Abstract
Persons with amnestic mild cognitive impairment (MCI) have subtle impairments in medical decision-making capacity (MDC). We examined the relationship between proton magnetic resonance spectroscopy (MRS) and MDC in MCI. Twenty-nine MCI patients and 42 controls underwent MRS to obtain ratios of N-acetylaspartate (NAA)/Creatine (Cr), Choline (Cho)/Cr, and myo-Inositol (mI)/Cr of the posterior cingulate. They also completed the Capacity to Consent to Treatment Instrument (CCTI), a vignette-based instrument measuring decisional standards of expressing choice, appreciating consequences of choice, providing rational reasons for choice, and understanding treatment choices. Patients showed abnormal MRS ratios of mI/Cr and Cho/Cr compared to controls, and impairments on the CCTI understanding and reasoning Standards. Performance on the Reasoning Standard of the CCTI was correlated with NAA/Cr (r = 0.46, p < 0.05). The relationship of NAA/Cr with decision-making suggests a role for posterior cortical neuronal functioning in performance of complex IADLs in MCI.
Keywords: magnetic resonance spectroscopy, mild cognitive impairment, decision making, posterior cingulate gyrus, hippocampus
INTRODUCTION
Amnestic mild cognitive impairment (MCI) often denotes the transitional phase between normal cognitive aging and Alzheimer’s disease (AD) (Petersen, Doody et al., 2001). The original criteria proposed by Petersen and colleagues indicate that patients with MCI have “generally intact” activities of daily living (Petersen, Stevens et al., 2001), although investigations of instrumental activities of daily life (IADLs) in MCI have revealed mild impairments in informant reported IADLs (Tabert et al., 2002) as well as laboratory assessed IADLs (Griffith et al., 2003). This current investigation focuses on medical decision-making capacity (MDC), an IADL critical to independence in older adults with ethical and public policy implications. Our research group has recently demonstrated that patients with MCI experience subtle deficits in conceptual Standards used to assess MDC, including understanding a treatment choice, providing rational reasons for a treatment choice, and appreciating consequences of treatment choices (Okonkwo et al., 2007). Furthermore, these MDC deficits in MCI patients are strongly associated with cognitive deficits in memory and executive function (Okonkwo, Griffith, Belue et al., 2008), suggesting that brain structures thought to play roles in memory and executive function may also show relationships with MDC performance.
The knowledge of functional neuroanatomy underlying IADL performance in AD and MCI is very limited. For instance, it is unclear whether neuropathological changes in certain brain regions might cause some IADLs, such as MDC, to be particularly vulnerable in MCI (Okonkwo et al., 2007) and prone to rapid declines in mild AD (Huthwaite et al., 2006) and MCI (Okonkwo, Griffith, Copeland et al., 2008). One such brain region of interest is the posterior cingulate gyrus. The posterior cingulate is one of the first regions to show atrophy in preclinical dementia (Fox et al., 2001), shows metabolic changes early in preclinical dementia (Godbolt et al., 2006), and is one of the most highly involved regions in amyolid deposition as measured by the Pittsburgh B compound (Shin, Lee, Kim, Kim, & Cho, 2008). Posterior cingulate metabolic changes can be observed in patients with MCI (Arnaiz et al., 2001) and are found to be intermediate between normal metabolic functioning as seen in healthy older adults and pathological metabolic abnormalities observed in mild AD (Kantarci et al., 2000). Cortical metabolic measures of the posterior cingulate are associated with memory (Nestor, Fryer, Smielewski, & Hodges, 2003) and executive function (Griffith, Hollander et al., 2007) in MCI and have recently been associated with changes in IADLs in patients with AD (Antuono, Jones, Wang, & Li, 2001; Griffith, Okonkwo et al., 2007). Thus, investigating the relationship of posterior cingulate metabolism with a measure of a complex IADL such as MDC appears warranted.
The current study preliminarily explored the relationship between proton magnetic resonance spectroscopy (MRS) measures of the posterior cingulate gyrus in comparison with performance on a vignette-based instrument assessing decisional abilities in patients with amnestic MCI.
METHODS
Participants
Twenty-nine MCI patients participated from those who presented for clinical evaluation to the UAB Memory Disorders Clinic, a tertiary care outpatient clinic. They were subsequently recruited into the UAB Alzheimer’s Disease Research Center (ADRC) and an associated study of functional change in amnestic MCI. The diagnosis of amnestic MCI was made in the ADRC consensus diagnostic conference using Mayo criteria (Petersen, Stevens et al., 2001).
