, 8, and 15 of each 28-day cycle prior to administration of chemotherapy while patients received induction therapy and every 3 weeks during maintenance trastuzumab. Measurement of cardiac function by MUGA scan or echocardiogram was performed after cycles 3 and 6 of induction therapy and recommended every 12 weeks while receiving maintenance trastuzumab.
Statistical design and methods
The primary endpoint of this study was the objective RR (PR + CR) for patients with cHER2+ MBC. A total of at least 49 eligible patients were required to be HER2 positive on central review. Based upon the RR of 57% (as reported by Robert et al., at the time of study design; later published to be 52%) for trastuzumab plus paclitaxel–carboplatin given every 3 weeks [6], we sought to determine whether this regimen would be associated with an RR of at least 75%. The regimen would be of considered promising if at least 33 of 49 cHER2+ patients had a response by RECIST criteria. This would occur with probability 9.2% if the true RR was 57%, and with probability 91.6% if the true RR was 75% in patients with tumors cHER2+. A total of up to 60 patients were entered in order to allow to an approximately 20% rate of HER2-negative on central review or ineligible for other reasons.
A 2-stage safety design was used to halt accrual if the regimen demonstrated excessive neutropenia or neurotoxicity. Descriptive statistics were used to characterize patients at study entry. Two sets of analyses were performed. The primary analysis included only eligible patients who were cHER2+ as specified in protocol. A post-hoc analysis was performed for all treated patients regardless of eligibility or central review results. An unplanned subgroup analysis of outcome in patients with and without prior history of adjuvant taxanes was also performed. Exact binomial confidence intervals were used to describe RR. The method of Kaplan–Meier was used to characterize the duration of response, TTP, time to treatment failure (TTF), and OS [22]. TTP was defined as the time from study entry to progression, censored at the date of last disease assessment for those who have not progressed. TTF was defined as the time from study entry to the date at which patient was removed from treatment due to progression, toxicity, refusal, or death. If the patient was considered to be a major treatment violation or was taken off study for reasons other than toxicity or disease progression, the patient would be censored on the date they were removed from treatment.
Results
Patients
The study was activated on March 19, 2004 and terminated on March 24, 2006 after accrual of 61 patients from 23 institutions, of whom 2 withdrew and never initiated therapy. The clinical characteristics of all 59 patients enrolled and 39 patients who were confirmed to have HER2/neu positive disease in a central lab are shown in Table 2. The characteristics of the two groups were similar. Of the patients who were cHER2+, 64% received prior adjuvant or neoadjuvant chemotherapy (including 36% who had adjuvant taxane chemotherapy), 46% received prior radiation therapy, and 38% had received prior hormonal therapy. Three patients (8%) had received adjuvant trastuzumab.
Table 2.
Patient characteristics
| Patients centrally confirmed HER2+ (N = 39)
|
All treated patients (N = 59)
|
|||
|---|---|---|---|---|
| N | % | N | % | |
| Age | ||||
| Median (Range) | 54(24–81) | 51(24–81) | ||
| Race | ||||
| White | 35 | 90 | 51 | 86 |
| Black | 3 | 8 | 6 | 10 |
| Asian | 1 | 3 | 1 | 2 |
| Unknown | 0 | 0 | 1 | 2 |
| ECOG performance status | ||||
| 0 | 21 | 54 | 36 | 61 |
| 1 | 18 | 46 | 23 | 39 |
| Site of disease at study entry | ||||
| Local–regional | 21 | 54 | 33 | 56 |
| Ipsilateral supraclavicular nodes | 10 | 26 | 12 | 20 |
| Opposite breast | 0 | 0 | 1 | 2 |
| Distant nodes | 16 | 41 | 21 | 36 |
| Distant skin/subcutaneous tissue | 2 | 5 | 3 | 5 |
| Bone | 16 | 41 | 25 | 42 |
| Lung | 19 | 49 | 30 | 51 |
| Liver | 23 | 59 | 32 | 54 |
| Pleura | 6 | 15 | 8 | 14 |
| Brain | 1 | 3 | 2 | 3 |
| Other | 6 | 15 | 8 | 14 |
| Number of sites involved median (range) | 3(1–6) | 3(1–6) | ||
| ER/PgR status at initial diagnosis | ||||
| ER−/PgR− | 21 | 54 | 31 | 53 |
| ER+ and/or PgR+ | 17 | 44 | 25 | 42 |
| Unknown | 1 | 3 | 3 | 5 |
| ER/PgR status at disease recurrence | ||||
| ER−/PgR− | 14 | 36 | 22 | 37 |
| ER+ and/or PgR+ | 4 | 10 | 7 | 12 |
| Unknown | 21 | 54 | 30 | 51 |
| Prior adjuvant/neoadjuvant chemotherapy | 25 | 64 | 35 | 59 |
| Prior taxane | 14 | 36 | 18 | 31 |
| Prior radiation therapy | 18 | 46 | 25 | 42 |
| Prior hormonal therapy | 15 | 38 | 21 | 36 |
Results of central HER2 testing
All patients treated had HER2+ breast cancer by local pathologic evaluation, and 42 tumor samples (71%) underwent centralized testing for HER2. Of the tumors centrally evaluated for HER2, 39 (93%) were confirmed positive. One patient was classified as ineligible because HER2 status was determined by Automated Cellular Imaging System instead of FISH at the outside institution; however, this patient was cHER2+ and thus was included in the primary analysis. Of the 20 patients who did not have cHER2+ tumors, 3 (15%) were negative for HER2 on central testing and 17 (85%) did not undergo centralized testing because of inadequate specimens or lack of available tissue for analysis.
Treatment administered
Among all treated patients, 45 (76%) received all six cycles of induction therapy and 14 (24%) discontinued therapy before cycle 6, after a median of three cycles (range 1–5). Thirty patients (51%) started maintenance therapy and received a median of five cycles (range 1–50). Of the 39 patients who were cHER2+, 28 (72%) received all six cycles of induction therapy and 11 patients (28%) discontinued treatment before cycle 6, after a median of three cycles (range 1–5). Seventeen patients (44%) started maintenance therapy and received a median of four cycles (range 1–50).
Disease progression was the most common reason for treatment discontinuation, accounting for 55% of patients cHER2+ and 66% of all treated patients. Discontinuation for adverse event was similar between cHER2+ and all treated patients (13 and 14%, respectively). Two patients were continuing maintenance therapy as of January 2009.
Efficacy
The RRs for all treated patients and those cHER+ are listed in Table 3. The objective RR for all treated patients was 44% (95% CI 31–58; CR = 7%, PR = 37%) and 41% (95% CI 26–58; CR = 8%, PR = 33%) for cHER2+. In all treated patients, nine had SD ≥ 6 months compared to five patients cHER+ for a clinical benefit rate of 59% (95% CI 46–72) and 56% (95% CI 40–72), respectively. The median duration of response was similar for all treated and cHER2+ patients (7.8 and 7.1 months, respectively). Based upon ixabepilone’s known activity in taxane-refractory breast cancer, we performed an unplanned subset analysis to compare response in patients who had received prior taxane-based adjuvant chemotherapy to those who had not received prior taxane therapy. Response (in all treated patients) was not significantly different for patients who had received adjuvant taxanes compared to patients who were taxane naive [RR = 41% (95% CI 26–58) for taxane-naive patients and 50% (95% CI 26–74) for patients who received prior adjuvant taxanes].
Table 3.