Control participants were 42 volunteers recruited from the community into the ADRC through newspaper advertisements or health fairs. These participants underwent ADRC neurological evaluation and neuropsychological testing to ensure the absence of medical and psychiatric conditions that could compromise cognition and characterized as cognitively normal following ADRC diagnostic consensus conference.
General exclusion criteria included a diagnosis of exclusively non-amnestic MCI, evidence of another neurodegenerative disease, history of stroke, another chronic debilitating neurological illness (i.e., cerebral palsy), severe organ disease, autoimmune disease, cancer (except skin cancer), alcoholism, or a terminal condition with less than 12 months to live. Participants were also excluded if they had untreated major depression or any other severe psychiatric disorder.
All participants gave informed consent according to UAB Institutional Review Board guidelines.
Capacity to Consent to Treatment Instrument (CCTI)
All participants completed the CCTI, a vignette-based direct assessment measure of MDC previously shown to be sensitive to changes in patients with MCI (Okonkwo et al., 2007). The development and psychometric properties of the CCTI are described in prior reports (Dymek, Atchison, Harrell, & Marson, 2001; Marson, Ingram, Cody, & Harrell, 1995). The two vignettes of the CCTI present simulated medical decision-making scenarios. The first vignette (A) examines decisions about treatment for a newly-diagnosed brain neoplasm whereas the second vignette (B) assesses decisions about treatment of a newly-diagnosed coronary artery blockage. The CCTI was administered by trained technicians and each participant’s responses were tape-recorded verbatim and subsequently rated according to five consent standards: expressing a treatment choice (S1), making a reasonable treatment choice (S2), appreciating the consequences of a treatment choice (S3), providing rational reasons for a treatment choice (S4), and understanding the treatment choices (S5). The reasonable treatment choice Standard (S2) was not analyzed in the current study as this Standard is simply scored yes or no and only assessed on Vignette A. For the other four Standards, scores from both vignettes were summed (e.g. S5A + S5B) to yield composite scores representing each Standard (e.g. S5 total). The CCTI raw scores are presented in Table 1.
Table 1.
Group Comparisons on Demographic, Clinical, MRS, and CCTI Variables
| Controls (n = 42) |
Amnestic MCI Pts (n= 29) |
P value | |
|---|---|---|---|
| Age - mean (SD) | 64.83 (8.34) | 70.31 (7.00) | .005 |
| Years of Education - mean (SD) | 15.00 (2.27) | 14.00 (2.84) | .105 |
| Gender: Female / Male | 31 / 11 | 19 / 10 | .452 |
| Race: White / Black | 34 / 8 | 22 / 7 | .606 |
| DRS Total Score (max = 144) - mean (SD) | 139.38 (2.94) | 131.14 (4.71) | .001 |
| MMSE (max = 30) - mean (SD) | 29.43 (1.04) | 28.00 (1.44) | .001 |
| CDR Staging: 0.0 / 0.5 | 42 / 0 | 0 / 29 | .001 |
| Cholinesterase Inhibitor Use: yes/ no | ---- | 11 / 18 | ---- |
| CCTI Standard 1: Expressing Choice (range: 0–4)* | 3.76 (0.48) | 3.86 (0.35) | .612 |
| CCTI Standard 3: Appreciating Consequences (range: 0–8) * | 7.50 (1.11) | 6.93 (1.39) | .116 |
| CCTI Standard 4: Reasoning about Treatment (range: 0–12) * | 10.93 (4.55) | 8.14 (4.09) | .027 |
| CCTI Standard 5: Understanding Treatment (range: 0–78)* | 63.90 (9.12) | 46.93 (12.93) | .001 |
| NAA/Cr * | 1.34 (0.12) | 1.31 (0.14) | .432 |
| Cho/Cr * | 0.64 (0.05) | 0.67 (0.06) | .035 |
| mI/Cr * | 0.90 (0.10) | 0.98 (0.11) | .006 |
values adjusted for age
MRS Methods
Single voxel MR spectra were obtained using a Philips Intera 3T MRI system with a quadrature TR head coil. Spectra were measured with PRESS sequences of 2048 samples and a spectral bandwidth of 2000 Hz using 128 acquisitions. A TR/TE = 2000/32 ms was used to maximize the mI signal (Kantarci et al., 2000). An automated higher-order shim procedure was applied to correct for magnetic field inhomogeneity over the voxel. Suppression of the water signal followed using a frequency selective inversion recovery sequence.
Spectra were obtained from a midline voxel of interest of 20 × 20 × 20mm located superior and posterior to the splenium of the corpus callosum and in the region of the posterior cingulate gyri, inferior to the cingulate sulci and superior to the parieto-occipital sulci. The placement of this voxel has been shown to be reliable in prior work (Griffith, Hollander et al., 2007).
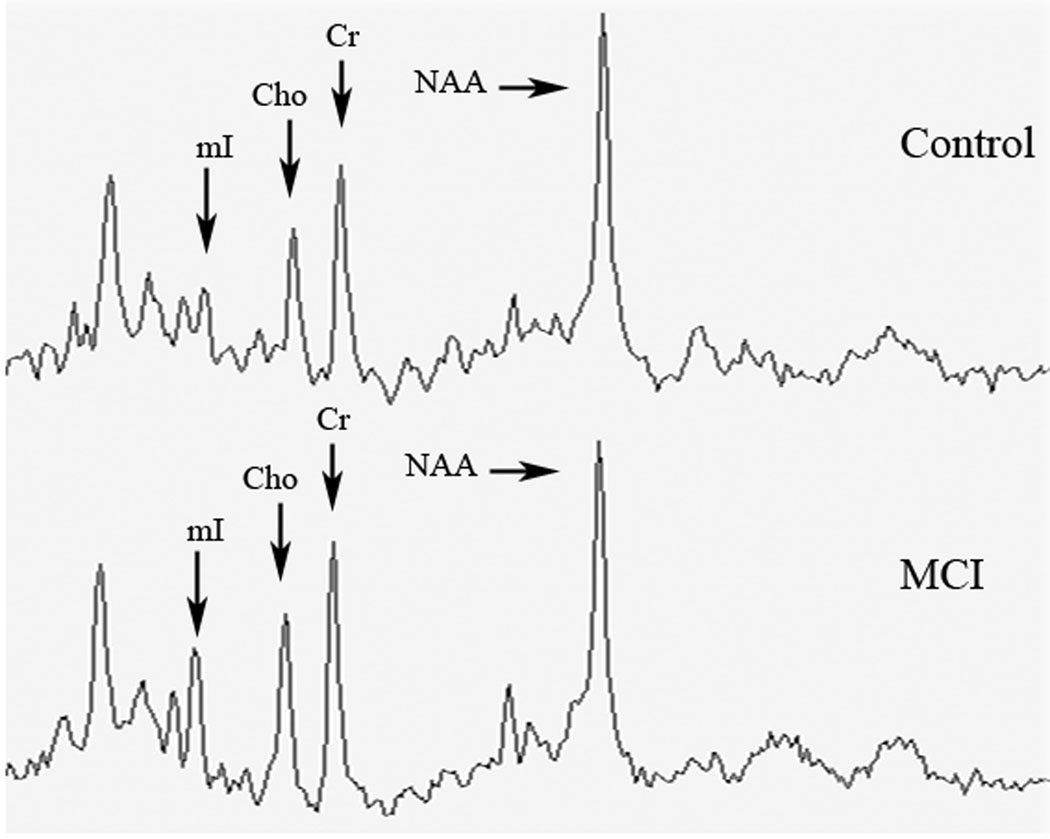
Following MRS data collection, a time domain fitting analysis that utilizes complete prior in vitro knowledge of all metabolites was performed (Lamb et al., 1996). This procedure allowed the derivation of metabolic ratios of N-acetylaspartate (NAA), choline-containing compounds (Cho), and myo-Inositol (mI) in reference to creatine (Cr). Representative spectra are shown in Figure 1.
Figure 1.
Representative posterior cingulate MRS spectra from control participant (top) and MCI participant (bottom) scaled to reference to the Creatine (Cr) peak. Metabolite peaks of interest are labeled (see text for abbreviations).
Statistical Analysis
Group-level analyses were performed to compare the amnestic MCI group to the control group on demographics and mental status scores using independent-samples t tests (continuous variables) or Chi square (categorical variables). Results of these analyses were then used to determine whether covariate-adjustment should be applied to the group comparisons on the CCTI and MRS variables using ANCOVA. Neuropsychological test scores were compared between the MCI patients and controls using ANCOVA as appropriate. Relationships of MRS ratios with the CCTI score was determined using Pearson correlation coefficients in the amnestic MCI patient group only. Secondary analyses using multiple regression within both the controls and MCI patients examined whether the relationship of MRS ratio for predicting CCTI scores differed in controls and MCI patients. Alpha was set at 0.05 (two-tailed) for significance.