Response as measured by RECIST
| Best response | All treated patients (N = 59)
|
Centrally confirmed HER2+ (N = 39)
|
||
|---|---|---|---|---|
| N | % | N | % | |
| Complete response | 4 | 7 | 3 | 8 |
| Partial response | 22 | 37 | 13 | 33 |
| No change/stable | 15 | 25 | 10 | 26 |
| SD ≥ 6 months | 9 | 15 | 5 | 13 |
| Progression | 16 | 27 | 11 | 28 |
| Unevaluable | 2 | 3 | 2 | 5 |
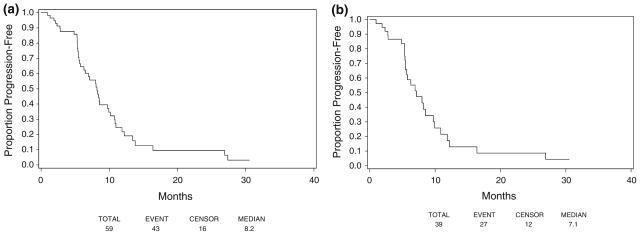
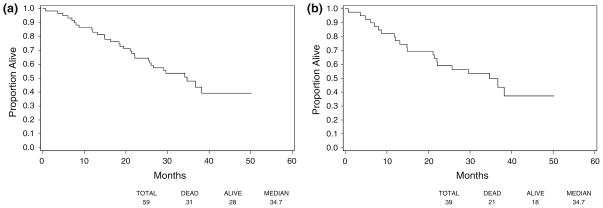
Median TTP for all treated patients was 8.2 months (95% CI 6.3–9.9 months) (Fig. 1a). The median TTP for cHER2+ patients was 7.1 months (95% CI 5.5–9.7 months) (Fig. 1b). Median TTF was 5.9 months for all treated patients (95% CI 5.3–7.6 months) and 5.4 months (95% CI 5.3–6.2 months) for cHER2+. The median OS was 34.7 months for all treated patients and those who were cHER2+ (95% CI 25.7 to [not reached], and 21.4 to [not reached], respectively) (Fig. 2a, b).
Fig. 1.
TTP. a All treated patients, b centrally confirmed HER2+
Fig. 2.
Overall survival. a All treated patients, b centrally confirmed HER2+
Toxicity
Because HER2 status should play no role in treatment-related toxicity, the data reported represent all treated patients (N = 59). Most treatment-related adverse events (TRAEs) were manageable and low grade (Table 4). The most common severe (grade 3 or 4) hematologic toxicities were neutropenia (grade 3 = 30.5%, grade 4 = 18.6%), thrombocytopenia (grade 3 = 11.9%, grade 4 = 1.7%), and anemia (grade 3 = 6.8%, grade 4 = 5.1%). One patient, who was 76 years of age, died from therapy-related complications of diarrhea, hypotension, and presumed sepsis approximately 1 week after receiving cycle 1, days 1 and 8 of therapy. One patient developed grade 1 skin infection in the presence of grades 3–4 neutropenia. There were no reports of febrile neutropenia.
Table 4.
All grade 3/4, and commonly occurring (≥10%) grade 1/2 TRAEs
| Toxicity type | All treated patients (n = 59)
|
|||
|---|---|---|---|---|
| Grade
| ||||
| 1, 2 (n) | 3 (n) | 4 (n) | 5 (n) | |
| Allergic reaction | 8 | 2 | – | – |
| Hemoglobin | 48 | 4 | 3 | – |
| Leukocytes | 35 | 18 | 1 | – |
| Neutrophils | 17 | 18 | 11 | – |
| Platelets | 29 | 7 | 1 | – |
| Hematologic-other | – | 1 | – | – |
| Hypertension | 1 | 1 | – | – |
| Hypotension | – | 1 | – | – |
| Left ventricular systolic dysfunction | 6 | – | – | – |
| Fatigue | 45 | 7 | – | – |
| Insomnia | 7 | – | – | – |
| Weight loss | 9 | 1 | – | – |
| Alopecia | 33 | – | – | – |
| Rash/desquamation | 10 | – | – | – |
| Death (presumed sepsis) | – | – | – | 1 |
| Anorexia | 20 | 3 | – | – |
| Constipation | 24 | – | – | – |
| Dehydration | – | 3 | – | – |
| Diarrhea w/o prior colostomy | 30 | 4 | – | – |
| Dyspepsia | 11 | – | – | – |
| Muco/stomatitis by exam, oral cavity | 17 | – | – | – |
| Nausea | 39 | 4 | – | – |
| Taste disturbance | 21 | – | – | – |
| Vomiting | 18 | 2 | – | – |
| Infection Gr0-2 neut, urinary tract | 3 | 2 | – | – |
| Edema limb | 10 | – | – | – |
| Alkaline phosphatase | 15 | 1 | – | – |
| ALT, SGPT | 24 | – | – | – |
| AST, SGOT | 19 | 1 | – | – |
| Hyperglycemia | 27 | 2 | – | – |
| Hypokalemia | 3 | 1 | – | – |
| Hyponatremia | 1 | 1 | – | – |
| Nonneuropathic generalized weakness | 1 | 1 | – | – |
| Dizziness | 7 | – | – | – |
| Encephalopathy | – | 1 | – | – |
| Neuropathy-motor | 4 | – | – | – |
| Neuropathy-sensory | 32 | 4 | – | – |
| Syncope | – | 1 | – | – |
| Abdomen, pain | 5 | 1 | – | – |
| Extremity-limb, pain | – | 1 | – | – |