RESULTS
Comparison of Amnestic MCI and Control Groups
The patient group was older than the control group, but the groups were similar in terms of years of education, gender, and race (Table 1). The amnestic MCI patient group performed worse than the controls on both the Dementia Rating Scale (DRS) and Mini Mental State Examination (MMSE) scores and had higher Clinical Dementia Rating (CDR) scores (p < .001). Nearly thirty-eight percent of the amnestic MCI participants were taking a cholinesterase inhibitor for treatment of memory loss at the time of the study (donepezil = 8, galantamine = 2, rivastigmine = 1).
Because amnestic MCI patients were older than controls, we adjusted for age while evaluating group differences on CCTI and MRS variables (Table 1). Patients with amnestic MCI performed significantly lower than controls on the CCTI Standards of Reasoning About Treatment (p < .05) and Understanding Treatment (p < .001) but were not significantly different from controls on the Expressing Choice and Appreciation Standards (see Table 2 for examples of responses). Comparison of MRS ratios demonstrated that the amnestic MCI patients had higher posterior cingulate ratios of mI/Cr (p < .01) and Cho/Cr (p < .05) compared to controls, but no significant differences in NAA/Cr.
Table 2.
Example of responses to the Cardiac Blockage vignette from study participants†
| Level of Performance |
Standard 4 Reasons for treatment choice |
Standard 5 Disadvantages of treatment option |
|---|---|---|
| Good response (control) | “I could return to my normal activity and not be confined and stay at home as much; I wouldn’t have to be on the medication for the rest of my life; it would remove the symptoms…To have a more complete life, not to have to depend on anyone.” - 9 point response |
“You would have to take the medication the rest of your life and you would not be able to do the same things you did before and have to stay at home.” - 6 point response |
| Marginal response (MCI) | “…it would cure it immediately; wouldn’t have to depend on the medicine the rest of my life and I would be better off, able to get around and do things a lot better… Consideration of the family.” - 5 point response |
“You would eventually get worse, you wouldn’t be in any pain; you wouldn’t be active and would deteriorate on down the line.” -2 point response |
| Very poor response (advanced MCI) | “Sometimes the medicine wouldn’t work right; valves might still close up. It would be better to get it done when you’re working right than to wait until you’re completely down to do it…You’d be hurting more with greater risk of stroke or heart attack; severe pain and tingling in your feet.” - 0 point response |
“Sometimes it would work, sometimes it wouldn’t. Probably work for a while and then the blockage would get worse. If you missed a dosage the valves would close up, might still need surgery, have a stroke or heart attack.” - 0 point response |
Participants are presented a hypothetical scenario where there are two courses of treatment (heart medicine, heart bypass surgery) each with its own incident risks and benefits. In all of the examples, the participants chose to have heart surgery.
Comparison of Controls and Amnestic MCI patients on Neuropsychological Tests
ANCOVAs adjusting for the age difference between the groups showed that the controls outperformed the amnestic MCI patients on nearly all measures (p values < 0.05) with the exception of simple visual construction, spontaneous clock drawing, and clock copy (Table 3).
Table 3.