| Head/headache | 8 | – | – | – |
| Joint pain | 13 | 1 | – | – |
| Muscle pain | 15 | 1 | – | – |
| Tumor pain | 2 | 1 | – | – |
| Cough | 6 | – | – | – |
| Dyspnea | 11 | 1 | – | – |
| Thrombosis/thrombus/embolism | – | 1 | 1 | – |
Non-hematologic toxicities were mostly mild (grades 1–2). One patient (1.7%) experienced a grade 4 thrombosis/embolism and this was the only grade 4 non-hematologic toxicity reported. The most common grade 3 toxicities were fatigue (11.9%), diarrhea (6.8%), nausea (6.8%), sensory neuropathy (6.8%), anorexia (5.1%), and dehydration (5.1%). Eight patients (13.6%) developed low-grade hypersensitivity reactions and two patients (3.4%) developed grade 3 hypersensitivity reactions. Low-grade (≤2) sensory or motor neuropathy was seen in the majority (61%) of patients treated. No patients developed congestive heart failure, but six patients (10.2%) were noted to have grades 1–2 left ventricular systolic dysfunction. Eight patients (14%) discontinued treatment because of TRAEs.
Discussion
This study was designed based upon the beneficial effects of carboplatin given in combination with paclitaxel and trastuzumab, specifically, the randomized trial reported by Robert et al., which demonstrated an RR of 52% (95% CI 42–62) and median PFS of 10.7 months, which was superior to paclitaxel and trastuzumab alone [6]. In this non-randomized phase II trial, ixabepilone, a novel epothilone, was given three times a weekly for a 4-week schedule combined with carboplatin and weekly trastuzumab as first-line therapy of HER2+ MBC. Although the combination was active, the RR of 41% seen in patients with cHER2+ tumors did not meet the anticipated RR of 75%, which was prespecified as the level of activity required to determine the regimen promising.
Given the limitations of cross-study comparisons, there are several potential reasons that this regimen did not reach anticipated endpoints. First, in the Robert study, paclitaxel and carboplatin were administered every 3 weeks with weekly trastuzumab. Based upon emerging data that weekly paclitaxel and carboplatin in combination with trastuzumab was associated with a higher rate of response than that reported for every 3-week chemotherapy regimens [5], a weekly administration schedule for carboplatin and ixabepilone was chosen for E2103; however, at the time of study design there were no data comparing weekly ixabepilone with a Q-3 week administration schedule for the treatment of MBC. Most recently, Rugo et al. [23] have presented the results of a randomized phase II trial suggesting that weekly ixabepilone given in combination with bevacizumab (N = 46) was associated with a lower RR compared to every 3-week ixabepilone in combination with bevacizumab (N = 45) (RR 50 vs. 71%).
This observation is not unique to ixabepilone. Previous clinical trials have demonstrated that although docetaxel and paclitaxel share similar mechanisms of action, paclitaxel is most effective when using a weekly administration schedule in MBC; yet, a large randomized phase III trial showed a trend for decreased RR (20.3 vs. 35.6%) when weekly docetaxel was compared to a Q-3 week docetaxel [7, 8, 24, 25]. The differences in efficacy between weekly and Q-3 week dosing schedules of docetaxel and paclitaxel have also been confirmed in the adjuvant setting where Q-3 week docetaxel and weekly paclitaxel, but not weekly docetaxel, demonstrated improved disease-free survival compared to Q-3 week paclitaxel [7].