Neuropsychological Test Performance of Study Groups
| Controls (n = 42) |
Amnestic MCI Pts (n= 29) |
P value* | |
|---|---|---|---|
| DRS Attention | 36.26 (1.01) | 34.83 (1.83) | .001 |
| Spatial Span Total (WMS-III) | 14.67 (3.24) | 12.55 (2.82) | .037 |
| Boston Naming Test (30-item) | 27.64 (1.89) | 24.28 (4.81) | .001 |
| Semantic Word Fluency | 59.24 (9.93) | 47.10 (6.93) | .001 |
| DRS Memory | 24.02 (1.00) | 20.79 (3.75) | .001 |
| Logical Memory I (WMS-R) | 26.90 (4.49) | 17.00 (7.45) | .001 |
| Logical Memory II (WMS-R) | 23.55 (4.87) | 10.62 (7.29) | .001 |
| Visual Reproduction I (WMS-III) | 77.57 (12.24) | 58.59 (15.79) | .001 |
| Visual Reproduction II (WMS-III) | 54.48 (18.37) | 26.86 (17.38) | .001 |
| CVLT-II Total Learning Trials 1–5 | 47.95 (7.94) | 31.69 (7.25) | .001 |
| CVLT-II Short Delay Free Recall | 9.52 (2.73) | 4.31 (2.42) | .001 |
| CVLT-II Long Delay Free Recall | 10.93 (2.65) | 4.62 (2.91) | .001 |
| DRS Construction | 5.74 (0.7) | 5.62 (0.8) | .419 |
| CLOX 2 | 13.52 (1.09) | 13.07 (1. 07) | .321 |
| DRS Conceptualization | 36.55 (2.29) | 34.28 (3.55) | .012 |
| DRS Initiation/Perseveration | 36.71 (0.60) | 35.38 (2.13) | .002 |
| Trail Making Test A (sec.) | 30.48 (9.89) | 42.93 (21.26) | .007 |
| Trail Making Test B (sec.) | 74.19 (19.63) | 141.41 (71.04) | .001 |
| CLOX 1 | 11.86 (1.78) | 11.10 (2.13) | .143 |
| Digit Symbol (WAIS-III) | 65.71 (14.65) | 44.71 (11.04) | .001 |
corrected for age
CLOX = Executive Clock Drawing Test; CVLT-II = California Verbal Learning Test II; DRS = Dementia Rating Scale; WAIS-III = Wechsler Adult Intelligence Scale III; WMS-III = Wechsler Memory Scale III; WMS-R = Wechsler Memory Scale - Revised
Correlation of CCTI Performance with MRS Ratios
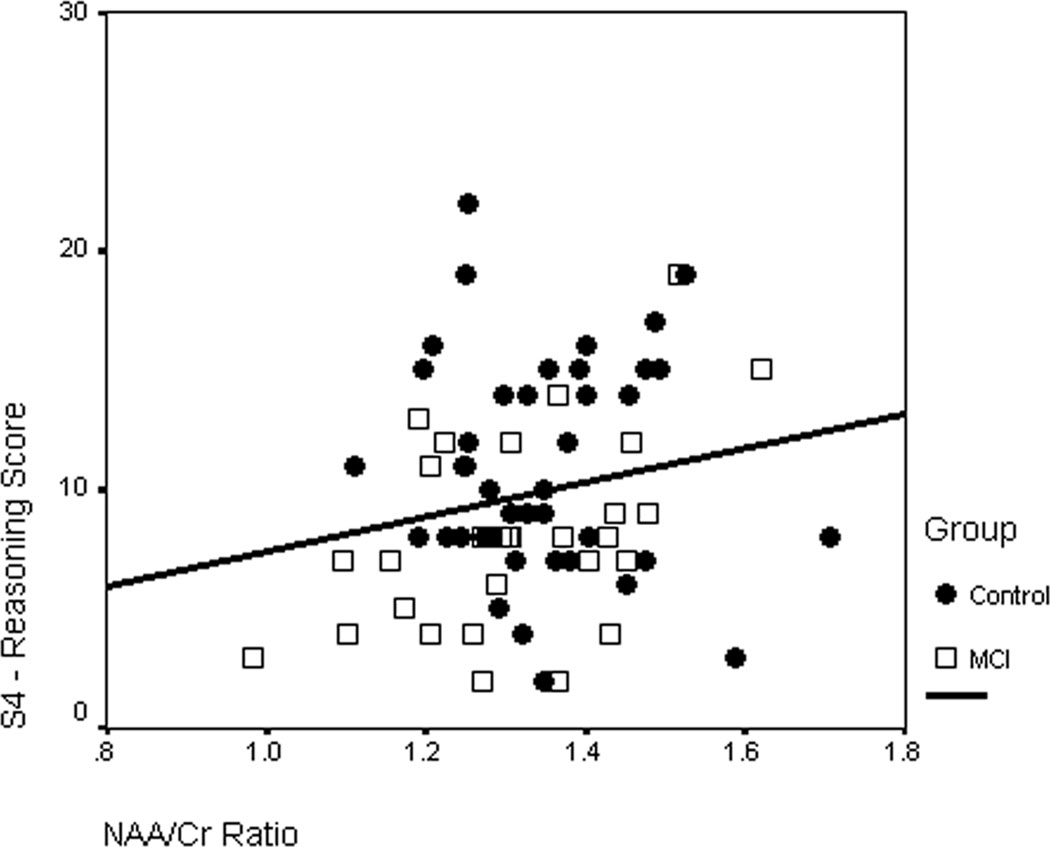
In the amnestic MCI group, the NAA/Cr ratio was positively correlated with Standard 4 (p < 0.05) (Table 4). No other significant correlations were observed between CCTI scores and MRS ratios.