These results may due to subtle structural differences between paclitaxel and docetaxel which influence anti-tumor activity. For example, docetaxel generates tubulin polymers that are structurally different than those induced by paclitaxel and is almost twice as potent as paclitaxel at inhibiting microtubule depolymerization [26, 27]. Preclinical studies of paclitaxel have demonstrated higher potency with prolonged drug exposure, but this was not the case with docetaxel, indicating docetaxel to be a schedule-independent drug [28]. Like docetaxel, ixabepilone is 2.5 times more potent than paclitaxel at microtubule stabilization and, thus, prolonged exposure via weekly dosing may not enhance efficacy [13]. Previous pharmacokinetic studies of ixabepilone have also demonstrated a rapid tissue distribution phase followed by a more prolonged terminal elimination phase similar to that seen with paclitaxel and docetaxel; however, paclitaxel clearance is decreased with increasing dose, suggesting a non-linear elimination process that is not shared by either docetaxel or ixabepilone [28]. As such, it is possible that the weekly administration of ixabepilone may be suboptimal; thus, further studies are needed to determine the best possible dosing regimens for ixabepilone for the treatment of MBC. It is also feasible that ongoing clinical trials of ixabepilone given every 3 weeks in combination with trastuzumab may demonstrate higher activity.
Additionally, although other non-randomized trials have reported response and TTP data similar to the study reported by Robert et al. for the addition of carboplatin to paclitaxel and trastuzumab [2, 3, 5], similar results were not seen in a phase III randomized trial comparing docetaxel and trastuzumab ± carboplatin [29]. Thus, it is clear that not all drugs which share a similar mechanism of action share similar benefit in combination regimens. It is, therefore, possible that carboplatin is not the optimal chemotherapy to combine with ixabepilone and trastuzumab.
Finally, because of concerns about peripheral neuropathy, patients were not allowed to receive more than six cycles of chemotherapy before entering a maintenance phase of single-agent trastuzumab. It is possible that a prolonged exposure to chemotherapy could improve the response to the combination, albeit at the potential cost of increased neuropathy.
It could also be argued that with only 71% of the patients undergoing centralized testing for HER2, the study was underpowered to meet its primary objective. However, similar RRs in all treated patients compared to those centrally confirmed, and the relatively low rate of discordance in HER2 status (7%) between the centralized lab and the outside pathology evaluation, suggest that the results would not be substantially improved with increased numbers of cHER2+ patients.
Toxicities for this regimen were manageable and included fatigue, neuropathy, and myelosuppression. Almost half of the patients treated developed grade 3 or 4 neutropenia, but no patients developed febrile neutropenia. One patient died from diarrhea, hypotension, and presumed septic shock after receiving cycle 1, days 1 and 8 of study treatment. Neuropathy was common, but was low grade in the majority (61%) of the patients treated. No patients developed congestive heart failure; however, six patients (10.2%) were noted to have low-grade impairment of left ventricular systolic dysfunction with serial cardiac imaging.
This trial is one of the first to combine ixabepilone with trastuzumab for the treatment of patients with HER2+ MBC. Although this study did not show significant improvement in efficacy when ixabepilone was given in combination with carboplatin and trastuzumab, the regimen was well tolerated with toxicity and efficacy similar to that seen with paclitaxel given in combination with carboplatin and trastuzumab.
Acknowledgments
This study was supported in part by grants from the Department of Health and Human Services and the National Institutes of Health (CA23318 to the ECOG statistical center, CA66636 to the ECOG data management center, CA21115 to the ECOG coordinating center, CA25224 to NCCTG).
Footnotes
Presented in part at the 2007 ASCO Breast Cancer Symposium, San Francisco CA, (abstract #152) and the 2007 San Antonio Breast Cancer Symposium, San Antonio, TX (abstract #6070).
Contributor Information
Stacy Moulder, Email: smoulder@mdanderson.org, Breast Medical Oncology, The University of Texas, M. D. Anderson Cancer Center, 1155 Pressler Street, Unit 1354, P.O. Box 301438, Houston, TX 77030, USA.
Hailun Li, Dana Farber Cancer Institute, Boston, MA, USA.
Molin Wang, Dana Farber Cancer Institute, Boston, MA, USA.
William J. Gradishar, Northwestern University, Evanston, IL, USA
Edith A. Perez, Mayo Clinic, Jacksonville, FL, USA
Joseph A. Sparano, Montefiore Medical Center, Bronx, NY, USA
Michael Pins, Northwestern University, Evanston, IL, USA.