Table 4.
Correlations between MRS Ratios and CCTI Scores in Patients with Amnestic MCI
| NAA/Cr | Cho/Cr | mI/Cr | |
|---|---|---|---|
| Standard 1: Expressing Choice | −.08 | −.03 | .04 |
| Standard 3: Appreciating Consequences | .06 | .13 | .08 |
| Standard 4: Reasoning about Treatment | .46* | .20 | −.10 |
| Standard 5: Understanding Treatment | .17 | .03 | −.18 |
P < .05
Regression of group and MRS ratios with CCTI scores
A linear regression model was fitted to determine whether the relationship between NAA/Cr and Standard 4 differed between the study groups. This model included terms for group (i.e., control vs. MCI), NAA/Cr, and group × NAA/Cr. The group × NAA/Cr interaction term was marginally significant (beta = −.30, SE = .94, t = −1.90, P = .062), indicating that the association between NAA/Cr and performance on Standard 4 was potentially dissimilar in control and MCI groups. Follow up simple main effects analyses revealed that whereas this association was significant among MCI patients (beta = .38, SE = .66, t = 2.34, P = .021), it was non-significant among controls (beta = −.05, SE = .67, t = −.33, P = .745). This suggests that the ability of NAA/Cr to predict Standard 4 scores is not a generalized phenomenon, but rather specific to MCI patients.
DISCUSSION
This study is one of the first to explore correlations between brain metabolic measures and complex higher-order decision-making in patients with amnestic MCI. Our preliminary findings suggest that posterior cortical brain metabolism of NAA/Cr was associated with verbal reasoning abilities related to medical decision-making in patients with amnestic MCI. These findings together with other recent preliminary work suggest that posterior cortical brain metabolism may play an important role in cognitively-mediated daily activities in patients with amnestic MCI.
The NAA/Cr ratio of the posterior cingulate gyrus was correlated with performance on the Reasoning Standard of the CCTI. NAA/Cr is thought to represent the integrity of neurons (Valenzuela & Sachdev, 2001) and is positively correlated with performance on cognitive measures such as the MMSE in patients with AD (Valenzuela & Sachdev, 2001) and the DRS in patients with MCI (Kantarci et al., 2002). We observed NAA/Cr ratios to be within normal limits in our MCI patients (Table 1). While NAA/Cr levels are often abnormal in patients with AD, relatively normal NAA/Cr levels are usually observed in MCI in comparison with AD (Kantarci et al., 2000). NAA/Cr in patients with MCI is usually higher than is observed in AD but may be lower than is observed in controls (Kantarci et al., 2003). Thus, the positive relationship of NAA/Cr with CCTI performance in MCI patients may reflect normal metabolic functioning of the posterior cingulate in contributing to a network involved in cognitively demanding decision-making abilities, as well as potential early declines in NAA/Cr in MCI patients who are close to transitioning to dementia.
The study’s preliminary findings, in conjunction with those from other research, suggest that the posterior cingulate gyrus may play a role in performance of cognitively demanding IADLs in patients with MCI and AD. Our group has previously demonstrated that NAA/Cr of the posterior cingulate is positively associated with performance on a direct assessment measure of financial abilities in patients with AD (Griffith, Okonkwo et al., 2007), and similar findings have been reported with measures of other IADLs (Antuono et al., 2001). Interestingly, preliminary results from another recent study by our group found that resting PET glucose metabolism of the posterior cingulate was associated with informant report of IADLs in AD (Clark, Griffith, & ADNI Study Group, 2008). The posterior cingulate is known to be interconnected with diverse brain regions involved in higher-order cognition, such as the frontal midline regions (Braak & Braak, 1993), orbitofrontal and dorsolateral prefrontal cortices (Baleydier & Mauguiere, 1980; Petrides & Pandya, 1999), and medial temporal lobes (Baleydier & Mauguiere, 1980). Due to its widespread interconnectivity, the posterior cingulate is well situated to be potentially involved in memory, executive function, judgment, and affective/motivational aspects of cognition (Baleydier & Mauguiere, 1980; Braak & Braak, 1993; Petrides & Pandya, 1999). Given that memory and executive function are the primary neurocognitive predictors of CCTI performance in MCI (Okonkwo, Griffith, Belue et al., 2008), the posterior cingulate’s role in memory could also help explain the association with the CCTI. Further investigation would thus appear warranted.