Ximing Yang, Northwestern University, Evanston, IL, USA.
George W. Sledge, Indiana University Medical Center, Indianapolis, IN, USA
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Burris H, III, Yardley D, Jones S, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol. 2004;22:1621–1629. doi: 10.1200/JCO.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz M, Salvador J, Bayo J, et al. Phase-II study of weekly schedule of trastuzumab, paclitaxel, and carboplatin followed by a week off every 28 days for HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62:1085–1090. doi: 10.1007/s00280-008-0709-7. [DOI] [PubMed] [Google Scholar]
- 4.Pegram MD, Pienkowski T, Northfelt DW, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst. 2004;96:759–769. doi: 10.1093/jnci/djh133. [DOI] [PubMed] [Google Scholar]
- 5.Perez EA, Suman VJ, Rowland KM, et al. Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer. 2005;6:425–432. doi: 10.3816/CBC.2005.n.047. [DOI] [PubMed] [Google Scholar]
- 6.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 9.Gori S, Mosconi AM, Basurtol C, et al. Weekly paclitaxel in metastatic breast cancer patients: a phase II study. Tumori. 2002;88:470–473. doi: 10.1177/030089160208800607. [DOI] [PubMed] [Google Scholar]
- 10.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 11.Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19:4216–4223. doi: 10.1200/JCO.2001.19.22.4216. [DOI] [PubMed] [Google Scholar]
- 12.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on alpha, beta-tubulin by electron crystallography. Science. 2004;305:866–869. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 13.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 14.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 15.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 16.Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005;23:2726–2734. doi: 10.1200/JCO.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Roche H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007;25:3415–3420. doi: 10.1200/JCO.2006.09.7535. [DOI] [PubMed] [Google Scholar]
- 18.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007;25:3421–3427. doi: 10.1200/JCO.2006.10.0784. [DOI] [PubMed] [Google Scholar]
- 19.Awada A, Piccart MJ, Jones SF, et al. Phase I dose escalation study of weekly ixabepilone, an epothilone analog, in patients with advanced solid tumors who have failed standard therapy. Cancer Chemother Pharmacol. 2009;63:417–425. doi: 10.1007/s00280-008-0751-5. [DOI] [PubMed] [Google Scholar]
- 20.Plummer R, Woll P, Fyfe D, et al. A phase I and pharmacokinetic study of ixabepilone in combination with carboplatin in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:8288–8294. doi: 10.1158/1078-0432.CCR-08-0471. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Rugo HS, Campone M, Amadori A, et al. Randomized phase II study of weekly versus every-3-week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first-line therapy for metastatic breast cancer (MBC) J Clin Oncol. 2009;27:15s. (suppl; abstr 1029) [Google Scholar]
- 24.Sparano JA. Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin Breast Cancer. 2000;1:32–40. doi: 10.3816/CBC.2000.n.002. discussion 1–2. [DOI] [PubMed] [Google Scholar]
- 25.Rivera E, Mejia JA, Arun BK, et al. Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer. 2008;112:1455–1461. doi: 10.1002/cncr.23321. [DOI] [PubMed] [Google Scholar]
- 26.Fromes Y, Gounon P, Veitia R, Bissery MC, Fellous A. Influence of microtubule-associated proteins on the differential effects of paclitaxel and docetaxel. J Protein Chem. 1996;15:377–388. doi: 10.1007/BF01886864. [DOI] [PubMed] [Google Scholar]
- 27.Gueritte-Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P. Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem. 1991;34:992–998. doi: 10.1021/jm00107a017. [DOI] [PubMed] [Google Scholar]
- 28.Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994;5:495–505. doi: 10.1093/oxfordjournals.annonc.a058903. [DOI] [PubMed] [Google Scholar]
- 29.Pegram MF, Forbes J, Pienkowski T, et al. BCIRG 007: first overall survival analysis of randomized phase III trial of trastuzumab plus docetaxel with or without carboplatin as first line therapy in HER2 amplified metastatic breast cancer (MBC) J Clin Oncol 2007; ASCO annual meeting proceedings. 2007;25 [Google Scholar]