Interestingly, this study did not find a correlation between mI/Cr and decisional abilities in amnestic MCI, despite finding that mI/Cr was higher in the MCI patients compared to controls, in keeping with other reports (Kantarci et al., 2000). The most common interpretation of increased mI/Cr in MCI is that this represents gliosis or immune responses to gliosis (Kantarci et al., 2000; Valenzuela & Sachdev, 2001). However, mI/Cr abnormalities do not appear to progress over time in MCI (Kantarci et al., 2007) and are not predictors of conversion to Alzheimer’s disease in MCI patients (Modrego, Fayed, & Pina, 2005). The NAA/Cr ratio has been shown to be sensitive to both progression in MCI and risk of conversion to AD. Given that declines in decision-making abilities correspond over time with declines in amnestic MCI and conversion to dementia (Okonkwo, Griffith, Copeland et al., 2008), the correspondence with NAA/Cr rather than mI/Cr might suggest that changes in decision-making in MCI would correspond over time with declines in NAA/Cr. Future studies could also address this hypothesis.
Eleven of the 29 participants in our amnestic MCI patient group were taking a cholinesterase inhibitor at the time of the study. We analyzed the study results in subgroups of amnestic MCI participants taking versus not taking cholinesterase inhibitors (data not shown). These findings suggest that the NAA/Cr ratio is lower in those MCI patients who are taking a cholinesterase inhibitor versus those not on a cholinesterase inhibitor. While such a finding seems counter-intuitive at first, we would point out that patients placed on a cholinesterase inhibitor may be more advanced in their MCI and thus closer to AD (Griffith et al., 2006), as NAA/Cr reductions are more characteristic of AD than MCI (Kantarci et al. 2000). As there were no group differences in MCI and controls on NAA/Cr, we believe that the effect of cholinesterase inhibitor use is unlikely to influence the study conclusions. However, further study into the long-term effects of cholinesterase inhibitors on brain metabolism would be of interest.
Possible limiting factors should be acknowledged. Persons with amnestic MCI in this study are undergoing longitudinal follow-up as part of their participation in the UAB ADRC. As this is a cross-sectional study, the ultimate clinical and neuropathological status of these participants is unknown as of yet. Our Center’s observed rates of conversion to AD from amnestic MCI is consistent with the annualized 15% rate of risk (Griffith et al., 2006), indicating that a majority of the participants in this study will likely clinically manifest AD in the future. However, this study’s findings could prove different if conducted in amnestic MCI patients who all eventually converted to AD. The CCTI is a clinical research instrument assessing decision-making abilities related to medical care. Although the instrument reflects medical consent scenarios common in the medical care of older adults, performance of the MCI patients on the CCTI does not necessarily reflect their actual consent capacity.
In conclusion, this study represents an initial attempt to link measures of brain metabolism with indicators of decisional abilities in a memory-disordered patient population. These findings suggest that NAA/Cr of the posterior cingulate is associated with decisional abilities in persons with amnestic MCI. Further work should examine the role of other brain regions in decisional abilities in amnestic MCI to help strengthen the current findings. However, this study, along with other studies recently reported in the literature, suggests that the posterior cingulate may play roles as part of a network involved in IADLs.
Figure 2.
Scatterplot of the relationship of NAA/Cr with CCTI Standard 4 (Reasoning about treatment) score in the amnestic MCI patients and controls.
ACKNOWLEDGEMENTS
This study was funded by grants from the National Institute of Aging (Alzheimer’s Disease Research Center - 1P50 AG16582-01: Harrell, PI); (1R01 AG021927-01: Marson, PI) and Alzheimer’s of Central Alabama. The funding sources had no involvement in the study design, collection, analysis, and interpretation of data or the report writing and decision regarding submission.
Footnotes
The authors acknowledge that there were no financial or other conflicts of interest.
LITERATURE CITED
- Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer's disease detected in vivo with 1H-MRS at 0.5 T. Neurology. 2001;56(6):737–742. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, Nordberg A. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001;12(4):851–855. doi: 10.1097/00001756-200103260-00045. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103(3):525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer neuropathology and limbic circuits. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. pp. 606–626. [Google Scholar]
- Clark DG, Griffith HR ADNI Study Group. Brain Glucose Metabolism is Associated with Everyday Functional Activities in Patients with Mild Cognitive Impairment and Patients with Alzheimer's Disease. Journal of the International Neuropsychological Society. 2008;14(S1):248. [Google Scholar]
- Dymek M, Atchison P, Harrell L, Marson DC. Competency to consent to medical treatment in cognitively impaired patients with Parkinson's disease. Neurology. 2001;56(1):17–24. doi: 10.1212/wnl.56.1.17. [DOI] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358(9277):201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Godbolt AK, Waldman AD, MacManus DG, Schott JM, Frost C, Cipolotti L, Fox NC, Rossor MN. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology. 2006;66(5):718–722. doi: 10.1212/01.wnl.0000201237.05869.df. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Belue K, Sicola A, Krzywanski S, Zamrini E, Harrell L, Marson DC. Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology. 2003;60(3):449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Hollander JA, Okonkwo O, Evanochko WT, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Executive function is associated with brain proton magnetic resonance spectroscopy in amnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29(6):599–609. doi: 10.1080/13803390600826595. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Netson KL, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Amnestic mild cognitive impairment: Diagnostic outcomes and clinical prediction over a two-year time period. Journal of the International Neuropsychological Society. 2006;12(2):166–175. doi: 10.1017/S1355617706060267. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Okonkwo OC, den Hollander JA, Belue K, Lanza S, Harrell LE, Brockington JC, Clark DG, Marson DC. Brain proton MRS is correlated with financial abilities in patients with Alzheimer's disease. Brain Imaging & Behavior. 2007;1:23–29. [Google Scholar]
- Huthwaite JS, Martin RC, Griffith HR, Anderson B, Harrell LE, Marson DC. Declining medical decision-making capacity in mild AD: a two-year longitudinal study. Behavioral Sciences and the Law. 2006;24(4):453–463. doi: 10.1002/bsl.701. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3 T. American Journal of Neuroradiology. 2003;24(5):843–849. [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Smith GE, Ivnik RJ, Petersen RC, Boeve BF, Knopman DS, Tangalos EG, Jack CR., Jr 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer's disease. Journal of the International Neuropsychological Society. 2002;8(7):934–942. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, Reyes D, Shiung M, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiology of Aging. 2007;28(9):1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb HJ, Doornbos J, den Hollander JA, Luyten PR, Beyerbacht HP, van der Wall EE, de Roos A. Reproducibility of human cardiac 31P-NMR spectroscopy. NMR in Biomedicine. 1996;9(5):217–227. doi: 10.1002/(SICI)1099-1492(199608)9:5<217::AID-NBM419>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Marson DC, Ingram KK, Cody HA, Harrell LE. Assessing the competency of patients with Alzheimer's disease under different legal standards. A prototype instrument. Archives of Neurology. 1995;52(10):949–954. doi: 10.1001/archneur.1995.00540340029010. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer's disease predicted by brain magnetic resonance spectroscopy. American Journal of Psychiatry. 2005;162(4):667–675. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Annals of Neurology. 2003;54(3):343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Okonkwo O, Griffith HR, Belue K, Lanza S, Zamrini EY, Harrell LE, Brockington JC, Clark D, Raman R, Marson DC. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–1535. doi: 10.1212/01.wnl.0000277639.90611.d9. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Griffith HR, Belue K, Lanza S, Zamrini EY, Harrell LE, Brockington JC, Clark D, Raman R, Marson DC. Cognitive models of medical decision-making capacity in patients with mild cognitive impairment. Journal of the International Neuropsychological Society. 2008;14(2):297–308. doi: 10.1017/S1355617708080338. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Griffith HR, Copeland JN, Belue K, Lanza S, Zamrini EY, Harrell LE, Brockington JC, Clark D, Raman R, Marson DC. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71(19):1474–1480. doi: 10.1212/01.wnl.0000334301.32358.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Shin J, Lee SY, Kim SH, Kim YB, Cho ZH. Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer's disease. Neuroimage. 2008;43(2):236–244. doi: 10.1016/j.neuroimage.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Tabert M, Albert S, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devenand D. Functional deficits in patients with mild cognitive impairment: Prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56(5):592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]